Bone Disease in Cystic Fibrosis: Insights into Etiopathogenesis and Advances in Treatment Management
Abstract
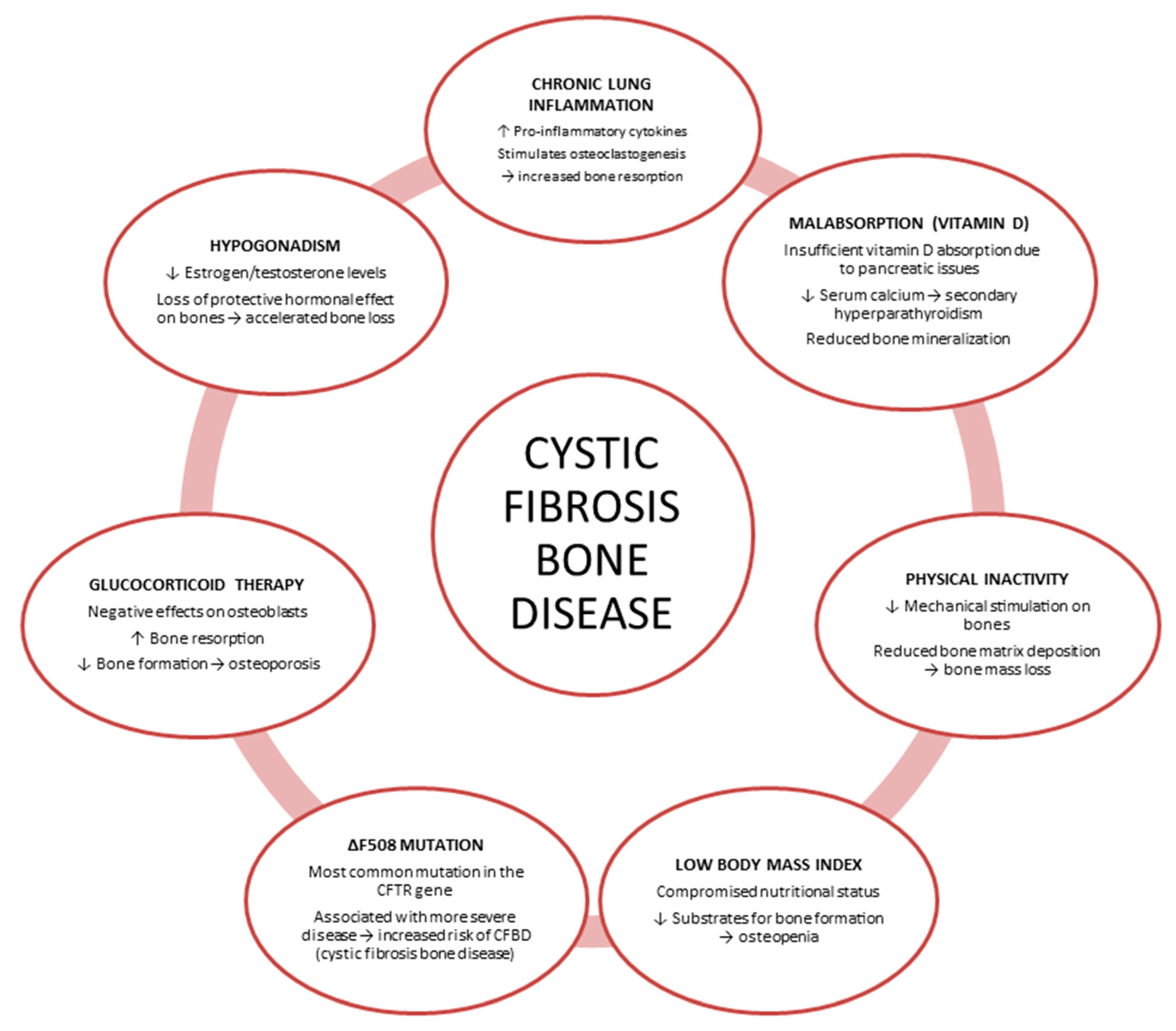
1. Introduction
2. Roles of Inflammatory Cytokines in CFBD
3. Impaired Glucose Metabolism in CF
4. Current State of Knowledge on CFBD: In Vitro, In Vivo, and Ex Vivo Studies
4.1. In Vitro Studies
4.2. In Vivo Studies
4.3. Ex Vivo Studies
5. Current and Future Therapeutic Interventions: Nutrition/Physical Activity/Pharmacological Treatments
5.1. Nutrition
5.2. Physical Activity
5.3. Pharmacological Treatments
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CF | Cystic fibrosis |
| CFTR | CF transmembrane conductance regulator |
| CFBD | CF-related bone disease |
| BMD | Bone mineral density |
| CFRD | CF-related diabetes |
References
- Ong, T.; Ramsey, B.W. Update in Cystic Fibrosis 2014. Am. J. Respir. Crit. Care Med. 2015, 192, 669–675. [Google Scholar] [CrossRef]
- Fuhrer, M.; Zampoli, M.; Abriel, H. Diagnosing Cystic Fibrosis in Low- and Middle-Income Countries: Challenges and Strategies. Orphanet J. Rare Dis. 2024, 19, 482. [Google Scholar] [CrossRef]
- Fonseca, Ó.; Gomes, M.S.; Amorim, M.A.; Gomes, A.C. Cystic Fibrosis Bone Disease: The Interplay between CFTR Dysfunction and Chronic Inflammation. Biomolecules 2023, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- Vavrina, K.; Griffin, T.B.; Jones, A.M.; Schindler, T.; Bui, T.N.; Sankararaman, S. Evolving Nutrition Therapy in Cystic Fibrosis: Adapting to the CFTR Modulator Era. Nutr. Clin. Pract. 2025, 40, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Mora Vallellano, J.; Delgado Pecellín, C.; Delgado Pecellín, I.; Quintana Gallego, E.; López-Campos, J.L. Evaluation of Bone Metabolism in Children with Cystic Fibrosis. Bone 2021, 147, 115929. [Google Scholar] [CrossRef] [PubMed]
- Jad, R.; Ma, X.; Stanojevic, S.; Illango, A.; Tullis, E.; Gilmour, J.; Goss, C.H.; Strug, L.J.; Stephenson, A.L. Longitudinal Changes in BMD in Adults with Cystic Fibrosis. J. Bone Miner. Res. 2024, 39, 1716–1721. [Google Scholar] [CrossRef]
- Cobb, C.; Wu, M.; Tangpricha, V. Cystic Fibrosis-Related Bone Disease: An Update on Screening, Diagnosis, and Treatment. Ther. Adv. Endocrinol. Metab. 2025, 16, 20420188251328210. [Google Scholar] [CrossRef]
- Conway, S.P. Osteoporosis and Osteopenia in Adults and Adolescents with Cystic Fibrosis: Prevalence and Associated Factors. Thorax 2000, 55, 798–804. [Google Scholar] [CrossRef]
- Ionescu, A.A.; Nixon, L.S.; Evans, W.D.; Stone, M.D.; Lewis-Jenkins, V.; Chatham, K.; Shale, D.J. Bone Density, Body Composition, and Inflammatory Status in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2000, 162, 789–794. [Google Scholar] [CrossRef]
