The Natural History and Clinical Outcomes of Transmembrane Protein 43 Cardiomyopathy: A Systematic Review
Abstract
1. Introduction
2. Method
2.1. Protocol Disclosure and Identification
2.2. The Search Strategy and Study Selection
2.3. Study Eligibility Criteria
2.4. Extraction of the Study Data
3. Results
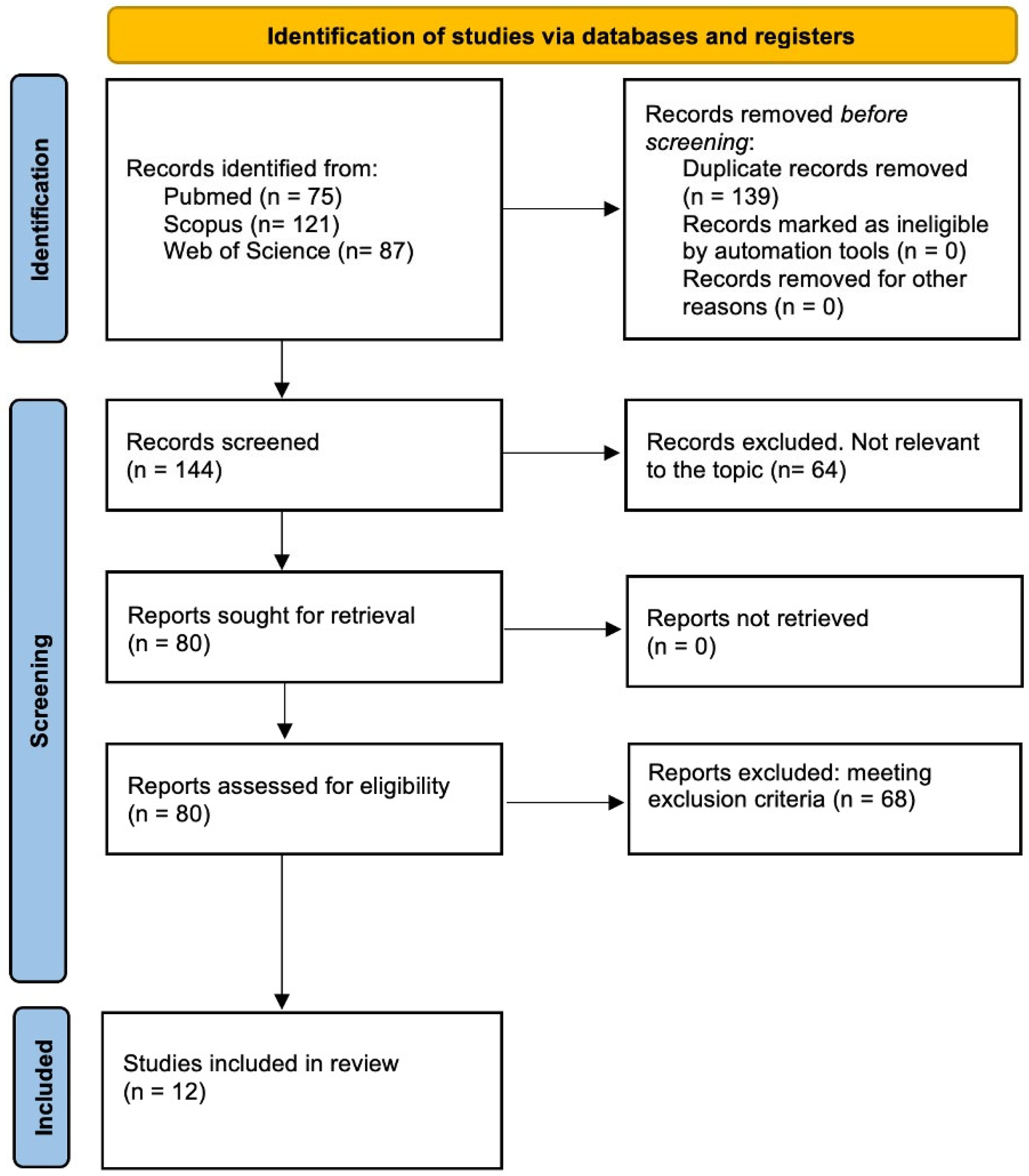
3.1. Study Retrieval
3.2. The Clinical Phenotype of TMEM43-Associated Cardiomyopathy
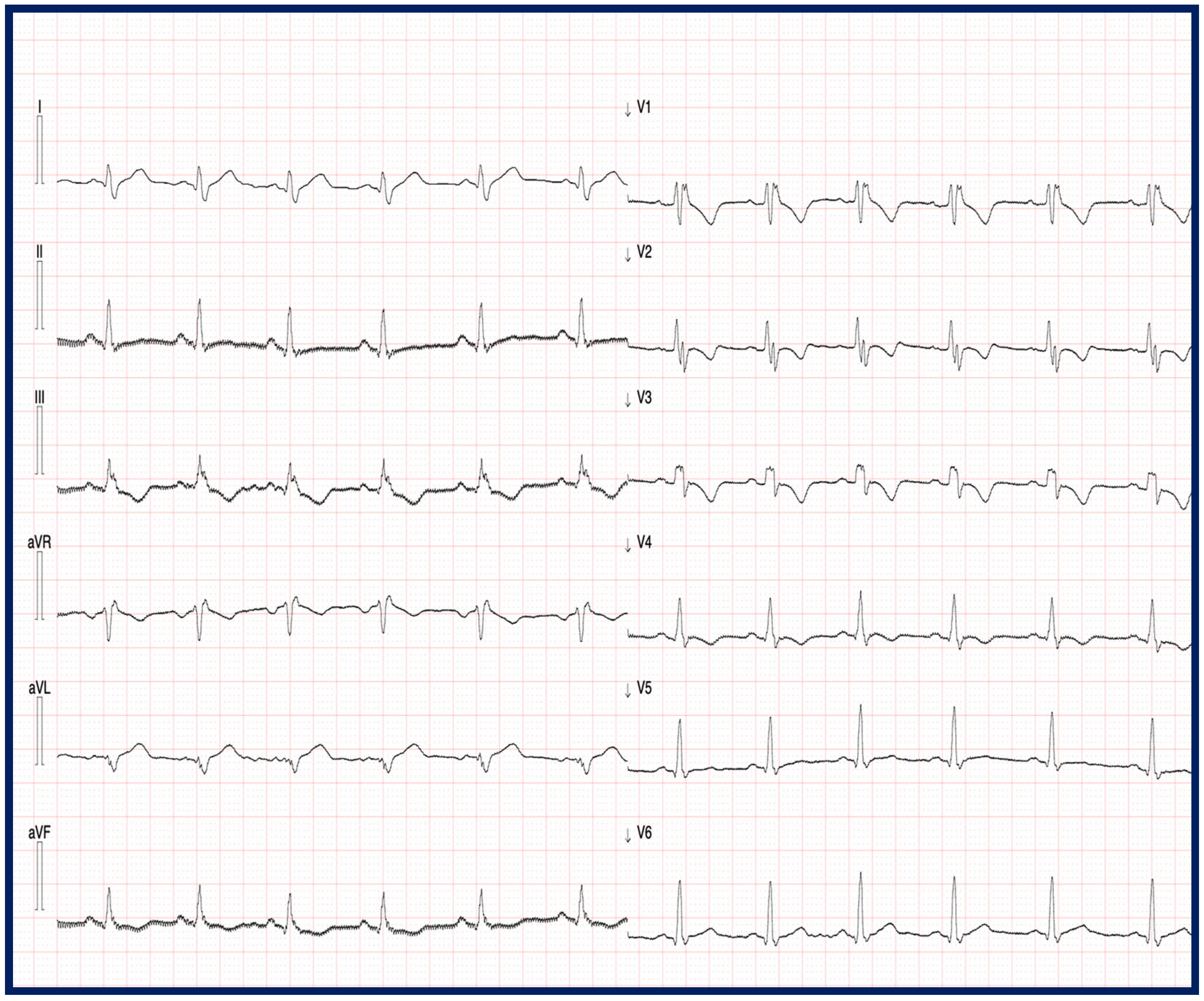
3.3. Electrocardiographic Characteristics of TMEM43-Related Cardiomyopathy
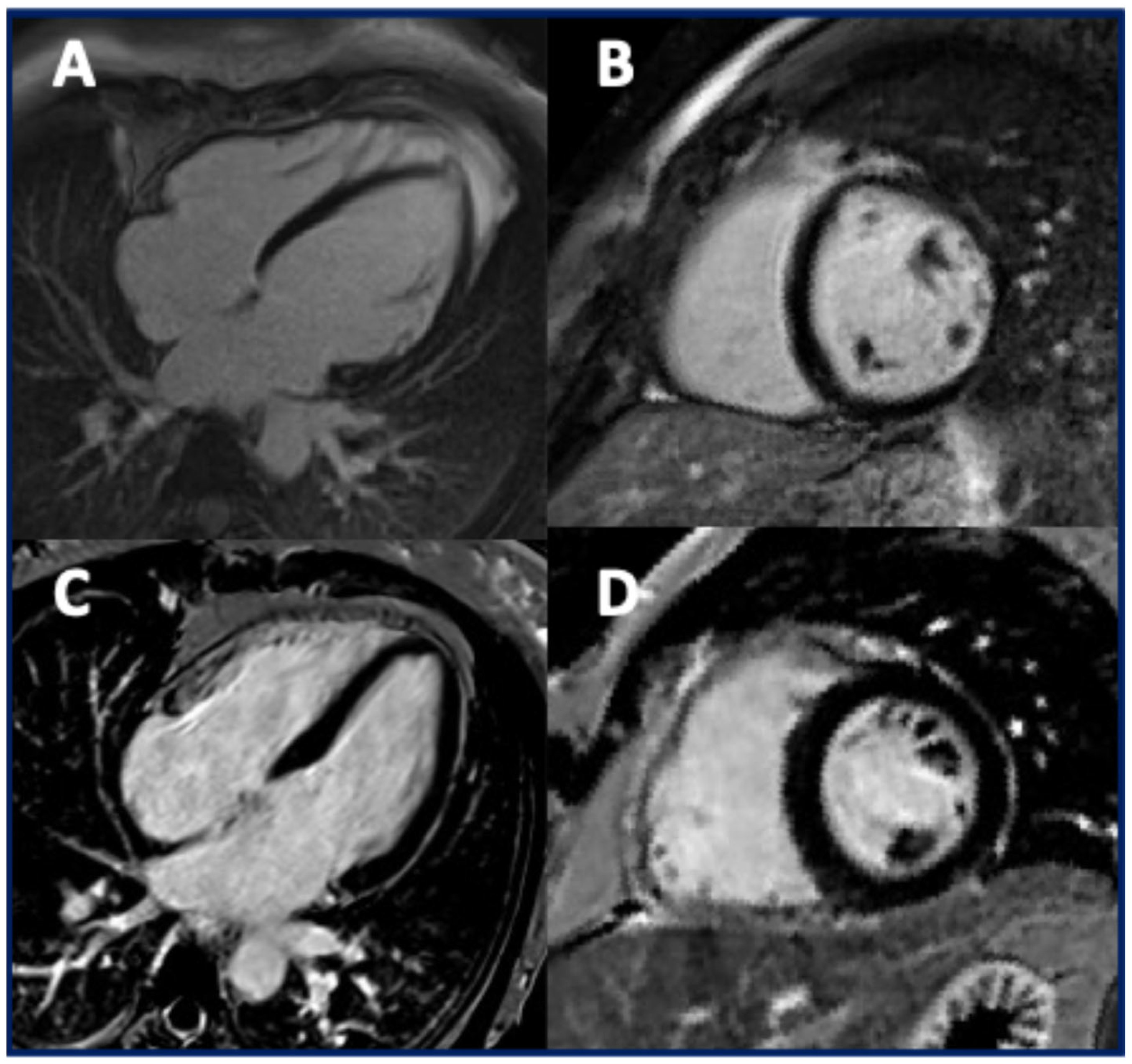
3.4. The Imaging Features of Patients with TMEM43 Cardiomyopathy
3.5. Clinical Outcomes in Patients with TMEM43 Cardiomyopathy
4. Discussion
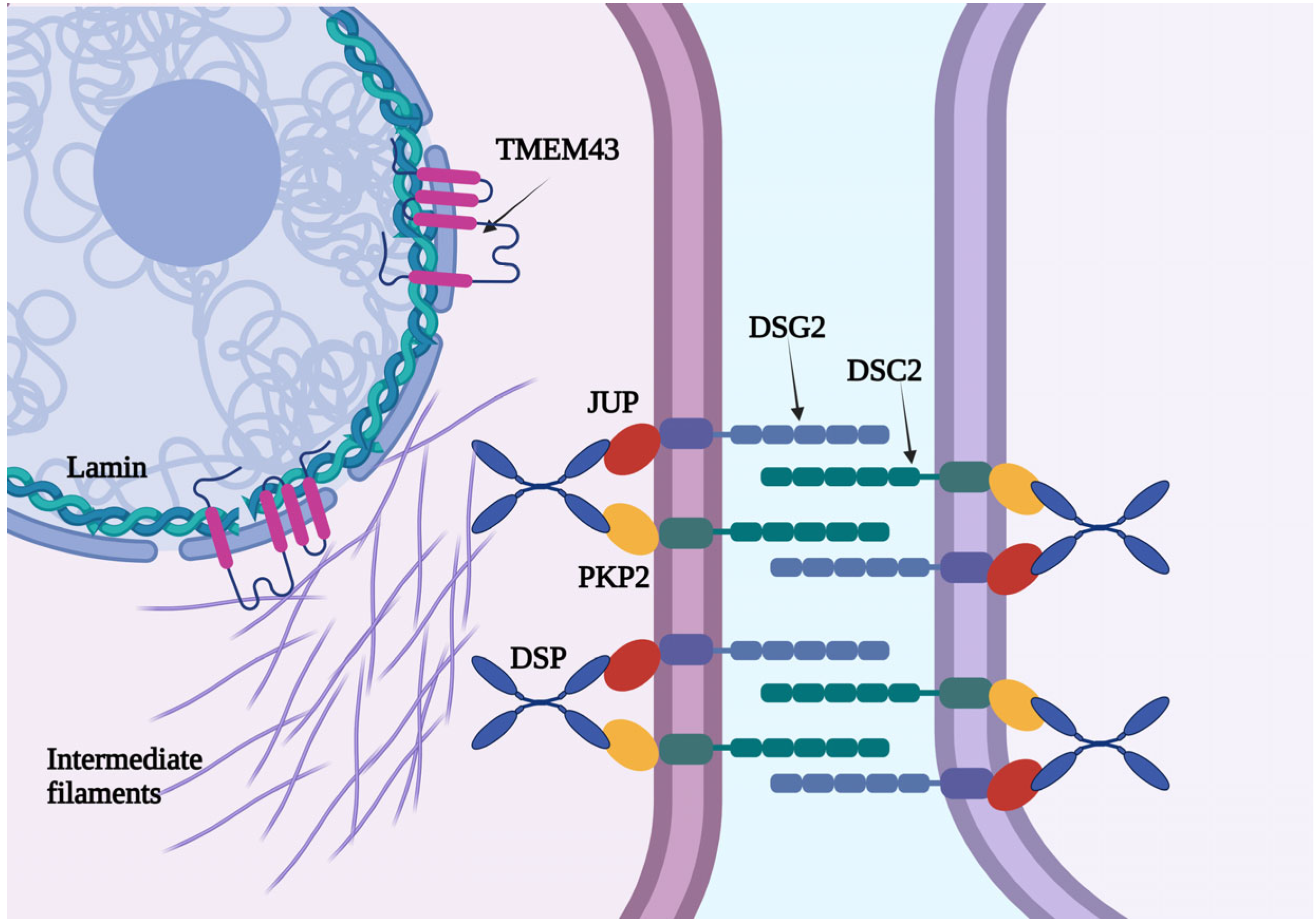
4.1. The Clinical Characteristics of TMEM43-Associated Cardiomyopathy
4.2. Electrocardiographic Features and VAs in Patients with TMEM43 Cardiomyopathy
4.3. The Imaging Findings in Patients with TMEM43 Cardiomyopathy
4.4. The Clinical Outcomes in Patients with TMEM43 Cardiomyopathy
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACM | Arrhythmogenic cardiomyopathy |
| VA | Ventricular arrhythmia |
| SCD | Sudden cardiac death |
| TMEM43 | Transmembrane protein 43 |
| ECG | Electrocardiogram |
| EPS | Electrophysiologic study |
| CMR | Cardiac magnetic resonance |
| HF | Heart failure |
| HT | Heart transplant |
| ICD | Implantable cardioverter–defibrillator |
| PRWS | Poor R wave progression |
| PVC | Premature ventricular contraction |
| SAEG | Signal-averaged ECG |
| LV | Left ventricular |
| LVEF | Left ventricular ejection fraction |
| RV | Right ventricular |
| LGE | Late gadolinium enhancement |
| JUP | Plakoglobin |
| DSP | Desmoplakin |
| PKP2 | Plakofillin |
| DSG2 | Desmoglein 2 |
| DSC2 | Desmocollin 2 |
