Surgical and Clinical Aspects Associated with Double-Valve Infective Endocarditis
Abstract
1. Introduction
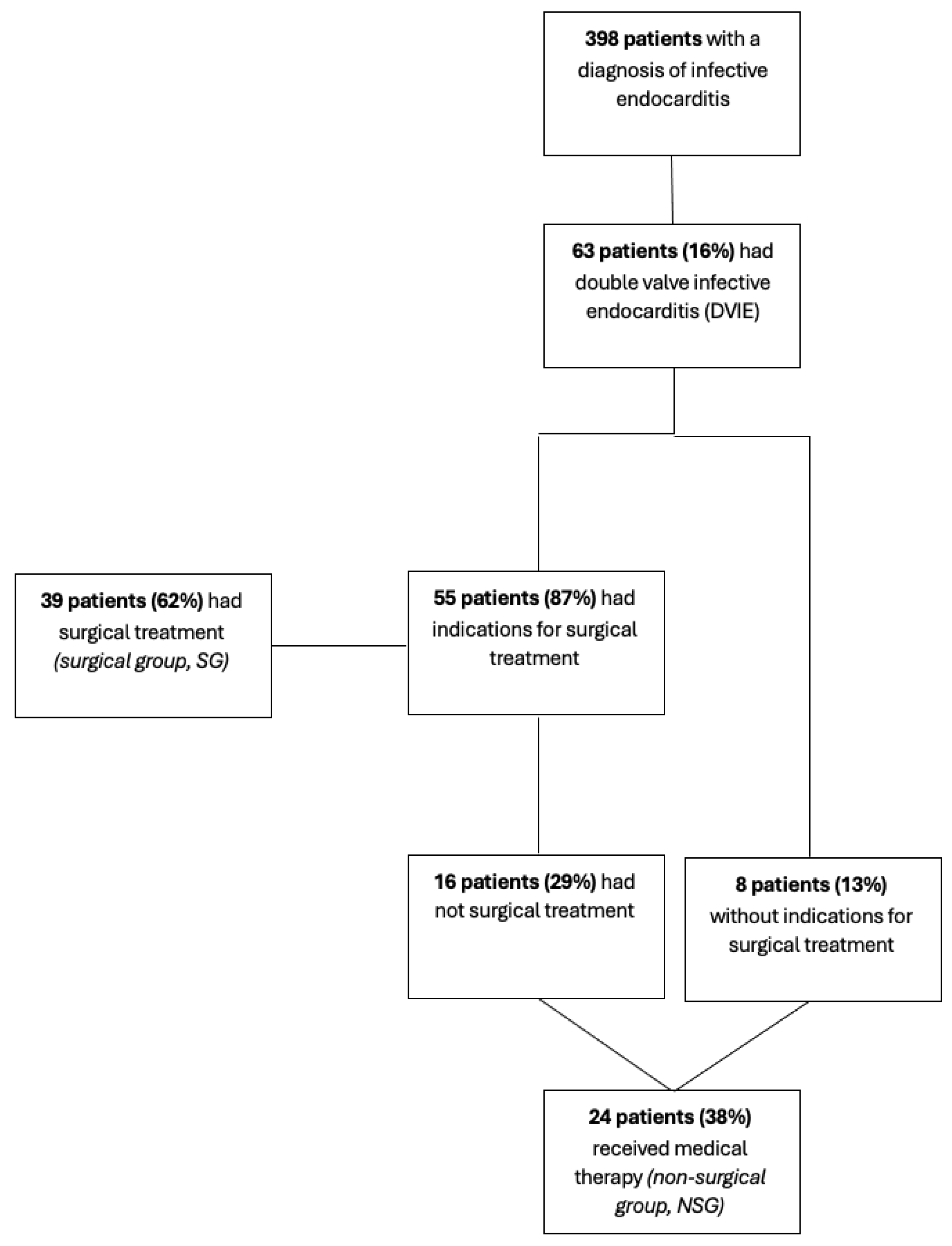
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Definition of Primary and Secondary Endpoints
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Microbiology Yield
3.3. Risk Factors for IE
3.4. Comorbidities
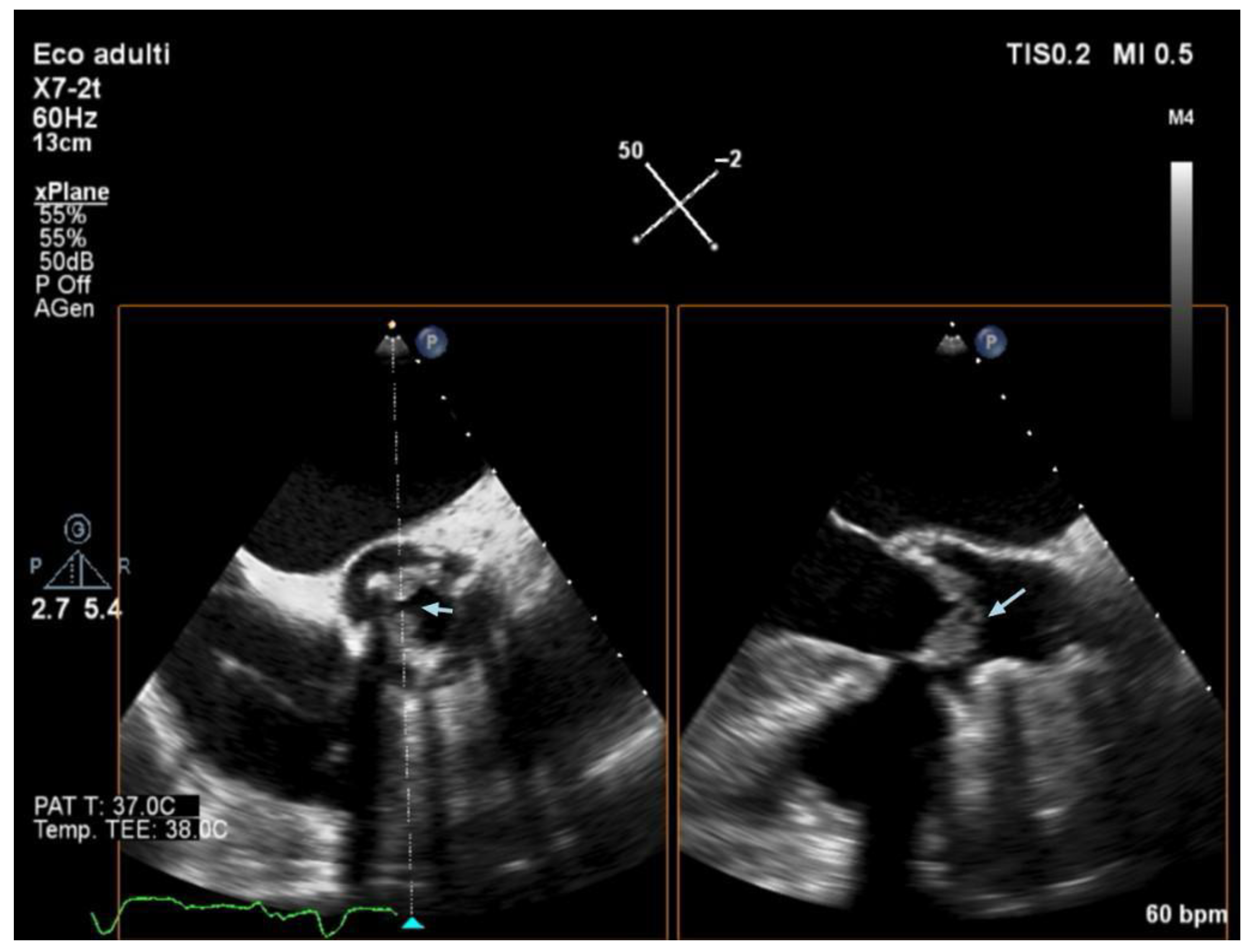
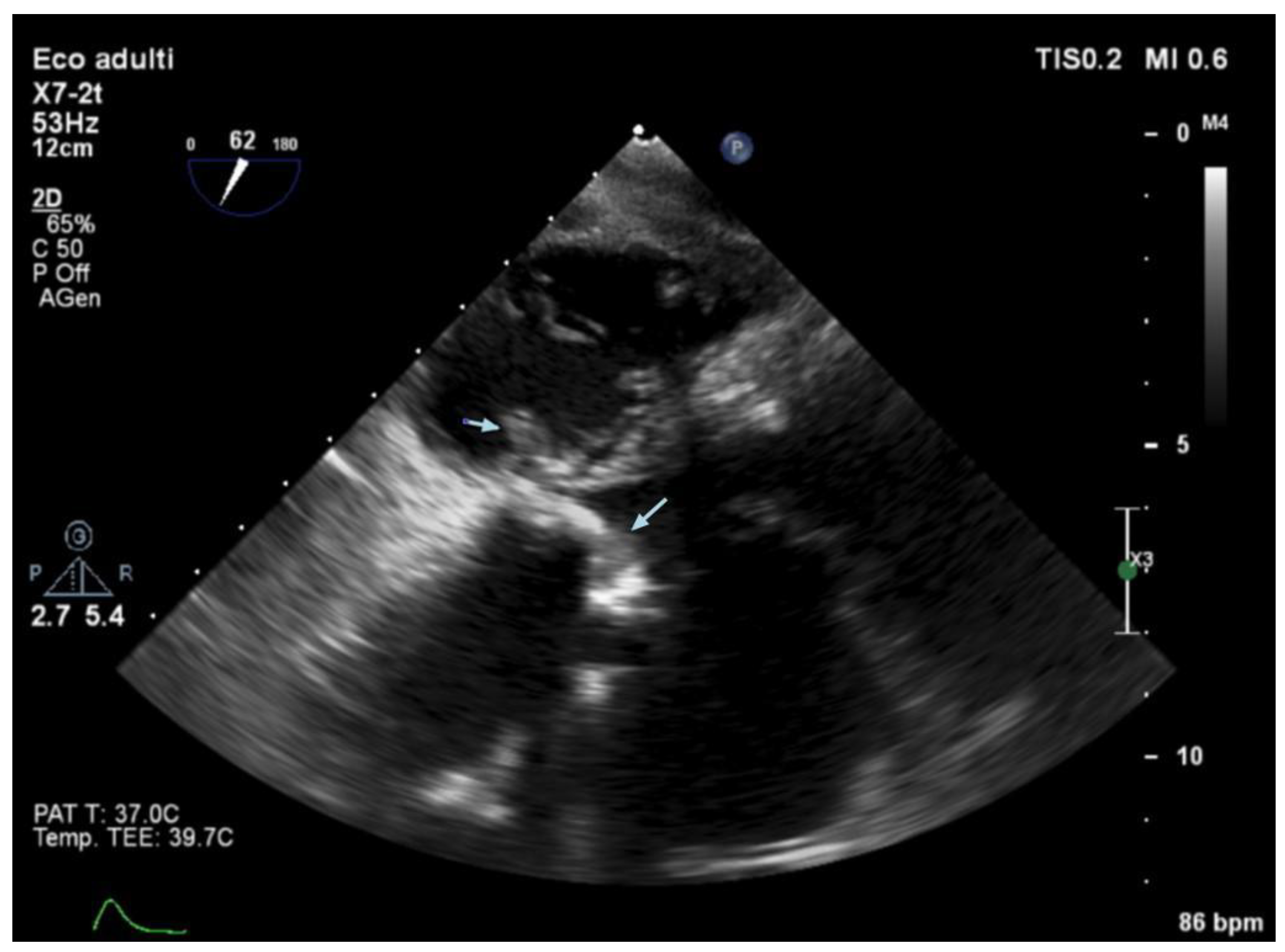
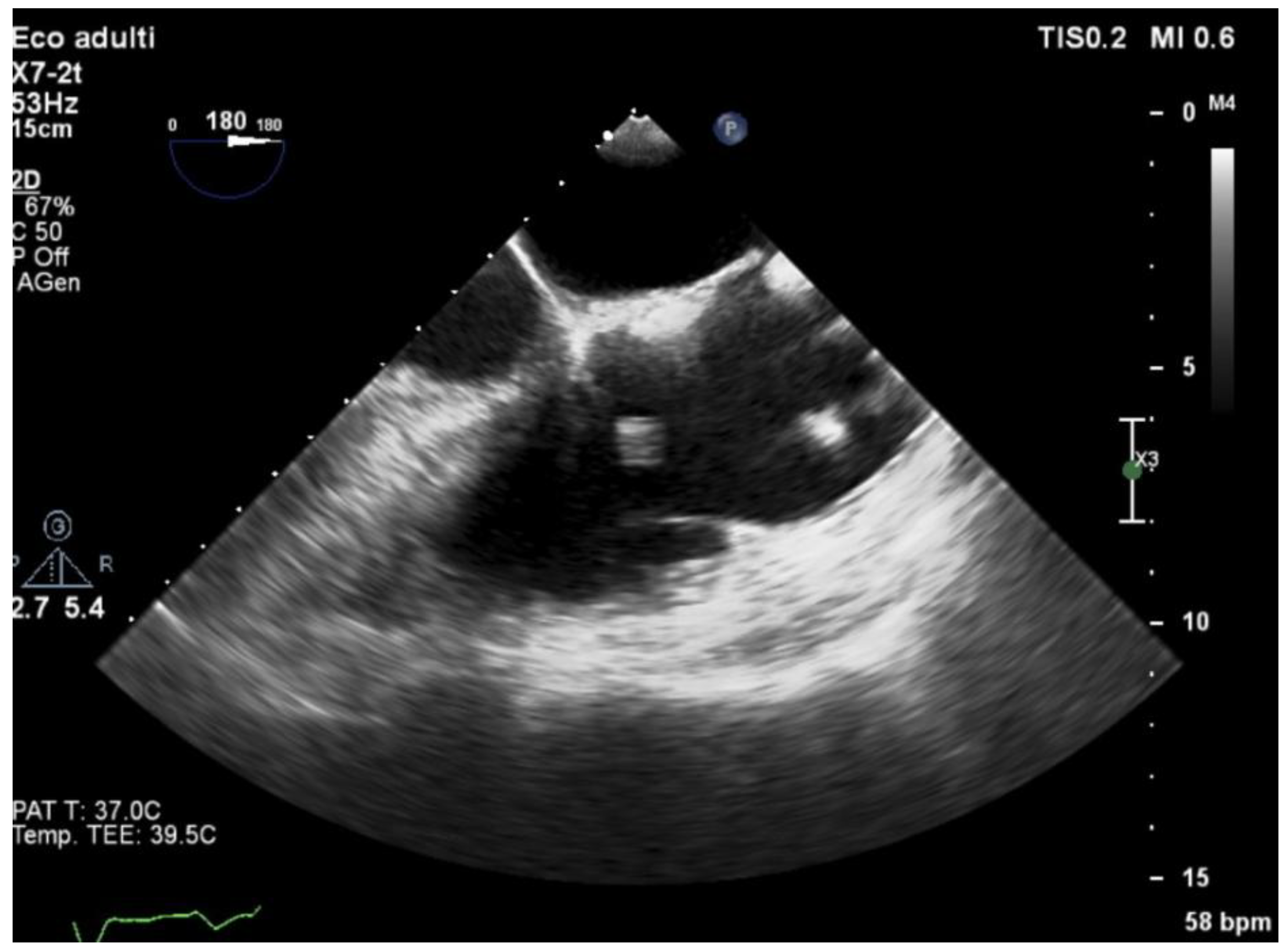
3.5. Echocardiography and Localization of IE
3.6. IE Complications
3.7. Clinical Course and Treatment
3.8. Surgical Procedures
3.9. Postoperative Complications
3.10. Outcomes
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado, V.; Marsan, N.A.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. ESC Scientific Document Group 2023 ESC Guidelines for the Management of Endocarditis. 2023 ESC Guidelines for the management of endocarditis: Developed by the task force on the management of endocarditis of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Bohbot, Y.; Peugnet, F.; Lieu, A.; Carbone, A.; Mouhat, B.; Philip, M.; Gouriet, F.; Arregle, F.; Chevalier, F.; Diouf, M.; et al. Characteristics and Prognosis of Patients with Left-Sided Native Bivalvular Infective Endocarditis. Can. J. Cardiol. 2021, 37, 292–299. [Google Scholar] [CrossRef]
- Rajani, R.; Klein, J.L. Infective endocarditis: A contemporary update. Clin. Med. 2020, 20, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Zaballos, S.; González-Ramallo, V.; Quintana, E.; Muñoz, P.; De La Villa-Martínez, S.; Fariñas, M.C.; Arnáiz-de Las Revillas, F.; De Alarcón, A.; Rodríguez-Esteban, M.Á.; Miró, J.M.; et al. Multivalvular Endocarditis: A Rare Condition with Poor Prognosis. J. Clin. Med. 2022, 11, 4736. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Lazar, J.M.; Cunha, B.A.; Liao, W.; Minnaganti, V. Multi-valvular endocarditis. Clin. Microbiol. Infect. 2000, 6, 207–212. [Google Scholar] [CrossRef]
