Abstract
As people with cystic fibrosis (PwCF) live longer, kidney disease is emerging as a significant comorbidity that is increasingly linked to cardiovascular complications and progression to end-stage kidney disease. In our recent review, we proposed the unifying term CF-related kidney disease (CFKD) to encompass the spectrum of kidney dysfunction observed in this population. Early detection of kidney injury is critical for improving long-term outcomes, yet remains challenging due to the limited sensitivity of conventional laboratory tests, particularly in individuals with altered muscle mass and unique CF pathophysiology. Emerging approaches, including novel blood and urinary biomarkers, urinary extracellular vesicles, and genetic risk profiling, offer promising avenues for identifying subclinical kidney damage. When integrated with machine learning algorithms, these tools may enable the development of personalized risk stratification models and targeted therapeutic strategies. This precision medicine approach has the potential to transform kidney disease management in PwCF, shifting care from reactive treatment of late-stage disease to proactive monitoring and early intervention.
1. Introduction
Cystic Fibrosis (CF) is a genetic disorder caused by mutations in the CF Transmembrane Conductance Regulator (CFTR) gene [1]. Since the discovery and clinical use of highly effective modulator therapy (HEMT), which improves mutant CFTR protein expression and/or function, life expectancy for people with CF (PwCF) has increased significantly and resulted in substantial improvements in lung function and quality of life [2,3]. Chronic kidney disease (CKD) has emerged as a notable morbidity in the aging population of PwCF [4,5]. CF-related kidney disease (CFKD) has been proposed as a term for kidney dysfunction affecting PwCF [6,7]. In addition to renal morbidity, CKD is an identified risk factor for cardiovascular events, subsequent end-stage kidney disease (ESKD), and death [8]. The known risk factors for acute kidney injury (AKI) in PwCF include nephrotoxic medications (e.g., aminoglycosides, non-steroidal anti-inflammatory drugs), severe pulmonary infections, systemic inflammation, hypoxia, and oxidative stress [9,10,11]. AKI is frequently undetected by conventional assays and may increase the risk of CKD [12]. Evidence-based interventions such as lifestyle modifications and blood pressure control, as well as monitoring medication use during the early stages of kidney injury, may prevent or delay CKD [13,14].
CFTR, abundantly expressed in most segments of the renal tubular epithelium, is implicated in diverse functions that differ from those in the lung [6,15,16,17]. In the proximal tubules of the kidney, CFTR regulates vesicular acidification and the endocytic uptake of low-molecular-weight proteins. In the collecting duct, it plays a role in bicarbonate secretion (reviewed in Hart et al., 2025) [6]. The mechanisms of CFKD are incompletely understood and may include factors associated with CFTR dysfunction in the lung and other organs, including the kidney, cumulative treatment side effects combined with an age-related kidney function decline, as well as genetic factors [6,18,19]. Here, we review conventional and emerging biomarkers for early detection of kidney injury and dysfunction, with a focus on PwCF. To guide future research on CF-specific biomarkers of kidney dysfunction, we describe genetic modifiers associated with worse outcomes in CF and emphasize their potential as CFKD contributors.
To identify relevant studies for this review, we conducted a literature search using the PubMed database. The search included combinations of the following keywords: “Cystic fibrosis,” “kidney injury,” “biomarkers,” “chronic kidney disease (CKD),” “acute kidney disease (AKD),” “CF-related kidney disease (CFKD),” and “CFTR.” We included original research articles, reviews, and clinical studies that focused on biomarkers associated with kidney disease in people with cystic fibrosis, as well as in the general population to offer a comprehensive view of the current biomarker landscape. Studies that did not address kidney disease or biomarker relevance were excluded. For emerging biomarkers, we focused on studies published within the past 10 years (2014–2024) to ensure relevance to current research and clinical developments. Additional references were included when necessary to provide background or foundational knowledge.
2. Conventional Laboratory Tests for Kidney Function and the Detection of Kidney Disease
Several laboratory tests performed on blood and urine specimens are typically used in clinical practice to evaluate kidney function. Most tests are not disease-specific and provide only an imperfect estimate of kidney function. Results of several of these tests have to be interpreted together to better understand kidney health and the potential etiology of dysfunction. For this reason, conventional laboratory tests do not meet the criteria of a biomarker, which is defined as a measurable and objectively evaluated characteristic that indicates normal physiological or pathological processes [20].