| DES | Desmin |
| PLN | Phospholamban |
| FLNC | Filamin C |
| CDH2 | Cadherin 2 |
| TJP1 | Tight Junction Protein 1 |
| LBBB | Left bundle branch block |
| RBBB | Right bundle branch block |
| VT | Ventricular tachycardia |
References
- Corrado, D.; Link, M.S.; Calkins, H. Arrhythmogenic Right Ventricular Cardiomyopathy. N. Engl. J. Med. 2017, 376, 61–72. [Google Scholar] [CrossRef]
- Corrado, D.; Thiene, G.; Bauce, B.; Calore, C.; Cipriani, A.; De Lazzari, M.; Migliore, F.; Perazzolo Marra, M.; Pilichou, K.; Rigato, I.; et al. The “Padua classification” of cardiomyopathies: Combining pathobiological basis and morpho-functional remodeling. Int. J. Cardiol. 2025, 418, 132571. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Bueno Marinas, M.; Cason, M.; Bariani, R.; Celeghin, R.; De Gaspari, M.; Pinci, S.; Cipriani, A.; Rigato, I.; Zorzi, A.; Rizzo, S.; et al. A Comprehensive Analysis of Non-Desmosomal Rare Genetic Variants in Arrhythmogenic Cardiomyopathy: Integrating in Padua Cohort Litera-ture-Derived Data. Int. J. Mol. Sci. 2024, 25, 6267. [Google Scholar] [CrossRef] [PubMed]
- Siragam, V.; Cui, X.; Masse, S.; Ackerley, C.; Aafaqi, S.; Strandberg, L.; Tropak, M.; Fridman, M.D.; Nanthakumar, K.; Liu, J.; et al. TMEM43 mutation p.S358L alters intercalated disc protein expression and reduces conduction velocity in arrhythmogenic right ventricular cardiomyopathy. PLoS ONE 2014, 9, e109128. [Google Scholar] [CrossRef] [PubMed]
- Merner, N.D.; Hodgkinson, K.A.; Haywood, A.F.M.; Connors, S.; French, V.M.; Drenckhahn, J.D.; Kupprion, C.; Ramadanova, K.; Thierfelder, L.; McKenna, W.; et al. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a mis-sense mutation in the TMEM43 gene. Am. J. Hum. Genet. 2008, 82, 809–821. [Google Scholar] [CrossRef]
