The Role of Glenoid Osteotomy in the Treatment of Shoulder Dysplasia in Brachial Plexus Birth Palsy: A Systematic Review of the Literature
Abstract
1. Introduction
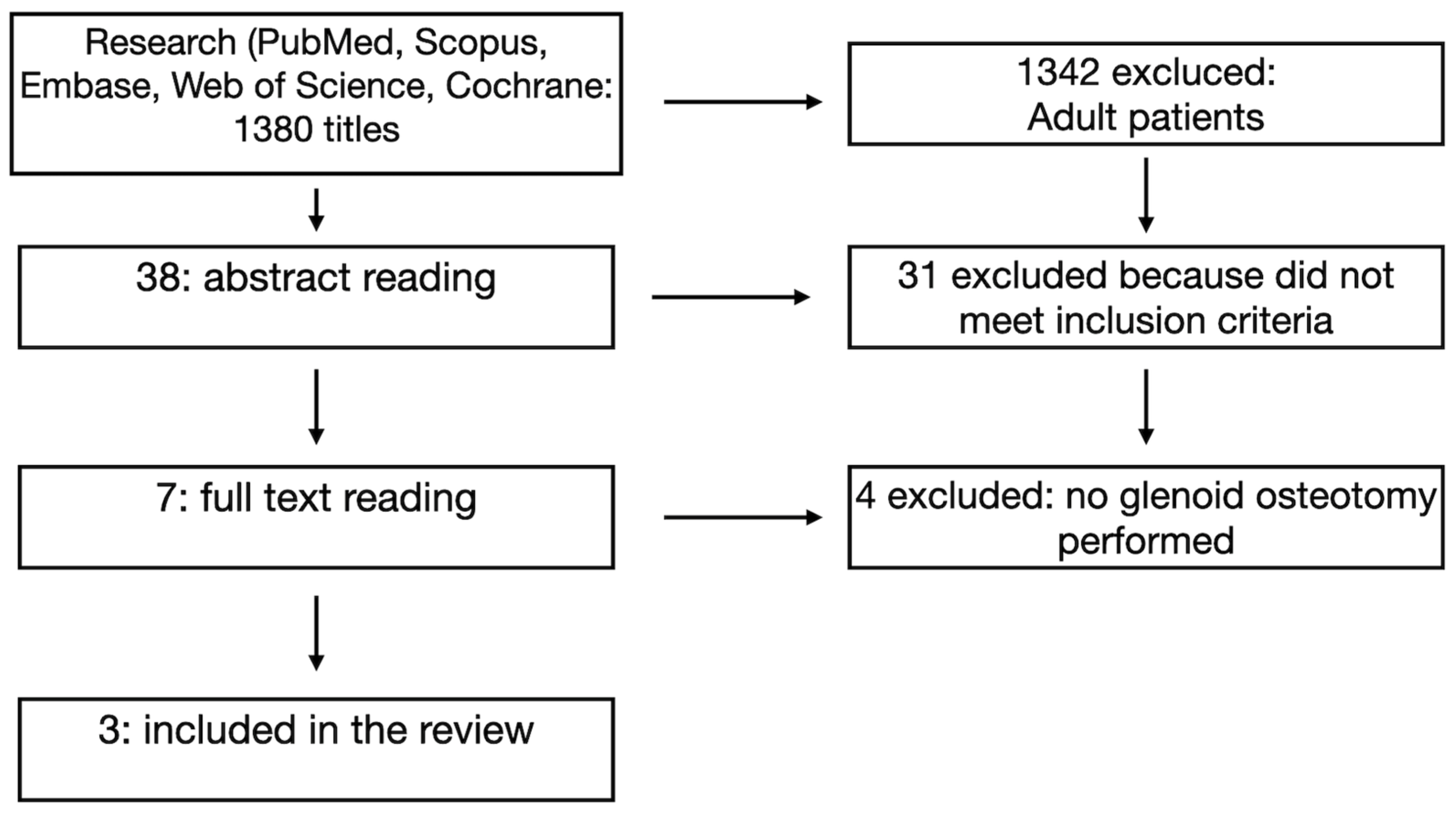
2. Materials and Methods
| Database | Search Terms | Filters | Results |
| Embase | (‘glenoid’/exp OR glenoid) AND (‘osteotomy’/exp OR osteotomy) AND [english]/lim AND [embase]/lim | English | 398-20 December 2024 |
| PubMed | (“glenoid” [All Fields] OR “glenoidal” [All Fields] OR “glenoids” [All Fields]) AND (“osteotomie” [All Fields] OR “osteotomied” [All Fields] OR “osteotomy” [MeSH Terms] OR “osteotomy” [All Fields] OR “osteotomies” [All Fields]) | 270-20 December 2024 | |
| Scopus | (TITLE-ABS-KEY (osteotomy)) AND (TITLE-ABS-KEY (glenoid)) | 439-20 December 2024 | |
| Web of Science | Topic Search = osteotomy AND Topic Search = glenoid | English | 265-20 December 2024 |
| Cochrane | ALL = glenoid ALL = osteotomy #1 AND #2 | English | 7 trials + 4 systematic reviews |
- Included pediatric patients (under the age of 18) with a diagnosis of BPBI;
- Described the use of glenoid osteotomy as part of the surgical management;
- Reported clinical or radiological outcomes.
- Studies focusing solely on adult patients;
- Reports lacking outcome data or clear methodology;
- Case reports with fewer than three patients;
- Review articles, editorials, and letters to the editor.
3. Results
3.1. Patient Demographics and Surgical Context
3.2. Surgical Techniques
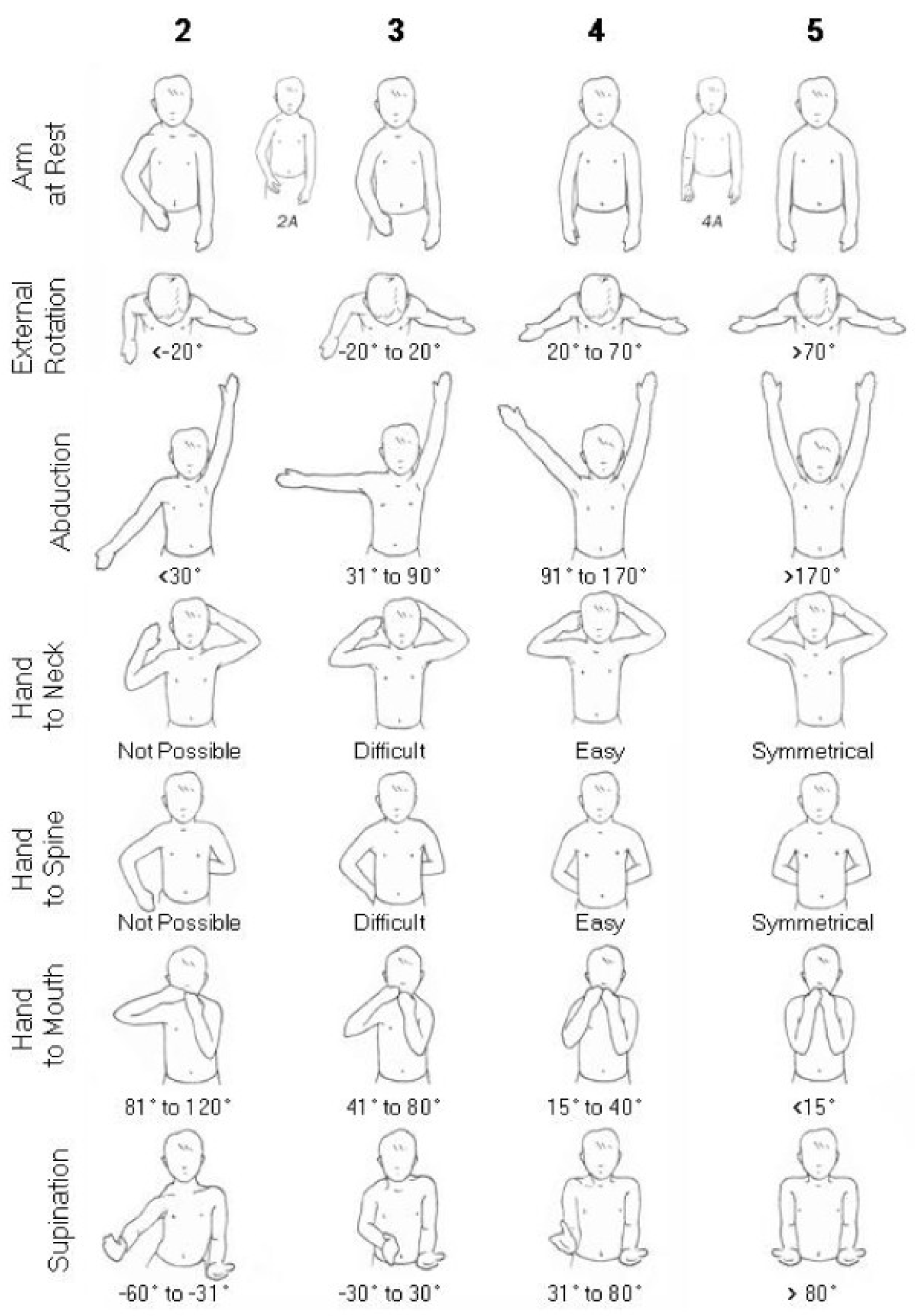
3.3. Functional Outcomes
3.4. Limitations in Data Reporting
4. Discussion
- The design and execution of prospective, multicenter trials involving large, diverse pediatric cohorts;
- The development of uniform imaging and clinical assessment protocols, including the use of 3D imaging for surgical planning and outcome evaluation;
- The integration of multidimensional outcome measures, such as the Mallet scale, objective strength assessments, and patient- or parent-reported quality-of-life instruments;
- The implementation of longitudinal follow-up extending into late adolescence or even early adulthood.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SD | Shoulder Dysplasia |