- Selton-Suty, C.; Doco-Lecompte, T.; Bernard, Y.; Duval, X.; Letranchant, L.; Delahaye, F.; Célard, M.; Alla, F.; Carteaux, J.P.; Hoen, B.; et al. Clinical and microbiologic features of multivalvular endocarditis. Curr. Infect. Dis. Rep. 2010, 12, 237–243. [Google Scholar] [CrossRef]
- Scheggi, V.; Del Pace, S.; Ceschia, N.; Vanni, F.; Merilli, I.; Zoppetti, N.; Alterini, B.; Marchionni, N.; Stefàno, P.L. Double-valve infective endocarditis: Clinical features and prognostic impact—A retrospective study in a surgical centre. Heart Vessel. 2022, 37, 895–901. [Google Scholar] [CrossRef]
- Gillinov, A.M.; Diaz, R.; Blackstone, E.H.; Pettersson, G.B.; Sabik, J.F.; Lytle, B.W.; Cosgrove, D.M. Double valve endocarditis. Ann. Thorac. Surg. 2001, 71, 1874–1879. [Google Scholar] [CrossRef]
- Caldonazo, T.; Hagel, S.; Doenst, T.; Kirov, H.; Sá, M.P.; Jacquemyn, X.; Tasoudis, P.; Franz, M.; Diab, M. Conservative Versus Surgical therapy in patients with infective endocarditis and surgical indication—Meta-analysis of reconstructed time-to-event data. J. Am. Heart Assoc. 2024, 13, e033404. [Google Scholar] [CrossRef]
- Miller, P.C.; Schulte, L.J.; Marghitu, T.; Huang, S.; Kaneko, T.; Damiano, R.J.; Kachroo, P. Outcomes of double-valve surgery for infective endocarditis are improving in the modern era. J. Thorac. Cardiovasc. Surg. 2024, 168, 832–842. [Google Scholar] [CrossRef]
- Husso, A.; Riekkinen, T.; Rissanen, A.; Ollila, J.; Valtola, A. Combined Mitral and Aortic Valve Surgery: 17-Year Experience in a Single Center. Scand. J. Surg. 2021, 110, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.M.; Elhenawy, A.M.; Maganti, M.; Armstrong, S.; David, T.E.; Feindel, C.M. Outcomes of Double Valve Surgery for Active Infective Endocarditis. J. Thorac. Cardiovasc. Surg. 2009, 138, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, S.; Salto-Alejandre, S.; Ström, A.; Olaison, L.; Rasmussen, M. Surgery Is Underused in Elderly Patients with Left-Sided Infective Endocarditis: A Nationwide Registry Study. J. Am. Heart Assoc. 2021, 10, e020221. [Google Scholar] [CrossRef] [PubMed]