2.1. Serum Creatinine Concentration and Glomerular Filtration Rate (eGFR)
Serum creatinine concentration and glomerular filtration rate (GFR) are commonly used measurements in clinical practice to estimate kidney function. GFR reflects the kidney’s ability to filter blood, but its direct measurement is often impractical in clinical settings. Serum creatinine concentration is used as a surrogate measure to estimate GFR (eGFR; normal range 90–120 mL/min/1.73 m2). Creatinine, a by-product of muscle metabolism, is filtered by the glomeruli. It is not an ideal substance for measuring GFR because it is also secreted by the proximal tubule, leading to an overestimation of GFR by 10–20% [21]. Creatinine levels are influenced by age, sex, muscle mass, nutritional and hydration status, diet, and the use of medications. Its production decreases during sepsis [22].
Serum creatinine is primarily measured using the Jaffe reaction, enzymatic assays, isotope dilution mass spectrometry (IDMS), or high-performance liquid chromatography (HPLC). The Jaffe reaction is a simple and cost-effective colorimetric assay, but it is also limited by susceptibility to interference [23]. Enzymatic methods utilize specific enzymes, providing greater specificity and less interference than the Jaffe method, although they are more expensive [24]. IDMS is considered the gold standard for creatinine measurement, providing high precision and serving as a reference method for standardization, but its high cost and limited availability restrict its routine clinical use [25]. HPLC separates creatinine from other serum components before detection, minimizing interference. While it is highly specific and accurate, it is time-consuming and requires specialized equipment [26]. GFR estimation methods rely on the assumption of a steady state—a condition in which the rate of creatinine production by the body equals the rate of its clearance by the kidneys. Creatinine reflects eGFR during the steady state, and the utility of serum creatinine declines as kidney disease progresses [27]. In response to injury, the kidney deploys compensatory mechanisms, leading to hyperfiltration through functioning glomeruli that maintain normal or even elevated eGFR, thereby masking the progressive loss of nephrons. Serum creatinine levels only begin to increase after significant kidney damage has occurred, with renal function declining by as much as 50% before noticeable changes in creatinine are detected [28]. Therefore, the correlation between kidney function and eGFR is non-linear; eGFR does not accurately predict early kidney disease or define the mechanisms of injury [29]. An overestimation of eGFR due to creatinine secretion by the kidney proximal tubule further complicates measurements.
2.2. Serum Cystatin C
Cystatin C, a substance released by all nucleated cells, has been used to measure eGFR (recommendations from the National Kidney Foundation & American Society of Nephrology NKF-ASN Task Force) [30]. It is particularly useful when combined with serum creatinine measurements [31]. Various eGFR formulas exist, based on measurements of serum creatinine and/or Cystatin C, age groups, sex, and population characteristics. Some of the most commonly used formulas include those from the Chronic Kidney Disease in Children (CKiD) Study and the CKD-EPI, which are tailored to specific groups for more accurate estimation of kidney function. For example, the CKiD U25 eGFR formula is specifically designed for children and young adults aged 1–25 years. Other formulas are optimized for specific populations, including those with CKD or older adults (Table 1) [32,33,34,35,36]. Two independent studies concluded that cystatin C clearance outperforms eGFR in PwCF [37,38]. However, none of the current formulas for eGFR are validated in PwCF, and thus the correlation with GFR in PwCF is unclear [39].

Table 1.
eGFR formulas based on age groups and sex.
Table 1.
eGFR formulas based on age groups and sex.
| eGFR Formula | Population | Age Group | Characteristics |
|---|---|---|---|
| CKiD U25 eGFR | CKiD | 1–25 years | Age, sex, height, serum creatinine, and serum Cystatin C [32,33] |
| CKD-EPI 2021 (Creatinine) | General adult population | ≥18 years | Age, sex, and serum creatinine [32] |
| MDRD Study | CKD | ≥18 years | Age, sex, race, and serum creatinine [34] |
| Schwartz Formula | Pediatric population (general) | 1–18 years | Height and serum creatinine [35] |
| CKD-EPI 2012 (Creatinine-Cystatin C) | General adult population | ≥18 years | Age, sex, race, serum creatinine, and serum Cystatin C [36] |
CKiD, chronic kidney disease in children; eGFR, estimated glomerular filtration rate; CKD-EPI, chronic kidney disease epidemiology collaboration; MDRD, modification of diet in renal disease; CKD, chronic kidney disease.