| BPBI | Brachial Plexus Birth Injury |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| DDH | Developmental Dysplasia of the Hip |
| LD | Latissimus Dorsi |
| CA | Chiara Arrigoni |
| RF | Roberto Facchi |
| NC | Nunzio Catena |
References
- Pagnotta, A.; Haerle, M.; Gilbert, A. Long-term results on abduction and external rotation of the shoulder after latissimus dorsi transfer for sequelae of obstetric palsy. Clin. Orthop. Relat. Res. 2004, 426, 199–205. [Google Scholar] [CrossRef]
- Nixon, M.; Trail, J. Management of shoulder problems following obstetric brachial plexus injury. Shoulder Elb. 2014, 6, 12–17. [Google Scholar] [CrossRef]
- Waters, P.M.; Smith, G.R.; Jaramillo, D. Glenohumeral deformity secondary to brachial plexus birth palsy. J. Bone Jt. Surg. Am. 1998, 80, 668–677. [Google Scholar] [CrossRef]
- Menashe, S.J.; Tse, R.; Nixon, J.N.; Ishak, G.E.; Thapa, M.M.; McBroom, J.A.; Iyer, R.S. Brachial plexus birth palsy: Multimodality imaging of spine and shoulder abnormalities in children. AJR Am. J. Roentgenol. 2015, 204, W199–W206. [Google Scholar] [CrossRef]
- Olofsson, P.N.; Chu, A.; McGrath, A.M. The Pathogenesis of Glenohumeral Deformity and Contracture Formation in Obstetric Brachial Plexus Palsy—A Review. J. Brachial Plex. Peripher. Nerve Inj. 2019, 14, e24–e34. [Google Scholar] [CrossRef]
- Pearl, M.L. Shoulder problems in children with brachial plexus birth palsy: Evaluation and management. J. Am. Acad. Orthop. Surg. 2009, 17, 242–254. [Google Scholar] [CrossRef]
- Waters, P.M.; Peljovich, A.E. Shoulder reconstruction in patients with chronic brachial plexus birth palsy. A case control study. Clin. Orthop. Relat. Res. 1999, 364, 144–152. [Google Scholar] [CrossRef]
- Waters, P.M.; Bae, D.S. The effect of derotational humeral osteotomy on global shoulder function in brachial plexus birth palsy. J. Bone Jt. Surg. Am. 2006, 88, 1035–1042. [Google Scholar] [CrossRef]
- Jönsson, K.; Roos, F.; Hultgren, T. Structures contributing to the shoulder contracture in brachial plexus birth palsy. An intraoperative biomechanical study. J. Hand Surg. Eur. Vol. 2022, 47, 237–242. [Google Scholar] [CrossRef]