- Ghanta, R.K.; Pettersson, G.B. Surgical treatment of infective endocarditis in elderly patients: The importance of shared decision making. J. Am. Heart Assoc. 2021, 10, e022186. [Google Scholar] [CrossRef]
- Lemos, L.H.B.; Silva, L.R.D.; Correa, M.G.; Golebiovski, W.; Weksler, C.; Garrido, R.Q.; Barbosa, G.F.; Lamas, C.D.C. Endocardite infecciosa em Idosos: Características distintas. Arq. Bras. Cardiol. 2021, 117, 775–781. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Corey proposed modifications to the duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the Management of Infective Endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Fowler, V.G.; Durack, D.T.; Selton-Suty, C.; Athan, E.; Bayer, A.S.; Chamis, A.L.; Dahl, A.; DiBernardo, L.; Durante-Mangoni, E.; Duval, X.; et al. The 2023 duke-international society for cardiovascular infectious diseases criteria for infective endocarditis: Updating the modified duke criteria. Clin. Infect. Dis. 2023, 77, 518–526. [Google Scholar] [CrossRef]
- Habib, G.; Hoen, B.; Tornos, P.; Thuny, F.; Prendergast, B.; Vilacosta, I.; Moreillon, P.; De Jesus Antunes, M.; Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and by the International Society of Chemotherapy (ISC) for Infection and Cancer; Authors/Task Force Members; et al. Guidelines on the Prevention, Diagnosis, and Treatment of Infective Endocarditis (New Version 2009): The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Eur. Heart J. 2009, 30, 2369–2413. [Google Scholar] [CrossRef]
- Madeira, S.; Rodrigues, R.; Tralhão, A.; Santos, M.; Almeida, C.; Marques, M.; Ferreira, J.; Raposo, L.; Neves, J.; Mendes, M. Assessment of perioperative mortality risk in patients with infective endocarditis undergoing cardiac surgery: Performance of the EuroSCORE I and II Logistic Models. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 141–148. [Google Scholar] [CrossRef]