2.3. Urine Protein Composition and Concentration
Urine protein composition and concentration are used to assess glomerular and tubular integrity. Urinary albumin excretion is typically classified into three categories: normoalbuminuria (<30 mg/day or UACR < 30 mg/g), microalbuminuria (30–300 mg/day or UACR 30–300 mg/g), and macroalbuminuria (>300 mg/day or UACR > 300 mg/g) [40]. Excessive urinary albumin concentration (macroalbuminuria) indicates glomerular damage. By contrast, tubular damage is suggested by elevated concentrations of urinary low molecular weight (LMW; molecular weight < 40 kDa) proteins, such as beta-2 microglobulin (β2MG) and retinol-binding protein (RBP) in the presence of normoalbuminuria [41]. Urinary LMW proteins are typically measured using various biochemical and electrophoretic techniques, including enzyme-linked immunosorbent assay (ELISA), sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), liquid chromatography-tandem mass spectrometry (LC-MS), and Urine Protein Electrophoresis (UPEP), which is a clinical laboratory test [42,43,44].
Microalbuminuria can be at least partially mitigated by tubular reabsorption of albumin. This process exerts metabolic stress on the renal proximal tubule where the reabsorption occurs, leading to tubular damage, tubulointerstitial fibrosis, and CKD progression over months to years. For all these reasons, worsening proteinuria is a test of established kidney injury and its progression.
According to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, AKI is defined by an increase in serum creatinine (within 2–7 days) and/or decrease in urinary output, while CKD is determined based on decreased eGFR < 60 mL/min/1.73 m2 and albuminuria persistent for more than 3 months [13]. The Fractional Excretion of Sodium (FeNa) and Fractional Excretion of Urea (FeUrea) are key tests for distinguishing prerenal from intrinsic renal AKI [45]. FeNa < 1% suggests prerenal AKI, while FeNa > 2% indicates intrinsic renal AKI. FeUrea < 35% suggests prerenal AKI, and FeUrea > 50% suggests intrinsic AKI, especially in patients on diuretics. Urine specific gravity and osmolality also aid in the diagnosis; high specific gravity (>1.020) and osmolality (>500 mOsm/kg) point to prerenal AKI, while low specific gravity (<1.010) and osmolality (<300 mOsm/kg) suggest intrinsic renal injury, such as acute tubular necrosis (ATN) [46].
In PwCF, data on urinary protein composition and concentration remain limited. However, recent studies have begun to shed light on renal involvement in CF. Notably, Rosner et al. found that total urinary protein, normalized to creatinine, was significantly elevated in PwCF compared to healthy controls [47]. Interestingly, this increase in proteinuria did not correlate with eGFR, and the urine albumin/creatinine ratio was similar between CF and control cohorts. These findings suggest that the elevated urinary protein observed in PwCF is not due to glomerular albumin leakage but may instead reflect subclinical tubular injury, potentially related to CFTR dysfunction in the renal epithelium.
2.4. Other Tests
Several other tests can provide valuable insight into kidney health. Urinalysis is a standard diagnostic tool used to evaluate kidney function and detect abnormalities in urine composition. The presence of red blood cells (RBCs) in urine (hematuria) can indicate glomerular disease or lower urinary tract abnormalities such as nephrolithiasis. Pyuria (the presence of white blood cells, WBCs) suggests urinary tract infection or interstitial nephritis. Cellular casts, including RBC casts, indicate glomerulonephritis, while WBC casts suggest interstitial nephritis or infection. Granular casts are frequently observed in ATN and indicate tubular injury, while hyaline casts, which can be found in concentrated urine or with dehydration, are less specific. Crystals in the urine can indicate metabolic disturbances and nephrolithiasis [48]. Kidney biopsy and histological evaluation of kidney tissue provide the most specific diagnosis and may predict the severity and prognosis of kidney disease. The kidney complications in PwCF have been recently reviewed [6]. Many of the standard clinical tests reviewed above are used for their diagnosis (Table 2) [6,49,50,51,52,53,54,55,56,57,58,59,60,61].

Table 2.
Standard tests used in clinical practice for the diagnosis of common kidney complications in PwCF (modified from Hart et al.) [6].
Table 2.
Standard tests used in clinical practice for the diagnosis of common kidney complications in PwCF (modified from Hart et al.) [6].
| Kidney Complication | Laboratory/Imaging Test for Detection |
|---|---|
| AKI | Serum creatinine, BUN, urinalysis, urine microscopy, FeNa, FeUrea, and urine output monitoring [49] |
| Pseudo-Bartter syndrome | Serum electrolytes, venous blood gas, and urine electrolytes [50] |
| AA amyloidosis | Serum amyloid A, kidney biopsy, and SAP scintigraphy [51,52] |
| IgA nephropathy | Urinalysis, urine microscopy, UPCR, and kidney biopsy [53] |
| Diabetic glomerulopathy | Urinalysis, UACR, eGFR, and kidney biopsy [54] |
| Tubulointerstitial nephritis | Urinalysis, kidney ultrasound, and kidney biopsy [55,56] |
| CKD | eGFR, UACR, UPCR, and urinalysis [57] |
| LMW proteinuria (non-glomerular) | Urine β2MG, and UPEP [58] |
| Hypercalciuria/ nephrolithiasis/ nephrocalcinosis | UCaCR, 24 h urinary analysis calcium excretion, urine microscopy, serum 25-hydroxyvitamin D, and kidney ultrasound [59,60,61] |
AKI, acute kidney injury; BUN, blood urea nitrogen, FeNa, fractional excretion of sodium; FeUrea, fractional excretion of urea, AA amyloidosis, secondary amyloidosis; SAP, serum amyloid P component); IgA, immunoglobulin A; UPCR, urine protein to creatinine ratio; UACR, urine albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; LMW, low molecular weight; β2MG, beta-2 microglobulin; UPEP, urine protein electrophoresis; UCaCR, urine calcium to creatinine ratio.