- Iorio, M.L.; Menashe, S.J.; Iyer, R.S.; Lewis, S.P.; Steinman, S.; Whitlock, K.B.; Tse, R.W. Glenohumeral Dysplasia Following Neonatal Brachial Plexus Palsy: Presentation and Predictive Features During Infancy. J. Hand Surg. Am. 2015, 40, 2345–2351.e1. [Google Scholar] [CrossRef]
- de Joode, S.G.C.J.; Meijer, R.; Samijo, S.; Heymans, M.J.L.F.; Chen, N.; van Rhijn, L.W.; Schotanus, M.G.M. Long-term functional outcome of secondary shoulder surgery in brachial plexus birth palsy patients. Bone Jt. J. 2023, 105-B, 455–464. [Google Scholar] [CrossRef]
- Senes, F.M.; Catena, N.; Arrigoni, C. Palliative Surgery in Obstetrical Brachial Plexus Palsy. In Pediatric Hand Surgery; Pajardi, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Birch, R. Late sequelae at the shoulder in obstetrical palsy in children. In Surgical Techniques in Orthopaedics and Traumatology: Shoulder; Randelli, M., Karlsson, J., Eds.; Elsevier: Paris, France, 2001; Volume 3, p. 55-200-E-210. [Google Scholar]
- Sénès, F.; Catena, N.; Sénès, J. Nerve Transfer in Delayed Obstetrical Palsy Repair. J. Brachial Plex. Peripher. Nerve Inj. 2015, 10, e2–e14. [Google Scholar] [CrossRef] [PubMed]
- Birch, R. Medial rotation contraction and posterior dislocation of the shoulder. In Brachial Plexus Injury, 1st ed.; Gilbert, A., Ed.; Martin Dunitz: London, UK, 2001. [Google Scholar]
- Zargarbashi, R.; Rabie, H.; Panjavi, B.; Kamran, H.; Mosalamiaghili, S.; Erfani, Z.; Mirghaderi, S.P.; Salimi, M. Glenoid osteotomy with various tendon transfers for brachial plexus birth palsy: Clinical outcomes. J. Shoulder Elb. Surg. 2023, 32, e60–e70. [Google Scholar] [CrossRef]
- Dodwell, E.; O’Callaghan, J.; Anthony, A.; Jellicoe, P.; Shah, M.; Curtis, C.; Clarke, H.; Hopyan, S. Combined glenoid anteversion osteotomy and tendon transfers for brachial plexus birth palsy: Early outcomes. J. Bone Jt. Surg. Am. 2012, 94, 2145–2152. [Google Scholar] [CrossRef]
- Di Mascio, L.; Chin, K.F.; Fox, M.; Sinisi, M. Glenoplasty for complex shoulder subluxation and dislocation in children with obstetric brachial plexus palsy. J. Bone Jt. Surg. Br. 2011, 93, 102–107. [Google Scholar] [CrossRef][Green Version]