- Hodgkinson, K.A.; Parfrey, P.S.; Bassett, A.S.; Kupprion, C.; Drenckhahn, J.; Norman, M.W.; Thierfelder, L.; Stuckless, S.N.; Dicks, E.L.; McKenna, W.J.; et al. The impact of implantable cardioverter-defibrillator therapy on survival in autosomal-dominant arrhythmogenic right ventricular cardiomyopathy (ARVD5). J. Am. Coll. Cardiol. 2005, 45, 400–408. [Google Scholar] [CrossRef]
- Hodgkinson, K.A.; Connors, S.P.; Merner, N.; Haywood, A.; Young, T.L.; McKenna, W.J.; Gallagher, B.; Curtis, F.; Bassett, A.S.; Parfrey, P.S. The natural history of a genetic subtype of arrhythmogenic right ventricular cardiomyopathy caused by a p.S358L mutation in TMEM43. Clin. Genet. 2013, 83, 321–331. [Google Scholar] [CrossRef]
- Milting, H.; Klauke, B.; Christensen, A.H.; Müsebeck, J.; Walhorn, V.; Grannemann, S.; Münnich, T.; Šarić, T.; Rasmussen, T.B.; Jensen, H.K.; et al. The TMEM43 Newfoundland mutation p.S358L causing ARVC-5 was imported from Europe and increases the stiff-ness of the cell nucleus. Eur. Heart J. 2015, 36, 872–881. [Google Scholar] [CrossRef]
- Hodgkinson, K.A.; Howes, A.J.; Boland, P.; Shen, X.S.; Stuckless, S.; Young, T.L.; Curtis, F.; Collier, A.; Parfrey, P.S.; Connors, S.P. Long-Term Clinical Outcome of Arrhythmogenic Right Ventricular Cardiomyopathy in Individuals With a p.S358L Mutation in TMEM43 Following Implantable Cardioverter Defibrillator Therapy. Circ. Arrhythmia Electrophysiol. 2016, 9, e003589. [Google Scholar] [CrossRef]
- AbdelWahab, A.; Gardner, M.; Parkash, R.; Gray, C.; Sapp, J. Ventricular tachycardia ablation in arrhythmogenic right ventricular cardiomyopathy patients with TMEM43 gene mutations. J. Cardiovasc. Electrophysiol. 2018, 29, 90–97. [Google Scholar] [CrossRef]