3. Emerging Biomarkers of Early Kidney Injury in the General Population and PwCF
As described above, conventional tests report already-established kidney damage and are insensitive to subclinical or early injury. In contrast, novel biomarkers in the blood and urine may provide insights into the timing, severity, and site of kidney injury [62]. Molecular alterations revealed in genomic, proteomic, and metabolomic studies may recognize renal damage in the preclinical phase and provide insight into the pathophysiology of early kidney disease. Urinary biomarkers have already been recommended for detecting drug-induced tubular injury in early clinical trials [63].
Kidney injury molecule (KIM)-1 showed a strong negative correlation with eGFR in both AKI and CKD settings [64,65,66]. KIM-1 is a phosphatidylserine receptor expressed on the proximal tubules and targets apoptotic cells to lysosomes. Interleukin-18 (IL-18) and neutrophil gelatinase-associated lipocalin (NGAL) are also considered potential biomarkers for kidney injury [47,67,68]. The cell cycle arrest biomarkers tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7) have emerged as promising indicators for detecting acute kidney injury (AKI), particularly in critically ill patients and those undergoing cardiac surgery. Recognizing its diagnostic potential, the NephroCheck™ test, which quantifies the product of [TIMP-2] × [IGFBP7], was approved by the U.S. Food and Drug Administration (FDA) in 2014 for clinical use in intensive care unit (ICU) settings to assess the risk of developing moderate to severe AKI [69]. Selenium-binding protein 1 (SBP1) plays a multifaceted role in cellular processes, exhibiting predominant expression in proximal tubular cells under normal conditions. Elevated urinary SBP1 levels have been identified as an early and sensitive biomarker of AKI, outperforming traditional markers like NGAL and TIMP-2 [70,71]. Kidney injury and inflammatory markers and tests across CKD stages are summarized in Table 3 [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85].

Table 3.
Kidney injury and inflammatory markers and tests across CKD stages. Levels of early kidney injury markers such as KIM-1, NGAL, and IL-18 increase first, while indicators such as serum creatinine, BUN, and Cystatin C increase in later stages.
Table 3.
Kidney injury and inflammatory markers and tests across CKD stages. Levels of early kidney injury markers such as KIM-1, NGAL, and IL-18 increase first, while indicators such as serum creatinine, BUN, and Cystatin C increase in later stages.
| Biomarker/Test | Type | Early Stage (Subclinical Injury) | Intermediate Stage (Functional Decline) |
Late Stage
(Established CKD) |
|---|---|---|---|---|
| Serum creatinine [70,71] | Functional decline | Normal | Starts increasing | High in late-stage CKD |
| BUN [72,73] | Functional decline | Normal | Increases moderately | High in severe CKD |
| Cystatin C [74,75] | Functional decline | Normal or slightly elevated | Moderately increased | High in severe CKD |
| eGFR [76] | Functional decline | Normal or even high | Progressive decline | Severely reduced in CKD |
| Proteinuria [77] | Glomerular dysfunction | Slight increase | Increases significantly | Severe elevation in CKD |
| KIM-1 [78] | Tubular injury marker | Early rise | Decreases with chronicity | Low but persists in CKD |
| β2MG [79,80] | Glomerular dysfunction | Normal or slightly elevated | Moderately increased | High in severe CKD |
| NGAL [81] | Tubular injury marker | Early and rapid increase | Fluctuates with injury | Persistent in progressive CKD |
| IL-18 [82,83] | Inflammatory marker | Early marker of tubular damage | Moderate increase | Can persist in CKD |
| TNFα [84,85] | Inflammatory marker | Low or normal | Elevated due to chronic inflammation | Persistently high in late CKD |
CKD, chronic kidney disease; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; KIM-1, kidney injury molecule-1; β2MG, beta-2 microglobulin; NGAL, neutrophil gelatinase-associated lipocalin; IL-18, interleukin-18; TNFα, tumor necrosis factor α.