- Pizzino, F.; Paradossi, U.; Trimarchi, G.; Benedetti, G.; Marchi, F.; Chiappino, S.; Conti, M.; Di Bella, G.; Murzi, M.; Di Sibio, S.; et al. Clinical features and patient outcomes in infective endocarditis with surgical indication: A single-centre experience. J. Cardiovasc. Dev. Dis. 2024, 11, 138. [Google Scholar] [CrossRef]
- Nallamothu, B.K.; Cohen, D.J. No “I” in heart team: Incentivizing multidisciplinary care in cardiovascular medicine. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 410–413. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: Executive summary. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef]
- Mangner, N.; Panagides, V.; Del Val, D.; Abdel-Wahab, M.; Crusius, L.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; et al. Incidence, clinical characteristics, and impact of absent echocardiographic signs in patients with infective endocarditis after transcatheter aortic valve implantation. Clin. Infect. Dis. 2023, 76, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Davierwala, P.M.; Marin-Cuartas, M.; Misfeld, M.; Borger, M.A. The value of an “Endocarditis Team ”. Ann. Cardiothorac. Surg. 2019, 8, 621. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Lee, H.J.; Lee, S.J.; Lee, K.H.; Lee, E.H.; Baek, Y.J.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; et al. Impact of Valve Culture Positivity on prognosis in patients with infective endocarditis who underwent valve surgery. Infect. Dis. Ther. 2022, 11, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- García-Granja, P.E.; López, J.; Vilacosta, I.; Sarriá, C.; Ladrón, R.; Olmos, C.; Sáez, C.; Maroto, L.; Di Stefano, S.; Gómez, I.; et al. Impact of valve culture in the prognosis of active left-sided infective endocarditis. Clin. Infect. Dis. 2019, 68, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