- Stein, J.; Laor, T.; Carr, P.; Zbojniewicz, A.; Cornwall, R. The Effect of Scapular Position on Magnetic Resonance Imaging Measurements of Glenohumeral Dysplasia Caused by Neonatal Brachial Plexus Palsy. J. Hand Surg. Am. 2017, 42, 1030.e1–1030.e11. [Google Scholar] [CrossRef]
- van de Bunt, F.; Pearl, M.L.; Lee, E.K.; Peng, L.; Didomenico, P. Analysis of normal and dysplastic glenohumeral morphology at magnetic resonance imaging in children with neonatal brachial plexus palsy. Pediatr. Radiol. 2017, 47, 1337–1344. [Google Scholar] [CrossRef]
- Frich, L.H.; Schmidt, P.H.; Torfing, T. Glenoid morphology in obstetrical brachial plexus lesion: A three-dimensional computed tomography study. J. Shoulder Elb. Surg. 2017, 26, 1374–1382. [Google Scholar] [CrossRef]
- Eismann, E.A.; Laor, T.; Cornwall, R. Three-Dimensional Magnetic Resonance Imaging of Glenohumeral Dysplasia in Neonatal Brachial Plexus Palsy. J. Bone Jt. Surg. Am. 2016, 98, 142–151. [Google Scholar] [CrossRef]
- Levidy, M.F.; Azer, A.; Shafei, J.; Srinivasan, N.; Mahajan, J.; Gupta, S.; Abdelmalek, G.; Pant, K.; Jain, K.; Shah, Y.; et al. Global trends in surgical approach to neonatal brachial plexus palsy: A systematic review. Front. Surg. 2025, 11, 1359719. [Google Scholar] [CrossRef]
- Jönsson, K.; Werner, M.; Roos, F.; Hultgren, T. Development of the glenohumeral joint after subscapular release and open relocation in children with brachial plexus birth palsy: Long-term results in 61 patients. J. Shoulder Elb. Surg. 2019, 28, 1983–1990. [Google Scholar] [CrossRef]
- Kozin, S.H.; Boardman, M.J.; Chafetz, R.S.; Williams, G.R.; Hanlon, A. Arthroscopic treatment of internal rotation contracture and glenohumeral dysplasia in children with brachial plexus birth palsy. J. Shoulder Elb. Surg. 2010, 19, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Alluri, R.K.; Lightdale-Miric, N.; Meisel, E.; Kim, G.; Kaplan, J.; Bougioukli, S.; Stevanovic, M. Functional outcomes of tendon transfer for brachial plexus birth palsy using the Hoffer technique. Bone Jt. J. 2020, 102-B, 246–253. [Google Scholar] [CrossRef]