Even in the absence of albuminuria and with normal eGFR, PwCF exhibit evidence of both tubular cell dysfunction and renal injury, including decreased levels of urinary EGF (uEGF), and elevated levels of urinary tubular injury markers such as KIM-1, TFF3, β2MG, Cystatin C, and N-acetyl-β-D-glucosaminidase (NAG) [47]. These findings confirm that renal tubular injury occurs before significant glomerular dysfunction becomes evident, and decreased uEGF may suggest early signs of CKD in some PwCF [47,86,87]. Levels of KIM-1 and β2MG are also increased in PwCF during pulmonary exacerbations and correlate with lung function decline, pointing to the role of lung infection and inflammation in contributing to renal injury [47]. Increased urinary NAG levels suggest subclinical tubular kidney injury in PwCF undergoing aminoglycoside therapy, including nebulized tobramycin in children [88,89]. Elevated urinary soluble Fas (sFas) concentrations during aminoglycoside treatment are associated with the development of CKD in PwCF [90]. While these observations were limited to a few tubular injury markers, which have yet to be systematically studied in PwCF, they support the concept of a novel tool for the rapid and early detection of kidney injury before elevation of serum creatinine or urinary protein. Changes in markers and tests levels across CKD stages are summarized in Figure 1.
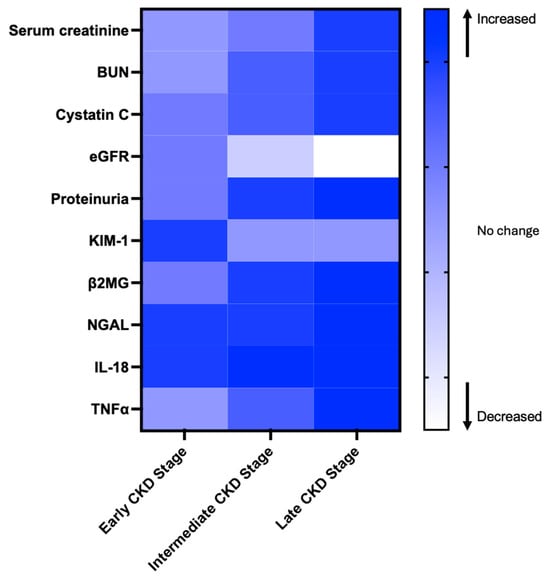
Figure 1.
The heatmap illustrates changes in biomarker levels across different stages of chronic kidney disease (CKD). Color changes represent whether a biomarker is reported to increase or decrease, based on literature evidence. This figure is for visualization purposes only and does not reflect actual biomarker concentrations [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85].
Extracellular vesicles (EVs) are a source of emerging site- and disease-specific kidney injury markers [91]. EVs are membrane-bound nanosized particles released by cells that play a significant role in intercellular communication by transferring signals capable of altering target cell function and influencing the pathophysiology of various diseases, including CF [92]. Urinary extracellular vesicles (uEVs), which are EVs present in urine, have been shown to mediate the crosstalk between different cell populations during nephrogenesis, amplification of kidney injury, inflammation, fibrosis, and regeneration [91]. Intercellular communication in the kidney occurs not only between mesangial, endothelial, and podocyte cells within the glomeruli, but also between glomerular and tubular compartments and among different tubular cell types [93].
Both the number of uEVs released and their contents (proteins, lipids, DNA, and RNA species) change under cellular stress and have been investigated as biomarkers for kidney disease [94]. It has been proposed that the characteristic signatures of the EV cargo may be leveraged as markers for location-specific kidney disease [95,96,97]. Because AKI frequently involves acute tubular damage that can progress to chronic injury characterized by interstitial fibrosis, the crosstalk between the tubular and interstitial compartments has become a key focus for uncovering new mechanisms underlying maladaptive repair processes and the progression from AKI to CKD [93]. A panel of proteins and micro(mi)RNAs have been associated explicitly with AKI versus CKD, glomerular versus tubular renal injury, specific kidney disease processes, and maladaptation after AKI [91]. Thus, elevated levels of specific EV markers may provide mechanistic information, and an early index of AKI may allow for monitoring of CKD progression in PwCF. However, further research is needed to validate these markers in PwCF and to explore their application in early diagnosis and monitoring of kidney disease progression.