- Dominguez, F.; Zorio, E.; Jimenez-Jaimez, J.; Salguero-Bodes, R.; Zwart, R.; Gonzalez-Lopez, E.; Molina, P.; Bermúdez-Jiménez, F.; Delgado, J.F.; Braza-Boïls, A.; et al. Clinical characteristics and determinants of the phenotype in TMEM43 arrhythmogenic right ventricular cardiomyopathy type 5. Heart Rhythm. 2020, 17, 945–954. [Google Scholar] [CrossRef]
- Paulin, F.L.; Hodgkinson, K.A.; MacLaughlan, S.; Stuckless, S.N.; Templeton, C.; Shah, S.; Bremner, H.; Roberts, J.D.; Young, T.L.; Parfrey, P.S.; et al. Exercise and arrhythmic risk in TMEM43 p.S358L arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2020, 17, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Fan, J.; Zhou, B.; Zhao, C.; Zhao, X.; Su, B.; Miao, Y.; Liao, Y.; Wang, L. Myocardial strain measured via two-dimensional speckle-tracking echocardiography in a family diagnosed with arrhythmogenic left ventricular cardiomyopathy. Cardiovasc. Ultrasound 2021, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Helle, E.; Care, M.; Moayedi, Y.; Gollob, M.H.; Thavendiranathan, P.; Spears, D.; Hanneman, K. Cardiac MRI and Clinical Outcomes in TMEM43 Arrhythmogenic Cardiomyopathy. Radiol. Cardiothorac. Imaging 2023, 5, e230155. [Google Scholar] [CrossRef]
- Cabrera-Borrego, E.; Bermúdez-Jiménez, F.J.; Gasperetti, A.; Tandri, H.; Sánchez-Millán, P.J.; Molina-Lerma, M.; Ro-ca-Luque, I.; Vázquez-Calvo, S.; Compagnucci, P.; Casella, M.; et al. Electro-physiological Phenotype-Genotype Study of Sustained Monomorphic Ventricular Tachycardia in Inherited, High Arrhythmic Risk, Left Ventricular Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2024, 17, e013145. [Google Scholar] [CrossRef]
- Marcus, F.I.; Edson, S.; Towbin, J.A. Genetics of arrhythmogenic right ventricular cardiomyopathy: A practical guide for physicians. J. Am. Coll. Cardiol. 2013, 61, 1945–1948. [Google Scholar] [CrossRef]