- Putman, M.S.; Anabtawi, A.; Le, T.; Tangpricha, V.; Sermet-Gaudelus, I. Cystic Fibrosis Bone Disease Treatment: Current Knowledge and Future Directions. J. Cyst. Fibros. 2019, 18, S56–S65. [Google Scholar] [CrossRef]
- Fenercioglu, A.K. The Anti-Inflammatory Roles of Vitamin D for Improving Human Health. Curr. Issues Mol. Biol. 2024, 46, 13514–13525. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in Bone Modeling and Remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Zhou, P.; Zheng, T.; Zhao, B. Cytokine-Mediated Immunomodulation of Osteoclastogenesis. Bone 2022, 164, 116540. [Google Scholar] [CrossRef]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt Pathway in Bone Repair and Regeneration—What Do We Know So Far. Front. Cell Dev. Biol. 2019, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Holmen, S.L.; Zylstra, C.R.; Mukherjee, A.; Sigler, R.E.; Faugere, M.-C.; Bouxsein, M.L.; Deng, L.; Clemens, T.L.; Williams, B.O. Essential Role of β-Catenin in Postnatal Bone Acquisition. J. Biol. Chem. 2005, 280, 21162–21168. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, G.; D’Amato, G.; Chiarito, M.; Tullo, A.; Colaianni, G.; Colucci, S.; Grano, M.; Faienza, M.F. An Update on the Role of RANKL–RANK/Osteoprotegerin and WNT-ß-Catenin Signaling Pathways in Pediatric Diseases. World J. Pediatr. 2019, 15, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Ventura, A.; Colucci, S.; Cavallo, L.; Grano, M.; Brunetti, G. Bone Fragility in Turner Syndrome: Mechanisms and Prevention Strategies. Front. Endocrinol. 2016, 7, 34. [Google Scholar] [CrossRef]
- Faienza, M.F.; Brunetti, G.; Ventura, A.; Piacente, L.; Messina, M.F.; De Luca, F.; Ciccarelli, M.; Oranger, A.; Mori, G.; Natale, M.P.; et al. Mechanisms of Enhanced Osteoclastogenesis in Girls and Young Women with Turner’s Syndrome. Bone 2015, 81, 228–236. [Google Scholar] [CrossRef]
- Roesch, E.A.; Nichols, D.P.; Chmiel, J.F. Inflammation in Cystic Fibrosis: An Update. Pediatr. Pulmonol. 2018, 53, S30–S50. [Google Scholar] [CrossRef]
- Ryan, B.M.; Russel, M.G.V.M.; Schurgers, L.; Wichers, M.; Sijbrandij, J.; Stockbrugger, R.W.; Schoon, E. Effect of Antitumour Necrosis Factor-α Therapy on Bone Turnover in Patients with Active Crohn’s Disease: A Prospective Study. Aliment. Pharmacol. Ther. 2004, 20, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Luce, V.; Ventura, A.; Colaianni, G.; Colucci, S.; Cavallo, L.; Grano, M.; Brunetti, G. Skeleton and Glucose Metabolism: A Bone-Pancreas Loop. Int. J. Endocrinol. 2015, 2015, 758148. [Google Scholar] [CrossRef]