| All | Groups | |||
|---|---|---|---|---|
| Characteristics | N = 63 | NSG 1, N = 24 | SG 1, N = 39 | p-Value 2 |
| Male sex, n (%) | 52 (83%) | 18 (75%) | 34 (87%) | 0.3 |
| Age, median (IQ) | 70.00 (17.50) | 71.50 (20.00) | 68.00 (15.50) | 0.3 |
| Age > 75 years old, n (%) | 16 (25%) | 9 (56%) | 7 (44%) | 0.08 |
| Length of hospital stay, median (IQ) | 36.50 (23.75) | 30.50 (23.50) | 37.50 (20.25) | 0.2 |
| Pathogens, n (%) | 0.3 | |||
| Coagulase-negative Staphylococci | 6 (9.5%) | 1 (4.2%) | 5 (13%) | |
| Enterococci | 19 (30%) | 9 (38%) | 10 (26%) | |
| Negative | 5 (7.9%) | 0 (0%) | 5 (13%) | |
| Others | 4 (6.3%) | 1 (4.2%) | 3 (7.7%) | |
| Staphylococcus aureus | 8 (13%) | 4 (17%) | 4 (10%) | |
| Streptococcus spp. | 21 (33%) | 9 (38%) | 12 (31%) | |
| Time to negative blood cultures, days (IQR) | 8.00 (9.25) | 9.50 (9.25) | 8.00 (7.00) | 0.7 |
| Valve culture performed, n (%) | / | / | 35 (90%) | |
| Valve culture positive, n (%) | / | / | 5 (14%) | |
| Localization, n (%) | 0.6 | |||
| AM [aortic–mitral] | 50 (79%) | 21 (88%) | 29 (74%) | |
| AT [aortic–tricuspidal] | 5 (7.9%) | 2 (8.3%) | 3 (7.7%) | |
| MT [mitral–tricuspidal] | 6 (9.5%) | 1 (4.2%) | 5 (13%) | |
| TP [tricuspidal–pulmonary] | 2 (3.2%) | 0 (0%) | 2 (5.1%) | |
| Type of valve, n (%) | 0.050 | |||
| NN [native–native] | 48 (76%) | 15 (63%) | 33 (85%) | |
| NP [native–prosthetic] | 12 (19%) | 6 (25%) | 6 (15%) | |
| PP [prosthetic–prosthetic] | 2 (3.2%) | 2 (8.3%) | 0 (0%) | |
| Unknown | 1 (1.6%) | 1 (4.2%) | 0 (0%) | |
| Risk factors for IE, n (%) | ||||
| Cardiovascular procedure history | 17 (27%) | 10 (42%) | 7 (18%) | 0.047 |
| ICD/PM at admission | 9 (14%) | 2 (8.3%) | 7 (18%) | 0.5 |
| Intravenous drug use | 8 (13%) | 3 (13%) | 5 (13%) | >0.9 |
| Anatomical alterations | 13 (21%) | 7 (29%) | 6 (15%) | 0.2 |