- Garcia-Gras, E.; Lombardi, R.; Giocondo, M.J.; Willerson, J.T.; Schneider, M.D.; Khoury, D.S.; Marian, A.J. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J. Clin. Investig. 2006, 116, 2012–2021. [Google Scholar] [CrossRef]
- Bengtsson, L.; Otto, H. LUMA interacts with emerin and influences its distribution at the inner nuclear membrane. J. Cell. Sci. 2008, 121, 536–548. [Google Scholar] [CrossRef]
- Shinomiya, H.; Kato, H.; Kuramoto, Y.; Watanabe, N.; Tsuruda, T.; Arimura, T.; Miyashita, Y.; Miyasaka, Y.; Mashimo, T.; Takuwa, A.; et al. Aberrant accumulation of TMEM43 accompanied by perturbed transmural gene expression in arrhythmogenic cardiomyopathy. FASEB J. 2021, 35, e21994. [Google Scholar] [CrossRef]
- Berte, B.; Sacher, F.; Venlet, J. VT Recurrence after ablation: Incomplete ablation or disease progression? A multicentric European study. J. Cardiovasc. Electrophysiol. 2016, 27, 80–87. [Google Scholar] [CrossRef]
- Alfakih, K.; Reid, S.; Jones, T.; Sivananthan, M. Assessment of ventricular function and mass by cardiac magnetic resonance imaging. Eur. Radiol. 2004, 14, 1813–1822. [Google Scholar] [CrossRef]
- Augusto, J.B.; Eiros, R.; Nakou, E.; Moura-Ferreira, S.; Treibel, T.A.; Captur, G.; Akhtar, M.M.; Protonotarios, A.; Gos-sios, T.D.; Savvatis, K.; et al. Dilated cardiomyopathy and arrhythmogenic left ventricular cardiomyopathy: A comprehensive genotype-imaging phenotype study. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 326–336. [Google Scholar] [CrossRef]
- Fatkin, D.; MacRae, C.; Sasaki, T.; Wolff, M.R.; Porcu, M.; Frenneaux, M.; Atherton, J.; Vidaillet, H.J., Jr.; Spudich, S.; De Girolami, U.; et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 1999, 341, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
| Citation | Aims | Patients with TMEM43 Mutation (NM_024334.3) | HGVSc | HGVSp | Conclusions |