- Brunetti, G.; D’Amato, G.; De Santis, S.; Grano, M.; Faienza, M.F. Mechanisms of Altered Bone Remodeling in Children with Type 1 Diabetes. World J. Diabetes 2021, 12, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Bronckers, A.; Kalogeraki, L.; Jorna, H.J.N.; Wilke, M.; Bervoets, T.J.; Lyaruu, D.M.; Zandieh-Doulabi, B.; DenBesten, P.; De Jonge, H. The Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Is Expressed in Maturation Stage Ameloblasts, Odontoblasts and Bone Cells. Bone 2010, 46, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Le Henaff, C.; Mansouri, R.; Modrowski, D.; Zarka, M.; Geoffroy, V.; Marty, C.; Tarantino, N.; Laplantine, E.; Marie, P.J. Increased NF-κB Activity and Decreased Wnt/β-Catenin Signaling Mediate Reduced Osteoblast Differentiation and Function in ΔF508 Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Mice. J. Biol. Chem. 2015, 290, 18009–18017. [Google Scholar] [CrossRef]
- Dumortier, C.; Frauenpreis, A.; Hoarau, A.; Ryan, A.L.; Gangloff, S.C.; Danopoulos, S.; Velard, F.; Al Alam, D. CFTR Mutation Is Associated with Bone Differentiation Abnormalities in Cystic Fibrosis. J. Cyst. Fibros. 2025, 24, 741–748. [Google Scholar] [CrossRef]
- Dif, F.; Marty, C.; Baudoin, C.; De Vernejoul, M.-C.; Levi, G. Severe Osteopenia in CFTR-Null Mice. Bone 2004, 35, 595–603. [Google Scholar] [CrossRef]
- Haston, C.K.; Li, W.; Li, A.; Lafleur, M.; Henderson, J.E. Persistent Osteopenia in Adult Cystic Fibrosis Transmembrane Conductance Regulator–Deficient Mice. Am. J. Respir. Crit. Care Med. 2008, 177, 309–315. [Google Scholar] [CrossRef]
- Paradis, J.; Wilke, M.; Haston, C.K. Osteopenia in Cftr-deltaF508 Mice. J. Cyst. Fibros. 2010, 9, 239–245. [Google Scholar] [CrossRef]
- Le Henaff, C.; Gimenez, A.; Haÿ, E.; Marty, C.; Marie, P.; Jacquot, J. The F508del Mutation in Cystic Fibrosis Transmembrane Conductance Regulator Gene Impacts Bone Formation. Am. J. Pathol. 2012, 180, 2068–2075. [Google Scholar] [CrossRef]
- Pashuck, T.D.; Franz, S.E.; Altman, M.K.; Wasserfall, C.H.; Atkinson, M.A.; Wronski, T.J.; Flotte, T.R.; Stalvey, M.S. Murine Model for Cystic Fibrosis Bone Disease Demonstrates Osteopenia and Sex-Related Differences in Bone Formation. Pediatr. Res. 2009, 65, 311–316. [Google Scholar] [CrossRef]
- Stalvey, M.S.; Clines, K.L.; Havasi, V.; McKibbin, C.R.; Dunn, L.K.; Chung, W.J.; Clines, G.A. Osteoblast CFTR Inactivation Reduces Differentiation and Osteoprotegerin Expression in a Mouse Model of Cystic Fibrosis-Related Bone Disease. PLoS ONE 2013, 8, e80098. [Google Scholar] [CrossRef]
- Le Henaff, C.; Faria Da Cunha, M.; Hatton, A.; Tondelier, D.; Marty, C.; Collet, C.; Zarka, M.; Geoffroy, V.; Zatloukal, K.; Laplantine, E.; et al. Genetic Deletion of Keratin 8 Corrects the Altered Bone Formation and Osteopenia in a Mouse Model of Cystic Fibrosis. Hum. Mol. Genet. 2016, 25, 1281–1293. [Google Scholar] [CrossRef]