| Skin lesions | 1 (1.6%) | 1 (4.2%) | 0 (0%) | 0.4 |
| Gastrointestinal alterations | 8 (13%) | 4 (17%) | 4 (10%) | 0.5 |
| History of rheumatic fever | 1 (1.6%) | 0 (0%) | 1 (2.6%) | >0.9 |
| Recent dental procedures | 1 (1.6%) | 0 (0%) | 1 (2.6%) | >0.9 |
| Comorbidities, n (%) | ||||
| Chronic hepatitis | 10 (16%) | 4 (17%) | 6 (15%) | >0.9 |
| Hemodialysis | 2 (3.2%) | 2 (8.3%) | 0 (0%) | 0.14 |
| Type 2 diabetes | 14 (22%) | 8 (33%) | 6 (15%) | 0.12 |
| HIV | 4 (6.3%) | 0 (0%) | 4 (10%) | 0.3 |
| Cancer | 10 (16%) | 7 (29%) | 3 (7.7%) | 0.034 |
| Immunosuppressive therapy | 9 (14%) | 7 (29%) | 2 (5.1%) | 0.021 |
| Anticoagulant therapy | 14 (22%) | 8 (33%) | 6 (15%) | 0.12 |
| Antiplatelet therapy | 10 (16%) | 6 (25%) | 4 (10%) | 0.2 |
| Complications of infection, n (%) | ||||
| Cardiac complications | 21 (33%) | 3 (13%) | 18 (46%) | 0.007 |
| Embolic complications | 31 (49%) | 9 (38%) | 22 (56%) | 0.2 |
| Cerebral emboli | 20 (32%) | 6 (25%) | 14 (36%) | 0.4 |
| Spleen/kidney emboli | 12 (19%) | 3 (13%) | 9 (23%) | 0.3 |
| Cutaneous emboli | 2 (3.2%) | 1 (4.2%) | 1 (2.6%) | >0.9 |
| Spondylodiscitis | 7 (11%) | 1 (4.2%) | 6 (15%) | 0.2 |
| Pulmonary emboli | 7 (11%) | 2 (8.3%) | 5 (13%) | 0.7 |
| Septic shock | 2 (3.2%) | 1 (4.2%) | 1 (2.6%) | >0.9 |
| Arrhythmias | 8 (13%) | 0 (0%) | 8 (21%) | 0.020 |
| EV antibiotic duration, days (IQ) | 38.00 (17.25) | 32.00 (12.00) | 42.00 (22.00) | 0.12 |
| Switch to oral antibiotics, n (%) | 11 (17%) | 5 (21%) | 6 (15%) | 0.7 |
| In-hospital mortality, n (%) | 7 (11%) | 5 (21%) | 2 (5.1%) | 0.095 |
| One-year survival, n (%) Unknown, n (%) | 41 (65%) 4 (6.3%) | 13 (54%) 0 (0%) | 28 (72%) 4 (10%) | 0.031 |
| Total | Groups | |||
|---|---|---|---|---|
| Characteristics | N = 63 | Native Valves N = 49 (77.8%) | Prosthetic Valve(s) N = 14 (22.2%) | p-Value 1 |
| Male sex, n (%) | 52 (82.5%) | 40 (81.6%) | 12 (85.7%) | 1.000 |