- Pehlivanoglu, T.; Erşen, A.; Bayram, S.; Atalar, A.C.; Demirhan, M. Arthroscopic versus open release of internal rotation contracture in the obstetrical brachial plexus paralysis (OBPP) sequela. J. Shoulder Elb. Surg. 2019, 28, 28–35. [Google Scholar] [CrossRef]
- Frade, F.; Gómez-Salgado, J.; Jacobsohn, L.; Florindo-Silva, F. Rehabilitation of Neonatal Brachial Plexus Palsy: Integrative Literature Review. J. Clin. Med. 2019, 8, 980. [Google Scholar] [CrossRef]
- Buchanan, P.J.; Grossman, J.A.I.; Price, A.E.; Reddy, C.; Chopan, M.; Chim, H. The Use of Botulinum Toxin Injection for Brachial Plexus Birth Injuries: A Systematic Review of the Literature. Hand 2019, 14, 150–154. [Google Scholar] [CrossRef]
- Waters, P.M.; Bae, D.S. The early effects of tendon transfers and open capsulorrhaphy on glenohumeral deformity in brachial plexus birth palsy. J. Bone Jt. Surg. Am. 2008, 90, 2171–2179. [Google Scholar] [CrossRef]
- Abzug, J.M.; Kozin, S.H.; Waters, P.M. Open Glenohumeral Joint Reduction and Latissimus Dorsi and Teres Major Tendon Transfers for Infants and Children Following Brachial Plexus Birth Palsy. Tech. Hand Up. Extrem. Surg. 2017, 21, 30–36. [Google Scholar] [CrossRef]
- Mintzer, C.M.; Waters, P.M.; Brown, D.J. Glenoid version in children. J. Pediatr. Orthop. 1996, 16, 563–566. [Google Scholar] [CrossRef]
- Van der Sluijs, J.A.; van Ouwerkerk, W.J.; de Gast, A.; Wuisman, P.; Nollet, F.; Manoliu, R.A. Retroversion of the humeral head in children with an obstetric brachial plexus lesion. J. Bone Jt. Surg. Br. 2002, 84, 583–587. [Google Scholar] [CrossRef]
- Le Hanneur, M.; Brahim, L.; Langlais, T.; Bouché, P.A.; Fitoussi, F. Age Influence Upon Glenohumeral Remodeling After Shoulder Axial Rebalancing Surgery in Brachial Plexus Birth Injury. J. Pediatr. Orthop. 2023, 43, e389–e395. [Google Scholar] [CrossRef] [PubMed]
- Pearl, M.L.; Edgerton, B.W. Glenoid deformity secondary to brachial plexus birth palsy. J. Bone Jt. Surg. Am. 1998, 80, 659–667. [Google Scholar] [CrossRef] [PubMed]
| N° of Patient | Median Age | Gender M/F | Side R/L | Previous Surgery | |
|---|---|---|---|---|---|