|---|---|---|---|---|---|
| Hodgkinson KA, JACC 2005 [7] | To evaluate the impact of ICD implantation in ARVD5 patients with 3p25-linked autosomal dominant disease vs controls | 48 pts with ARVD5 linked to 3p25 (obligate carriers, n = 8; DNA haplotype, n = 36; SCD or VT, n = 4) | NA | NA | ICDs significantly reduced the mortality in male patients with an ARVD5 linked to 3p25. |
| Merner ND, Am J Hum Genet 2008 [6] | To identify the ARVD5 gene and conduct a penetrance analysis of clinical features | 257 affected pts (144 mutation carriers and 113 clinically affected patients) | c.1073C>T | p.Ser458Leu | Mutation at locus ARVD5 causes a lethal, fully penetrant, sex-influenced cardiomyopathy. |
| Hodgkinson KA, Clin Genet 2013 [8] | To determine the phenotype and natural history of TMEM43-related ARVC | 258 affected pts (genetic mutation, n = 83; obligate carrier, n = 118; SCD or VT, n = 57) | c.1073C>T | p.Ser458Leu | TMEM43-related ARVC shows a sex-influenced malignant phenotype, with earlier and more severe expression in males. |
| Milting H, EHJ 2015 [9] | To evaluate the presence of the missense mutation TMEM43 in a German ARVC family | 11 pts with a TMEM43 mutation | c.1073C>T | p.Ser458Leu | TMEM43 screening is recommended in ARVC patients; the mutation may cause nuclear stiffness and cell death. |
| Hodgkinson KA, Circ Arrhythm Electrophysiol 2016 [10] | To assess the long-term outcomes after ICD implantation in ARVC patients with the TMEM43 mutation | 148 affected pts (genetic mutation, n = 146; VF or SCD, n = 2) who underwent ICD implantation | c.1073C>T | p.Ser458Leu | ICDs are recommended in young males with TMEM43; in females, delayed implantation may be appropriate. |