- Shead, E.F.; Haworth, C.S.; Condliffe, A.M.; McKeon, D.J.; Scott, M.A.; Compston, J.E. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Is Expressed in Human Bone. Thorax 2007, 62, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Divangahi, M.; Balghi, H.; Danialou, G.; Comtois, A.S.; Demoule, A.; Ernest, S.; Haston, C.; Robert, R.; Hanrahan, J.W.; Radzioch, D.; et al. Lack of CFTR in Skeletal Muscle Predisposes to Muscle Wasting and Diaphragm Muscle Pump Failure in Cystic Fibrosis Mice. PLoS Genet. 2009, 5, e1000586. [Google Scholar] [CrossRef] [PubMed]
- Le Heron, L.; Guillaume, C.; Velard, F.; Braux, J.; Touqui, L.; Moriceau, S.; Sermet-Gaudelus, I.; Laurent-Maquin, D.; Jacquot, J. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Regulates the Production of Osteoprotegerin (OPG) and Prostaglandin (PG) E2 in Human Bone. J. Cyst. Fibros. 2010, 9, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Scholte, B.J.; Davidson, D.J.; Wilke, M.; De Jonge, H.R. Animal Models of Cystic Fibrosis. J. Cyst. Fibros. 2004, 3, 183–190. [Google Scholar] [CrossRef]
- King, S.J.; Topliss, D.J.; Kotsimbos, T.; Nyulasi, I.B.; Bailey, M.; Ebeling, P.R.; Wilson, J.W. Reduced Bone Density in Cystic Fibrosis: ΔF508 Mutation Is an Independent Risk Factor. Eur. Respir. J. 2005, 25, 54–61. [Google Scholar] [CrossRef]
- Szulc, P.; Naylor, K.; Pickering, M.-E.; Hoyle, N.; Eastell, R.; Leary, E. Use of CTX-I and PINP as Bone Turnover Markers: National Bone Health Alliance Recommendations to Standardize Sample Handling and Patient Preparation to Reduce Pre-Analytical Variability. Ann. Biol. Clin. 2018, 76, 373–391. [Google Scholar] [CrossRef]
- Shevroja, E.; Lamy, O.; Kohlmeier, L.; Koromani, F.; Rivadeneira, F.; Hans, D. Use of Trabecular Bone Score (TBS) as a Complementary Approach to Dual-Energy X-Ray Absorptiometry (DXA) for Fracture Risk Assessment in Clinical Practice. J. Clin. Densitom. 2017, 20, 334–345. [Google Scholar] [CrossRef]
- Bergagnini-Kolev, M.C.; Hsu, S.; Aitken, M.L.; Goss, C.H.; Hoofnagle, A.N.; Zelnick, L.R.; Lum, D.; Best, C.M.; Thummel, K.E.; Kestenbaum, B.R.; et al. Metabolism and Pharmacokinetics of Vitamin D in Patients with Cystic Fibrosis. J. Steroid Biochem. Mol. Biol. 2023, 232, 106332. [Google Scholar] [CrossRef]
- Farahbakhsh, N.; Fatahi, S.; Shirvani, A.; Motaharifard, M.S.; Mohkam, M.; Tabatabaii, S.A.; Khanbabaee, G.; Yaghoobpoor, S.; Davoodi, S.Z.; Hosseini, A.H. Vitamin D Deficiency in Patients with Cystic Fibrosis: A Systematic Review and Meta-Analysis. J. Health Popul. Nutr. 2024, 43, 11. [Google Scholar] [CrossRef]
- Wilschanski, M.; Munck, A.; Carrion, E.; Cipolli, M.; Collins, S.; Colombo, C.; Declercq, D.; Hatziagorou, E.; Hulst, J.; Kalnins, D.; et al. ESPEN-ESPGHAN-ECFS Guideline on Nutrition Care for Cystic Fibrosis. Clin. Nutr. 2024, 43, 413–445. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, F.; Cianferotti, L.; Di Monaco, M.; Falchetti, A.; Fassio, A.; Gatti, D.; Gennari, L.; Giannini, S.; Girasole, G.; Gonnelli, S.; et al. Definition, Assessment, and Management of Vitamin D Inadequacy: Suggestions, Recommendations, and Warnings from the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Nutrients 2022, 14, 4148. [Google Scholar] [CrossRef] [PubMed]
- Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011; p. 13050. [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Tartara, A.; Gasparri, C.; Perna, S.; Infantino, V.; Riva, A.; Petrangolini, G.; Peroni, G. An Update on Magnesium and Bone Health. BioMetals 2021, 34, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Serna, J.; Bergwitz, C. Importance of Dietary Phosphorus for Bone Metabolism and Healthy Aging. Nutrients 2020, 12, 3001. [Google Scholar] [CrossRef]
- Kanazawa, I.; Sugimoto, T. Diabetes Mellitus-Induced Bone Fragility. Intern. Med. 2018, 57, 2773–2785. [Google Scholar] [CrossRef]
- Sermet-Gaudelus, I.; Bianchi, M.L.; Garabédian, M.; Aris, R.M.; Morton, A.; Hardin, D.S.; Elkin, S.L.; Compston, J.E.; Conway, S.P.; Castanet, M.; et al. European Cystic Fibrosis Bone Mineralisation Guidelines. J. Cyst. Fibros. 2011, 10, S16–S23. [Google Scholar] [CrossRef]
- Faienza, M.F.; Urbano, F.; Chiarito, M.; Lassandro, G.; Giordano, P. Musculoskeletal Health in Children and Adolescents. Front. Pediatr. 2023, 11, 1226524. [Google Scholar] [CrossRef]
- Birzniece, V. Exercise and the Growth Hormone–Insulin-like Growth Factor Axis. Curr. Opin. Endocr. Metab. Res. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Conwell, L.S.; Chang, A.B. Bisphosphonates for Osteoporosis in People with Cystic Fibrosis. Cochrane Database Syst. Rev. 2014, 1, CD002010. [Google Scholar] [CrossRef]
- Ullal, J.; Kutney, K.; Williams, K.M.; Weber, D.R. Treatment of Cystic Fibrosis Related Bone Disease. J. Clin. Transl. Endocrinol. 2022, 27, 100291. [Google Scholar] [CrossRef]
- Hildebrand, G.K.; Patel, P.; Kasi, A. Denosumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Lamy, O.; Stoll, D.; Aubry-Rozier, B.; Rodriguez, E.G. Stopping Denosumab. Curr. Osteoporos. Rep. 2019, 17, 8–15. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Black, D.M.; Greenspan, S.L.; Ensrud, K.E.; Palermo, L.; McGowan, J.A.; Lang, T.F.; Garnero, P.; Bouxsein, M.L.; Bilezikian, J.P.; Rosen, C.J. The Effects of Parathyroid Hormone and Alendronate Alone or in Combination in Postmenopausal Osteoporosis. N. Engl. J. Med. 2003, 349, 1207–1215. [Google Scholar] [CrossRef]
| Author and Year | Model Used | Outcomes |
|---|---|---|
| Bronckers et al., 2010 [24] | Animals and human tissues | CFTR dysfunction may contribute to osteopenia in both CF patients and CFTR-deficient mice due to its role on bone cells. |
| Le Henaff et al., 2015 [25] | Mouse cell line | The prevalent ΔF508-CFTR mutation impairs osteoblast differentiation and function by increasing NF-κB signaling and reducing Wnt/β-catenin signaling. |