| Zargarbashi 2023 | 33 | 27.5 +/− 14 months (range 0.8–4.4) | 18 vs. 15 | 12 vs. 21 | Not included in the study |
| Dodwell 2012 | 32 | 6.8 years (range 2.1–16.2) | 13 vs. 19 | 32 vs. 0 | 21 |
| Di Mascio 2011 | 29 | 5 years (range 1–18) | 12 vs. 17 | 14 vs. 15 | 14 |
| Anterior Release | LD Transfer | LD and Teres Major Transfer | LD and Teres Minor Transfer | Lower Trapezium Transfer | |
|---|---|---|---|---|---|
| Zargarbashi 2023 | 33 | 3 | 0 | 17 | 13 |
| Dodwell 2012 | 32 | 0 | 32 | 0 | 0 |
| Di Mascio 2011 | 28 | 0 | 0 | 0 | 0 |
| Pre-Mallet Score | Post-Mallet Score | Pre-Abduction | Post-Abduction | Pre-External Rotation | Post-External Rotation | |
|---|---|---|---|---|---|---|
| Zargarbashi 2023 | 13.5 +/− 1.02 degrees | 18.91 +/− 1.50 degrees | 86.61 +/− 17.17 degrees | 155 +/− 20.99 degrees | 9.26 +/− 7.50 degrees | 43.23 +/− 7.16 degrees |
| Dodwell 2012 | 11.05 degrees | 15.04 degrees | 104 degrees | 129 degrees | 66 degrees | 98 degrees |
| Di Mascio 2011 | 8.25 degrees | 11.75 degrees | 132 degrees | 156 degrees | 1 degrees | 56 degrees |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrigoni, C.; Facchi, R.; Catena, N. The Role of Glenoid Osteotomy in the Treatment of Shoulder Dysplasia in Brachial Plexus Birth Palsy: A Systematic Review of the Literature. J. Clin. Med. 2025, 14, 5610. https://doi.org/10.3390/jcm14165610
Arrigoni C, Facchi R, Catena N. The Role of Glenoid Osteotomy in the Treatment of Shoulder Dysplasia in Brachial Plexus Birth Palsy: A Systematic Review of the Literature. Journal of Clinical Medicine. 2025; 14(16):5610. https://doi.org/10.3390/jcm14165610
Chicago/Turabian StyleArrigoni, Chiara, Roberto Facchi, and Nunzio Catena. 2025. "The Role of Glenoid Osteotomy in the Treatment of Shoulder Dysplasia in Brachial Plexus Birth Palsy: A Systematic Review of the Literature" Journal of Clinical Medicine 14, no. 16: 5610. https://doi.org/10.3390/jcm14165610
APA StyleArrigoni, C., Facchi, R., & Catena, N. (2025). The Role of Glenoid Osteotomy in the Treatment of Shoulder Dysplasia in Brachial Plexus Birth Palsy: A Systematic Review of the Literature. Journal of Clinical Medicine, 14(16), 5610. https://doi.org/10.3390/jcm14165610






