Echo-Doppler Predictors of Residual Pulmonary Hypertension After Pulmonary Thromboendarterectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Hemodynamic Data
2.3. Transthoracic Echocardiography
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics Before Surgery
3.2. Hemodynamic Data
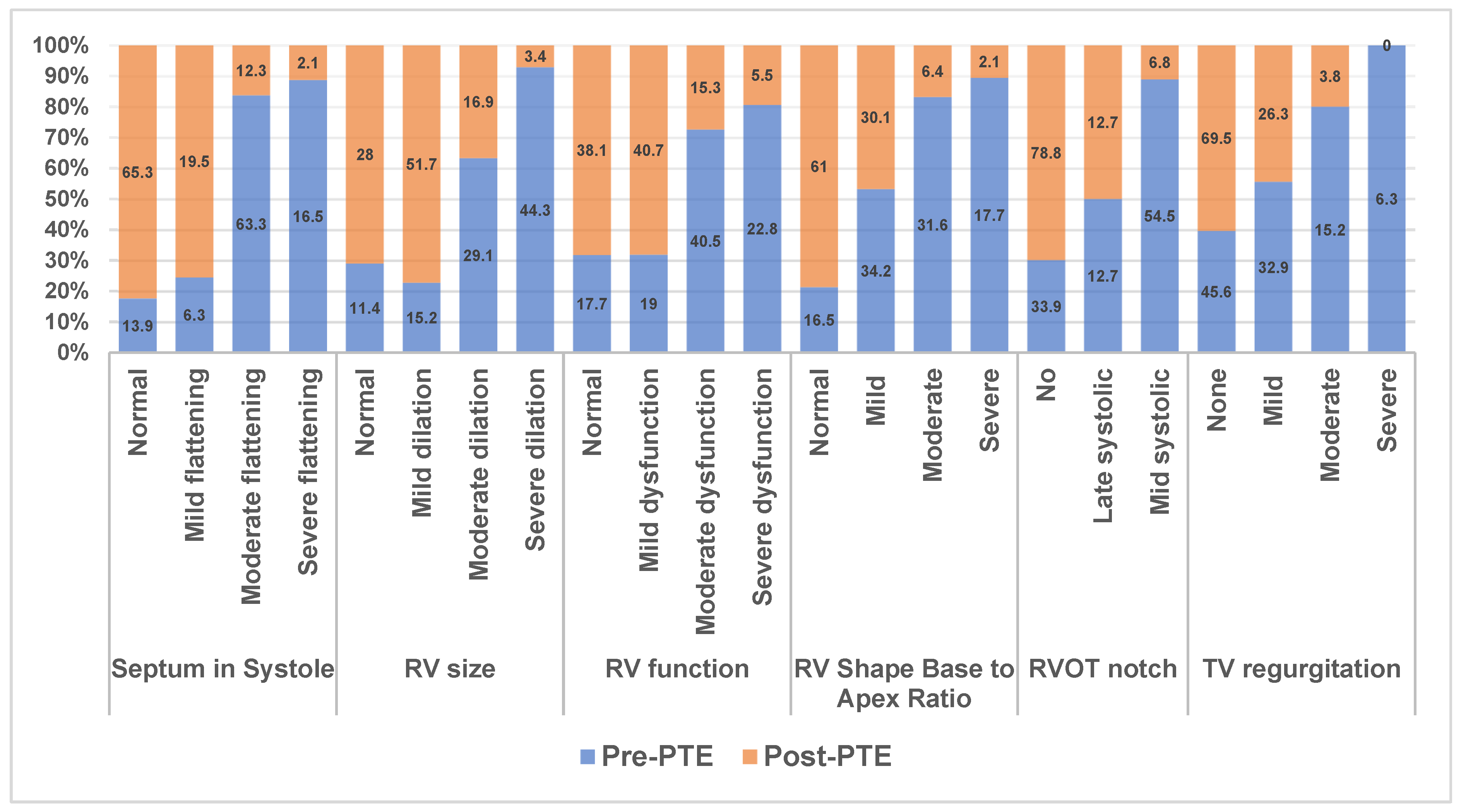
3.3. Echocardiographic Data
3.4. Inter-Observer Variability Agreement
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lang, I.M.; Pesavento, R.; Bonderman, D.; Yuan, J.X. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: A current understanding. Eur. Respir. J. 2013, 41, 462–468. [Google Scholar] [CrossRef]
- Ende-Verhaar, Y.M.; Cannegieter, S.C.; Vonk Noordegraaf, A.; Delcroix, M.; Pruszczyk, P.; Mairuhu, A.T.; Huisman, M.V.; Klok, F.A. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: A contemporary view of the published literature. Eur. Respir. J. 2017, 49, 1601792. [Google Scholar] [CrossRef]
- Brookes, J.D.L.; Li, C.; Chung, S.T.W.; Brookes, E.M.; Williams, M.L.; McNamara, N.; Martin-Suarez, S.; Loforte, A. Pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension: A systematic review. Ann. Cardiothorac. Surg. 2022, 11, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Rahnavardi, M.; Yan, T.D.; Cao, C.; Vallely, M.P.; Bannon, P.G.; Wilson, M.K. Pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension: A systematic review. Ann. Thorac. Cardiovasc. Surg. 2011, 17, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Madani, M.M. Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: State-of-the-art 2020. Pulm. Circ. 2021, 11, 20458940211007372. [Google Scholar] [CrossRef]
- Kerr, K.M.; Elliott, C.G.; Chin, K.; Benza, R.L.; Channick, R.N.; Davis, R.D.; He, F.; LaCroix, A.; Madani, M.M.; McLaughlin, V.V.; et al. Results From the United States Chronic Thromboembolic Pulmonary Hypertension Registry: Enrollment Characteristics and 1-Year Follow-up. Chest 2021, 160, 1822–1831. [Google Scholar] [CrossRef]