| Age, median (IQR) | 70.0 (57.0–74.5) | 65.0 (56.0–74.0) | 71.5 (69.2–77.0) | 0.053 |
| Length of hospital stay, days, median (IQR) | 36.5 (26.2–50.0) | 34.5 (26.0–50.0) | 38.5 (31.2–45.5) | 0.923 |
| One-year survival | 41 (65.1%) | 37 (75.5%) | 4 (28.6%) | 0.003 |
| Emboli | 31 (49.2%) | 24 (49.0%) | 7 (50.0%) | 1.000 |
| Cerebral emboli | 20 (31.7%) | 16 (32.7%) | 4 (28.6%) | 1.000 |
| Spleen or kidney emboli | 12 (19.0%) | 10 (20.4%) | 2 (14.3%) | 0.898 |
| Skin emboli | 1 (1.6%) | 1 (2.0%) | 0 (0.0%) | 1.000 |
| Osteomyelitis | 7 (11.1%) | 5 (10.2%) | 2 (14.3%) | 1.000 |
| Pulmonary emboli | 7 (11.1%) | 6 (12.2%) | 1 (7.1%) | 0.957 |
| Surgically treated | 39 (61.9%) | 33 (67.3%) | 6 (42.9%) | 0.176 |
| In-hospital mortality | 7 (11.1%) | 3 (6.8%) | 4 (28.6%) | 0.052 |
| Localization | Surgical Procedure | No. |
|---|---|---|
| Aortic–mitral [AM] (n = 29) | Double prosthetic valve Aortic prosthesis + mitral repair Mitral prosthesis + aortic repair Mitral prosthesis + tricuspidal repair | 16 (14 biological, 2 mechanical) 11 (9 biological, 2 mechanical) 1 (mechanical) 1 (mechanical) |
| Mitral–tricuspidal [MT] (n = 5) | Mitral prosthesis + tricuspidal repair Tricuspid prosthesis + mitral repair Double repair | 2 (1 mechanical) 1 (biological) 2 |
| Aortic–tricuspidal [AT] (n = 3) | Aortic prosthesis + tricuspidal repair | 3 (biological) |
| Tricuspidal–pulmonary [TP] (n = 2) | Tricuspid prosthesis + pulmonary valve repair Pulmonary prosthesis + tricuspidal repair | 1 (biological) 1 (biological) |
| Additional procedures | CABG Ascending aorta replacement Aortic root replacement | 8 (23%) 2 (6%) 1 (3%) |
| Characteristics of SG | N = 39 |
|---|---|
| ICU stay, days (IQ) Cardiac surgery department stay, days (IQ) In-hospital mortality, n (%) | 2.00 (5.00) 8.00 (4.75) 2 (5%) |
| Complications, n (%) AVB/Arrhythmias Post-op PM implantation Periprosthetic leak PNX Cardiac tamponade Pleural effusion Post-op dialysis | 16 (41%) 9 (25%) 1 (2.6%) 1 (2.6%) 2 (5.1%) 1 (2.6%) 1 (2.6%) |