Altered levels of specific proteins in uEVs have been linked to kidney injury. NGAL in uEVs, but not in free urine, leads to increases in delayed graft function post-transplant, indicating EVs may protect and enrich certain biomarkers [98]. Panich et al. identified exosomal activating transcription factor-3 (ATF3) as an early marker of sepsis-induced AKI [99]. Increased aquaporin-2 (AQP2) in uEVs signals collecting duct injury after transplantation, while low preoperative podocalyxin levels suggest early podocyte damage and risk of postoperative AKI [100,101]. Decreased CD133 (Prominin-1), a renal progenitor cell marker, in uEVs is seen in both AKI and CKD, reflecting reduced regenerative capacity [102]. MicroRNAs such as miR-125a-5p and miR-10a-5p are also reduced in uEVs of patients who later develop severe AKI, highlighting their potential in risk stratification [101]. Classical exosomal markers CD9 and CD63 are often elevated in uEVs of kidney transplant recipients with AKI, indicating increased vesicular trafficking during injury [100]. Wilms tumor-1 (WT-1), essential for podocyte integrity, is detected in uEVs as a marker of glomerular damage in various kidney diseases [103]. In chronic kidney injury, p16INK4a, a cellular senescence marker, is found in uEVs and linked to hypertensive nephropathy, providing insights into renal aging and fibrosis [104]. Takizawa et al. used a Tim4-based ELISA to measure uEV markers, including distal tubule/collecting duct-specific Mucin 1 (MUC1) and proximal tubule-specific maltase-glucoamylase (MGAM), showing the MGAM/MUC1 ratio rises as kidney function declines in CKD patients [105]. Together, these uEV-associated markers provide valuable insights into molecular events in AKI and CKD and show promise for precision nephrology. However, they have not yet been validated in PwCF.
Clinical Utility of Biomarkers in CFKD
Several biomarkers discussed in this review demonstrate varying clinical applicability in kidney injury. Traditional markers such as serum creatinine, BUN, and eGFR are routinely used in clinical practice to assess kidney function and are applicable to CFKD. However, have limited sensitivity for detecting early kidney injury. Cystatin C, a low molecular weight protein less influenced by muscle mass, has also been increasingly utilized in clinical settings for more accurate eGFR estimation Proteinuria and β2-microglobulin (β2MG) are established but not disease-specific indicators of kidney dysfunction and may aid in detecting renal involvement in CF. Emerging tubular injury markers like KIM-1 and NGAL have demonstrated potential for identifying early subclinical injury and could help monitoring CFKD progression, though they are not yet standard in clinical care. Similarly, inflammatory markers such as IL-18 and TNFα are being studied for their potential to detect ongoing kidney inflammation in CF. Together, these biomarkers provide a foundation for understanding and potentially stratifying CFKD progression. However, broader clinical adoption of newer candidate markers will require further validation in CF-specific patient cohorts.
4. Potential Genetic Modifiers of CFKD in PwCF
CKD and CF share an extensive literature regarding genetic modifiers of disease progression, with potential overlapping mechanisms. Although the genetic modifiers influencing disease severity have been extensively studied and separately validated in CF and CKD, it remains unknown how the modifiers associated with CF lung disease or CF-related diabetes affect the risk of CF-related kidney disease [106,107,108,109]. It is also unclear whether the risks of CKD progression identified in the general population have a similar effect in PwCF. Below, we review several gene polymorphisms associated with a potential for influencing the risk and severity of CKD in PwCF (Table 4).
Transforming growth factor β1 (TGFβ1) is an established genetic modifier in CF. Several TGFβ1 polymorphisms are associated with CF progression and P. aeruginosa infection [110]. Elevated TGFβ1 levels downregulate CFTR mRNA levels through miR-145-5p [111]. In addition to CFTR, TGFβ1 also regulates Calcium-activated Chloride Conductance (CaCC), thereby maintaining normal hydration of epithelial surfaces, including the airways and colon [112]. Aberrant TGFβ1 signaling drives HNF1B-Related Autosomal Dominant Tubulointerstitial Kidney Disease characterized by tubular cysts, renal fibrosis, and progressive decline in kidney function [113]. Genetic variations in TGFβ1 are also associated with susceptibility to IgA nephropathy [114], autosomal dominant polycystic kidney disease (ADPKD) [115], and CKD [116]. Furthermore, IgA nephropathy is the most common glomerular disease as one of the kidney complications in PwCF [6].

Table 4.
Common genetic determinants in CF and CKD.
Table 4.