- Corsico, A.G.; D’Armini, A.M.; Cerveri, I.; Klersy, C.; Ansaldo, E.; Niniano, R.; Gatto, E.; Monterosso, C.; Morsolini, M.; Nicolardi, S.; et al. Long-term outcome after pulmonary endarterectomy. Am. J. Respir. Crit. Care Med. 2008, 178, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Freed, D.H.; Thomson, B.M.; Berman, M.; Tsui, S.S.; Dunning, J.; Sheares, K.K.; Pepke-Zaba, J.; Jenkins, D.P. Survival after pulmonary thromboendarterectomy: Effect of residual pulmonary hypertension. J. Thorac. Cardiovasc. Surg. 2011, 141, 383–387. [Google Scholar] [CrossRef]
- Bonderman, D.; Skoro-Sajer, N.; Jakowitsch, J.; Adlbrecht, C.; Dunkler, D.; Taghavi, S.; Klepetko, W.; Kneussl, M.; Lang, I.M. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation 2007, 115, 2153–2158. [Google Scholar] [CrossRef]
- Cannon, J.E.; Su, L.; Kiely, D.G.; Page, K.; Toshner, M.; Swietlik, E.; Treacy, C.; Ponnaberanam, A.; Condliffe, R.; Sheares, K.; et al. Dynamic Risk Stratification of Patient Long-Term Outcome After Pulmonary Endarterectomy: Results From the United Kingdom National Cohort. Circulation 2016, 133, 1761–1771. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Forfia, P.R.; Vachiery, J.L. Echocardiography in pulmonary arterial hypertension. Am. J. Cardiol. 2012, 110, 16S–24S. [Google Scholar] [CrossRef]
- Augustine, D.X.; Coates-Bradshaw, L.D.; Willis, J.; Harkness, A.; Ring, L.; Grapsa, J.; Coghlan, G.; Kaye, N.; Oxborough, D.; Robinson, S.; et al. Echocardiographic assessment of pulmonary hypertension: A guideline protocol from the British Society of Echocardiography. Echo Res. Pract. 2018, 5, G11–G24. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Ginghina, C.; Easaw, J.; Muraru, D.; Grillo, M.T.; Lancellotti, P.; Pinamonti, B.; Coghlan, G.; Marra, M.P.; Popescu, B.A.; et al. Right ventricle in pulmonary arterial hypertension: Haemodynamics, structural changes, imaging, and proposal of a study protocol aimed to assess remodelling and treatment effects. Eur. J. Echocardiogr. 2010, 11, 27–37. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713; quiz 786–788. [Google Scholar] [PubMed]
- Raza, F.; Dillane, C.; Mirza, A.; Brailovsky, Y.; Weaver, S.; Keane, M.G.; Forfia, P. Differences in right ventricular morphology, not function, indicate the nature of increased afterload in pulmonary hypertensive subjects with normal left ventricular function. Echocardiography 2017, 34, 1584–1592. [Google Scholar] [CrossRef]