| Dumortier et al., 2025 [26] | Human cell line | CFTR mutation delays osteoblast differentiation and regeneration. |
| Dif et al., 2004 [27] | Mouse model | CFTR mutation is directly linked to osteopenia. |
| Haston et al., 2008 [28] | Mouse model | CFTR loss causes persistent osteopenia and bone abnormalities, independent of sex or haplotype. |
| Paradis et al., 2010 [29] | Mouse model | ΔF508-CFTR expression causes osteopenia, indicating CFTR dysfunction directly contributes to CF bone disease. |
| Le Henaff et al., 2012 [30] | Mouse model | F508del-CFTR reduces bone formation and causes osteopenia and bone architecture defects. |
| Pashuck et al., 2009 [31] | Mouse model | Reduced bone formation in female CF mice, increased in males. |
| Stalvey et al., 2013 [32] | Mouse model | CFTR inactivation impairs osteoblast function, increases osteoclastogenesis, and disrupts Wnt signaling, contributing to CF-related bone disease. |
| Le Henaff et al., 2016 [33] | Mouse model | Krt8 targeting restores bone formation and reduces osteopenia in F508del-CFTR mice. |
| Shead et al., 2007 [34] | Human tissue and cell line | CFTR is expressed in human osteoblasts, osteocytes, and osteoclasts |
| Divangahi et al., 2009 [35] | Human and mouse tissues and cell line | Unrecognized role of CFTR in skeletal muscle linked to cachexia and respiratory dysfunction in CF. |
| Le Heron et al., 2010 [36] | Human cell line | CFTR loss may increase inflammation-driven bone resorption, contributing to early bone loss in CF children. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, P.; Linguiti, G.; Leonetti, G.; Casolino, R.M.P.; Granberg, V.; Faienza, M.F. Bone Disease in Cystic Fibrosis: Insights into Etiopathogenesis and Advances in Treatment Management. J. Clin. Med. 2025, 14, 5657. https://doi.org/10.3390/jcm14165657
Giordano P, Linguiti G, Leonetti G, Casolino RMP, Granberg V, Faienza MF. Bone Disease in Cystic Fibrosis: Insights into Etiopathogenesis and Advances in Treatment Management. Journal of Clinical Medicine. 2025; 14(16):5657. https://doi.org/10.3390/jcm14165657
Chicago/Turabian StyleGiordano, Paola, Giovanna Linguiti, Giuseppina Leonetti, Rosa Maria Pia Casolino, Vanja Granberg, and Maria Felicia Faienza. 2025. "Bone Disease in Cystic Fibrosis: Insights into Etiopathogenesis and Advances in Treatment Management" Journal of Clinical Medicine 14, no. 16: 5657. https://doi.org/10.3390/jcm14165657
APA StyleGiordano, P., Linguiti, G., Leonetti, G., Casolino, R. M. P., Granberg, V., & Faienza, M. F. (2025). Bone Disease in Cystic Fibrosis: Insights into Etiopathogenesis and Advances in Treatment Management. Journal of Clinical Medicine, 14(16), 5657. https://doi.org/10.3390/jcm14165657