| EuroSCORE II, median (IQ) ECC time, minutes (IQ) Aortic cross-clamping time, minutes (IQ) | 7.00 (4.00) 156.00 (70.00) 119.00 (68.25) |
| Timing of surgery, n (%) Non urgent Urgent | 21 (54%) 18 (46%) |
| Variable | OR | IC 95% | p-Value |
|---|---|---|---|
| Cerebral emboli | 0.632 | 0.116–3.44 | 0.595 |
| Cardiac complications | 2.17 | 0.358–13.11 | 0.400 |
| Prosthetic valve | 0.300 | 0.0394–2.29 | 0.245 |
| Age (by 1 year) | 0.957 | 0.879–1.04 | 0.957 |
| Spleen/kidney emboli | 1.64 | 0.164–16.34 | 0.675 |
| Urgent surgery | 0.222 | 0.0363–1.36 | 0.104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerta, S.; Sangaletti, G.; Villano, V.A.; Puci, F.; Kushta, E.; Totaro, P.; Amoroso, F.; Magrini, G.; Valsecchi, P.; Bruno, R.; et al. Surgical and Clinical Aspects Associated with Double-Valve Infective Endocarditis. J. Clin. Med. 2025, 14, 5589. https://doi.org/10.3390/jcm14155589
Lerta S, Sangaletti G, Villano VA, Puci F, Kushta E, Totaro P, Amoroso F, Magrini G, Valsecchi P, Bruno R, et al. Surgical and Clinical Aspects Associated with Double-Valve Infective Endocarditis. Journal of Clinical Medicine. 2025; 14(15):5589. https://doi.org/10.3390/jcm14155589
Chicago/Turabian StyleLerta, Sonia, Gloria Sangaletti, Vincenzo Antonio Villano, Flavia Puci, Eraldo Kushta, Pasquale Totaro, Filippo Amoroso, Giulia Magrini, Pietro Valsecchi, Raffaele Bruno, and et al. 2025. "Surgical and Clinical Aspects Associated with Double-Valve Infective Endocarditis" Journal of Clinical Medicine 14, no. 15: 5589. https://doi.org/10.3390/jcm14155589
APA StyleLerta, S., Sangaletti, G., Villano, V. A., Puci, F., Kushta, E., Totaro, P., Amoroso, F., Magrini, G., Valsecchi, P., Bruno, R., & Seminari, E. (2025). Surgical and Clinical Aspects Associated with Double-Valve Infective Endocarditis. Journal of Clinical Medicine, 14(15), 5589. https://doi.org/10.3390/jcm14155589