- Arkles, J.S.; Opotowsky, A.R.; Ojeda, J.; Rogers, F.; Liu, T.; Prassana, V.; Marzec, L.; Palevsky, H.I.; Ferrari, V.A.; Forfia, P.R. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2011, 183, 268–276. [Google Scholar] [CrossRef]
- Mayer, E.; Jenkins, D.; Lindner, J.; D’Armini, A.; Kloek, J.; Meyns, B.; Ilkjaer, L.B.; Klepetko, W.; Delcroix, M.; Lang, I.; et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J. Thorac. Cardiovasc. Surg. 2011, 141, 702–710. [Google Scholar] [CrossRef]
- Reesink, H.J.; van der Plas, M.N.; Verhey, N.E.; van Steenwijk, R.P.; Kloek, J.J.; Bresser, P. Six-minute walk distance as parameter of functional outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J. Thorac. Cardiovasc. Surg. 2007, 133, 510–516. [Google Scholar] [CrossRef]
- Naeije, R.; Torbicki, A. More on the noninvasive diagnosis of pulmonary hypertension: Doppler echocardiography revisited. Eur. Respir. J. 1995, 8, 1445–1449. [Google Scholar] [CrossRef]
- Rubens, F.D.; Bourke, M.; Hynes, M.; Nicholson, D.; Kotrec, M.; Boodhwani, M.; Ruel, M.; Dennie, C.J.; Mesana, T. Surgery for chronic thromboembolic pulmonary hypertension--inclusive experience from a national referral center. Ann. Thorac. Surg. 2007, 83, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- D’Armini, A.M.; Cattadori, B.; Monterosso, C.; Klersy, C.; Emmi, V.; Piovella, F.; Minzioni, G.; Vigano, M. Pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension: Hemodynamic characteristics and changes. Eur. J. Cardiothorac. Surg. 2000, 18, 696–701; discussion 701–702. [Google Scholar] [CrossRef]
- Raza, F.; Vaidya, A.; Lacharite-Roberge, A.S.; Lakhter, V.; Al-Maluli, H.; Ahsan, I.; Boodram, P.; Dass, C.; Rogers, F.; Keane, M.G.; et al. Initial clinical and hemodynamic results of a regional pulmonary thromboendarterectomy program. J. Cardiovasc. Surg. 2018, 59, 428–437. [Google Scholar] [CrossRef]
- D’Armini, A.M.; Zanotti, G.; Ghio, S.; Magrini, G.; Pozzi, M.; Scelsi, L.; Meloni, G.; Klersy, C.; Vigano, M. Reverse right ventricular remodeling after pulmonary endarterectomy. J. Thorac. Cardiovasc. Surg. 2007, 133, 162–168. [Google Scholar] [CrossRef]
- Giusca, S.; Dambrauskaite, V.; Scheurwegs, C.; D’Hooge, J.; Claus, P.; Herbots, L.; Magro, M.; Rademakers, F.; Meyns, B.; Delcroix, M.; et al. Deformation imaging describes right ventricular function better than longitudinal displacement of the tricuspid ring. Heart 2010, 96, 281–288. [Google Scholar] [CrossRef]