| AbdelWahab A, J Cardiovasc Electrophysiol 2018 [11] | To compare the VT ablation outcomes in ARVC patients with vs. without a TMEM43 mutation | 5 pts with a TMEM43 mutation | c.1073C>T | p.Ser458Leu | TMEM43-related ARVC shows a more severe morphology and higher arrhythmic burden during ablation. |
| Dominguez F, Heart Rhythm 2020 [12] | To evaluate the phenotype, clinical course, and impact of exercise in pts with a TMEM43 mutation in non-Newfoundland-related families | 62 affected pts (genetic carriers, n = 30; obligate carriers, n = 14; SCD, n = 18) | c.1073C>T | p.Ser458Leu | TMEM43 causes severe ARVC, particularly in males; vigorous exercise may worsen arrhythmias, especially in females. |
| Paulin FL, Heart Rhythm 2020 [13] | To evaluate the impact of high-level physical activity in TMEM43-p.S358L ARVC patients after an ICD for primary prevention | 80 pts with a TMEM43 mutation | c.1073C>T | p.Ser458Leu | High-intensity exercise increases the risk of malignant VAs and earlier ICD shock in TMEM43 ARVC patients. |
| Ma C, Cardiovasc Ultrasound 2021 [14] | To assess the longitudinal myocardial strain in a family with TMEM43 mutations | 8 pts with a TMEM43 mutation | NA | NA | TMEM43 mutation carriers showed impaired LV layer-specific longitudinal strain vs. that in healthy controls. |
| Matos J, Radiol Cardiothorac Imaging 2023 [15] | To describe the CMR findings in ARVC patients with TMEM43 pathogenic or VUS variants using the 2020 Padua Criteria | 14 ARVC pts with a pathogenic variant (n = 8) and a VUS (n = 7) of TMEM43 | c.1073C>T and other VUS | p.Ser458Leu and other | TMEM43-related ARVC shows progressive LV dysfunction and subepicardial fibrosis, requiring longitudinal clinical and imaging follow-up. |
| Bueno Marinas M, Int J Mol Sci 2024 [4] | To assess rare genetic variants in non-desmosomal genes in pts with arrhythmogenic cardiomyopathy | 6 pts with a TMEM43 mutation | c.1073C>T; c.349A>G | p.Ser458Leu; p.Arg117Gly | TMEM43 showed 3.8-fold enrichment; one-third of ALVC patients had rare non-desmosomal variants. |