Common genetic determinants in CF and CKD.
| Modifier | Role in PwCF | Role in CKD |
|---|---|---|
| TGFβ1 | The most established genetic modifier in CF. Several TGFβ1 polymorphisms are associated with CF progression and P. aeruginosa infection [110]. | Drives HNF1β-induced ADTKD [113]. Susceptibility to IgAN [114], polycystic kidney diseases [115], and CKD [116]. |
| ACE | The D/I polymorphism is associated with disease severity [117]. Expression and localization are controlled by CFTR [118,119]. | DD genotype is a risk factor for CKD [120]. ID/DD genotypes are associated with chronic lesions, such as capsular adhesions or glomerulosclerosis and proteinuria in severe IgAN [121]. |
| MBL2 | Decreased survival and increased susceptibility to infections to P. aeruginosa and worse lung functions [122,123]. The pathogenic variants are Gly54Asp (the B allele, rs1800450), Gly57Glu (the C allele, rs1800451), and Arg52Cys (the D allele, rs5030737), which together referred to as 0 allele [124]. | Glomerular deposition of MBL has been consistently observed in kidney biopsy specimens in people with IgAN [125,126]. High serum levels are also associated with the development and progression of diabetic nephropathy [127]. |
| AAT | The most abundant proteinase inhibitor in the lung with anti-inflammatory effects. Genetic modifier protecting against disease progression [128,129,130,131]. M (normal), S (264Glu → Val) and Z (342Glu → Lys) [132]. Contradictory results for the effect of S and Z alleles [130,132,133,134]. | Rapid rise in serum levels predicts AKI in experimental and clinical settings [135,136]. S and Z alleles were associated with high levels of the antigen of ANCA in Granulomatosis with polyangiitis [137]. In CKD, AAT has a protective effect [138]. |
| β2AR | Stimulation results in improved lung function [139]. The Gly16Glu27 genetic variant upregulates CFTR activity [140,141]. | Expressed in proximal tubules, glomeruli, and podocytes [142]. Anti-inflammatory [143]. |
| TNFα | (-308 GA, rs1800629) polymorphism is associated with CF [124]. +691g ins/del polymorphic locus is associated with the severity of lung disease and. aeruginosa infection [144]. TNFα -308GA promoter polymorphism (rs1800629) that were associated with high TNFα transcription, CF and AKI severity [145,146,147]. | High levels disrupt the localization of PC2 to the plasma membrane and primary cilia in ADPKD [148,149]. |
| IL-10 | Anti-inflammatory cytokines present at low levels in PwCF. The haplotype GCC/ACC is significantly associated with P. aeruginosa infection and CF severity [150]. A significant association was found between the −1082GG genotype and colonization with A. fumigatus and allergic bronchopulmonary aspergillosis [151]. | Important role in normal physiology, AKI and CKD progression [152]. Polymorphisms are associated with AKI [147]. L-10 -1082 A/G polymorphism was associated with an increased risk of AKI [153] and primary glomerulonephritis [154]. |
| NOS | Low levels of exhaled NO [155,156]. NOS1 and NOS2 polymorphisms are associated with disease severity and inflammation [157,158]. G847T polymorphism in the NOS3 gene, is associated with high NO production had a slower decline in lung function [159,160]. | Levels are reduced in CKD. NOS inhibition causes systemic and glomerular hypertension, glomerular ischemia, glomerulosclerosis, tubulointerstitial injury, and proteinuria [161]. Presence of the two NOS3 gene polymorphisms, Glu298Asp polymorphisms 4 b/a and -786T > C is a risk of ESKD in patients with CKD and ADPKD [162,163]. |
| GST | M1 (GSTM1) allele associated with worse lung disease [164]. GSTM3*B allele contributes to clinical severity in CF [165]. | GSTM1, GSTT1, and GSTP1 polymorphisms are risk of ESKD [166]. GSTM1 deletion is associated with more rapid progression of pediatric CKD [167]. |
TGFβ1, transforming growth factor β1; CF, cystic fibrosis; HNF1β, hepatocyte nuclear factor 1-beta; ADTKD, autosomal dominant tubulointerstitial kidney disease; IgAN, immunoglobulin A nephropathy; ACE, angiotensin-converting enzyme; CFTR, CF transmembrane conductance regulator; MBL2, mannose-binding lectin; AAT, α1-antitrypsin; AKI, acute kidney injury; ANCA, antineutrophil cytoplasm antibodies; CKD, chronic kidney disease; β2AR, beta-2-adrenergic receptor; TNFα, tumor necrosis factor α; PC2, polycystin 2; ADPKD, autosomal dominant polycystic kidney disease; IL-10, interleukin-10; PwCF, people with cystic fibrosis; NOS, nitric oxide synthase; NO, nitric oxide; ESKD, end-stage kidney disease; GST, glutathione S-transferase.