- Wong, D.J.; Sampat, U.; Gibson, M.A.; Auger, W.R.; Madani, M.M.; Daniels, L.B.; Raisinghani, A.B.; DeMaria, A.N.; Blanchard, D.G. Tricuspid annular plane systolic excursion in chronic thromboembolic pulmonary hypertension before and after pulmonary thromboendarterectomy. Echocardiography 2016, 33, 1805–1809. [Google Scholar] [CrossRef] [PubMed]
- Marston, N.; Brown, J.P.; Olson, N.; Auger, W.R.; Madani, M.M.; Wong, D.; Raisinghani, A.B.; DeMaria, A.N.; Blanchard, D.G. Right ventricular strain before and after pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension. Echocardiography 2015, 32, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.C.; Madhan, A.S.; Kislitsina, O.; Elenbaas, C.; Nishtala, A.; Freed, B.; Schimmel, D.; Thomas, J.D.; Cuttica, M.; Malaisrie, S.C. Temporal trends in right heart strain in patients undergoing pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension. Echocardiography 2021, 38, 1932–1940. [Google Scholar] [CrossRef]
- Thistlethwaite, P.A.; Mo, M.; Madani, M.M.; Deutsch, R.; Blanchard, D.; Kapelanski, D.P.; Jamieson, S.W. Operative classification of thromboembolic disease determines outcome after pulmonary endarterectomy. J. Thorac. Cardiovasc. Surg. 2002, 124, 1203–1211. [Google Scholar] [CrossRef]



| Variables | Mean ± SD |
|---|---|
| Age (years) | 59.5 ± 14.7 |
| BMI (kg/m2) | 31.9 ± 7.9 |
| 6 min walk distance (meters) | 339.2 ± 136.2 |
| Variables | n (%) |
| Race/Ethnicity | |
| White | 126 (53.4%) |
| Latinx | 15 (6.4%) |
| Black | 78 (33.1%) |
| WHO Functional Class | |
| I | 18 (7.6%) |
| II | 64 (27.1%) |
| III | 124 (52.5%) |
| IV | 21 (8.9%) |
| Pre-PH therapy | 77 (32%) |
| DOAC | 106 (44.9%) |
| Tobacco use | 104 (44%) |
| History of thromboembolic events | 189 (80.1%) |
| History of DVT | 124 (53%) |
| RHC Parameters | Baseline Hemodynamics Mean ± SD | Early Post-PTE Hemodynamics Mean ± SD | p Value |
|---|---|---|---|
| RAP (mmHg) | 10.2 ± 5 | 7.9 ± 3.7 | <0.001 |
| Systolic PA (mmHg) | 73.9 ± 20.7 | 39.8 ± 15.2 | <0.001 |
| Diastolic PA (mmHg) | 26.3 ± 8.2 | 15.9 ± 6.3 | <0.001 |
| Mean PA (mmHg) | 43.8 ± 11.7 | 23.9 ± 8.5 | <0.001 |