| Cabrera-Borrego E, Circ Arrhythm Electrophysiol 2024 [16] | To evaluate the genotype–substrate correlation in LV-involved inherited cardiomyopathies | 6 pts with a TMEM43 mutation | c.1073C>T | p.Ser458Leu | In TMEM43 mutation carriers, the arrhythmic substrate is localized in the RV outflow tract, with biventricular involvement, an LBBB pattern, and LV fibrosis. Nuclear gene mutations are associated with higher arrhythmic and HF risk. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecere, A.; Martini, M.; Bueno Marinas, M.; Rigato, I.; Parodi, A.; Pilichou, K.; Bauce, B. The Natural History and Clinical Outcomes of Transmembrane Protein 43 Cardiomyopathy: A Systematic Review. J. Clin. Med. 2025, 14, 5611. https://doi.org/10.3390/jcm14165611
Cecere A, Martini M, Bueno Marinas M, Rigato I, Parodi A, Pilichou K, Bauce B. The Natural History and Clinical Outcomes of Transmembrane Protein 43 Cardiomyopathy: A Systematic Review. Journal of Clinical Medicine. 2025; 14(16):5611. https://doi.org/10.3390/jcm14165611
Chicago/Turabian StyleCecere, Annagrazia, Marika Martini, Maria Bueno Marinas, Ilaria Rigato, Alessandro Parodi, Kalliopi Pilichou, and Barbara Bauce. 2025. "The Natural History and Clinical Outcomes of Transmembrane Protein 43 Cardiomyopathy: A Systematic Review" Journal of Clinical Medicine 14, no. 16: 5611. https://doi.org/10.3390/jcm14165611
APA StyleCecere, A., Martini, M., Bueno Marinas, M., Rigato, I., Parodi, A., Pilichou, K., & Bauce, B. (2025). The Natural History and Clinical Outcomes of Transmembrane Protein 43 Cardiomyopathy: A Systematic Review. Journal of Clinical Medicine, 14(16), 5611. https://doi.org/10.3390/jcm14165611