| PCWP (mmHg) | 11.9 ± 5.1 | NA | |
| CO (lpm) | 4.4 ± 1.2 | 5.6 ± 1.3 | <0.001 |
| CI (lpm/m2) | 2.1 ± 0.5 | 2.7 ± 0.5 | <0.001 |
| TPR (WU) | 11.1 ± 4.8 | 4.5 ± 1.9 | <0.001 |
| PVR (WU) | 8.4 ± 4.4 |
| Variables | Group A No Intervention | Group B Intervention | p Value |
|---|---|---|---|
| Age (years) | 58 ± 14 | 61 ± 14 | 0.204 |
| BMI (kg/m2) | 32.1 ± 7.9 | 30.5 ± 8 | 0.218 |
| Pre-PTE Mean PA pressure (mmHg) | 43 ± 12 | 48 ± 10 | 0.357 |
| CI (L/min/m2) | 2.2 ± 0.5 | 1.9 ± 0.5 | 0.580 |
| TPR (WU) | 10.5 ± 4.8 | 13.3 ± 4.8 | 0.177 |
| Mean DESS | 9.1 ± 4.3 | 9.2 ± 4.8 | 0.9 |
| Post-PTE Mean PA pressure (mmHg) | 22.4 ± 7.7 | 29.9 ± 9.04 | 0.421 |
| CI (L/min/m2) | 2.7 ± 0.5 | 2.7 ± 0.5 | 0.457 |
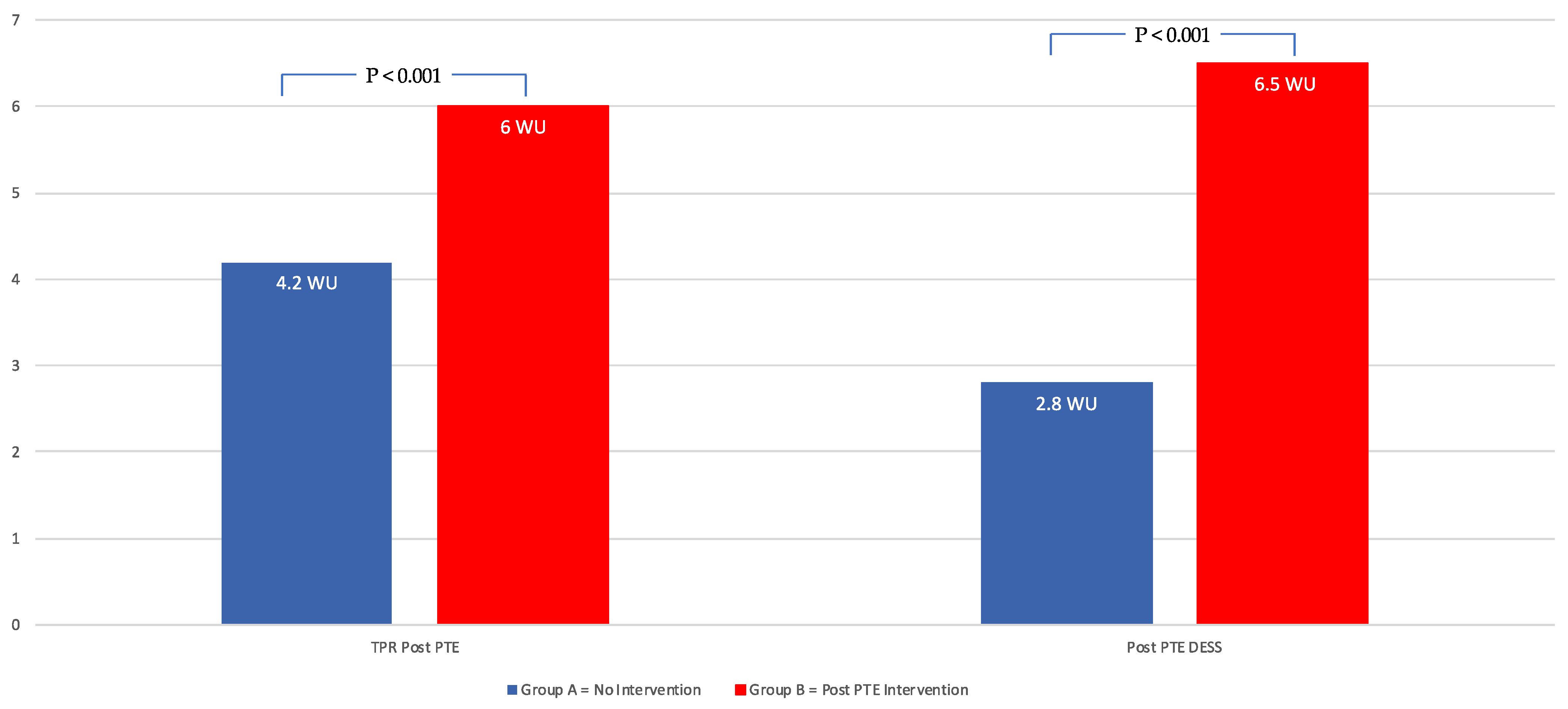
| TPR (WU) | 4.2 ± 1.6 | 6 ± 2.5 | <0.001 |
| Mean DESS | 2.8 ± 2.5 | 6.5 ± 4.6 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveros, E.; Jonnalagadda, A.; Pietrowicz, R.; Mauri, M.; Zhao, H.; Maruthi, R.; Saunders, H.; Lakhter, V.; Brailovsky, Y.; Bashir, R.; et al. Echo-Doppler Predictors of Residual Pulmonary Hypertension After Pulmonary Thromboendarterectomy. J. Clin. Med. 2025, 14, 5705. https://doi.org/10.3390/jcm14165705
Oliveros E, Jonnalagadda A, Pietrowicz R, Mauri M, Zhao H, Maruthi R, Saunders H, Lakhter V, Brailovsky Y, Bashir R, et al. Echo-Doppler Predictors of Residual Pulmonary Hypertension After Pulmonary Thromboendarterectomy. Journal of Clinical Medicine. 2025; 14(16):5705. https://doi.org/10.3390/jcm14165705
Chicago/Turabian StyleOliveros, Estefania, Anil Jonnalagadda, Rylie Pietrowicz, Madeline Mauri, Huaqing Zhao, Rohit Maruthi, Hollie Saunders, Vladimir Lakhter, Yevgeniy Brailovsky, Riyaz Bashir, and et al. 2025. "Echo-Doppler Predictors of Residual Pulmonary Hypertension After Pulmonary Thromboendarterectomy" Journal of Clinical Medicine 14, no. 16: 5705. https://doi.org/10.3390/jcm14165705
APA StyleOliveros, E., Jonnalagadda, A., Pietrowicz, R., Mauri, M., Zhao, H., Maruthi, R., Saunders, H., Lakhter, V., Brailovsky, Y., Bashir, R., Sadek, A., Vaidya, A., & Forfia, P. (2025). Echo-Doppler Predictors of Residual Pulmonary Hypertension After Pulmonary Thromboendarterectomy. Journal of Clinical Medicine, 14(16), 5705. https://doi.org/10.3390/jcm14165705