TNFα-308 GA polymorphism is associated with CF in diverse populations [124]. The TNFα +691g ins/del polymorphic locus is associated with the severity of CF lung disease and the age of onset of P. aeruginosa infection [144]. Different studies have found TNFα-308 GA promoter polymorphism (rs1800629) that was associated with high TNFα transcription, CF, and AKI severity [145,146,147]. High levels of TNFα induce scaffold protein FIP2, which disrupts the localization of polycystin 2 (PC2) to the plasma membrane and primary cilia in ADPKD [148,149].
Angiotensin-converting enzyme (ACE) is an enzyme in the renin–angiotensin–aldosterone system that is essential for regulating blood pressure and fluid balance. ACE gene D/I polymorphism is a modulator of the severity of CF [117]. ACE2 expression and localization are regulated by the CFTR gene, suggesting a possible role in the progression of CF disease [118,119]. ACE also regulates kidney function, and ACE inhibitors are commonly used to treat kidney disease [168]. Among hypertensive patients, the ACE-DD genotype has been shown to be a risk factor for the causation and development of chronic kidney failure [120]. In severe forms of IgA nephropathy (IgAN), ID/DD genotypes are associated with chronic lesions, such as capsular adhesions or glomerulosclerosis and proteinuria [121].
Mannose-binding lectin (MBL2), a member of C-type lectin family activates the complement system during inflammation and can cause both pathogen clearance and tissue injury [166]. In CF, low MBL2 levels are associated with decreased survival and increased susceptibility to P. aeruginosa infection and worse lung function [122,123]. A non-linear association between MBL levels and renal outcome has been found in IgAN, with both MBL deficiency and excess independently linked to poor renal outcomes, suggesting that MBL contributes to IgAN progression through multiple mechanisms [125,126]. High serum MBL levels are also associated with the development and progression of diabetic nephropathy [127].
α1-antitrypsin (AAT) is the most abundant proteinase inhibitor within the lung and prevents tissue damage caused by inflammation. AAT is considered a genetic modifier in CF, and exogenous AAT has been proposed as a potential therapy for CF [128,129]. However, two separate clinical studies suggest a protective effect of low to moderate levels of AAT on the progression of CF [130,131]. There are three alleles for AAT: M (normal), S (264Glu → Val), and Z (342Glu → Lys). The prevalence of S and Z alleles is approximately 12% in the CF population. Homozygous S and Z alleles result in 60% and 10% of plasma AAT levels in the homozygous state when compared with the homozygous M allele [132]. In CKD, AAT has a protective effect [138]. Two studies showed contradictory results regarding the effect of S and Z alleles on the age of onset of chronic P. aeruginosa acquisition in patients with CF [133,134]. Another polymorphism in the 3′UTR of AAT (G1237A) is associated with a small rise in AAT levels during the acute inflammatory conditions in CF [132], although a later study with a large sample size found no association between G1237A and lung functions in CF patients [130]. A rapid rise in AAT levels can predict AKI in experimental and clinical settings [135,136]. S and Z alleles have been associated with high levels of the antigen of antineutrophil cytoplasm antibodies (ANCA) in Granulomatosis with polyangiitis [137]. In conclusion, despite the considerable amount of data, the impact of AAT on CF phenotype is still unclear, and its role in CF and CKD warrants further investigations.
It has been shown that stimulation of β-adrenoceptor (β-AR) results in increased alveolar fluid clearance and ciliary beat frequency in PwCF [139]. The Gly16Glu27 β2AR genetic variant upregulates CFTR activity in adult CF patients [140]. A similar effect is observed with β2AR agonist salbutamol and ritodrine, suggesting that the effect increases β2AR activity [141]. In the kidney, β2AR is expressed in proximal tubules, glomeruli, and podocytes [142]. Similarly to its role in CF, β2AR agonist reduces the proinflammatory response to renal inflammation [143].
Interleukin-10 (IL-10) is an anti-inflammatory cytokine present in low levels in CF patients. In a study of 220 CF patients, IL-10 haplotype GCC/ACC was significantly associated with P. aeruginosa infection and CF severity [150]. In another study of 378 patients with CF, a significant association was found between the −1082GG genotype and colonization with A. fumigatus and allergic bronchopulmonary aspergillosis [151]. IL-10 plays an important role in renal physiology, AKI, and progression of chronic renal failure [152]. Polymorphism in IL-10 is associated with AKI (AKI) [147]. IL-10-1082 A/G polymorphism was associated with increased risks of AKI [153] and primary glomerulonephritis [154].
Nitric oxide synthase (NOS) is an immune modulator and vasodilator. PwCF typically show below normal levels of exhaled NO [155,156]. NOS1 and NOS2 polymorphisms are associated with disease severity and inflammation. More specifically, the number of AAT repeats in the NOS1 gene is negatively correlated with nasal and expired NO in PwCF and positively correlated with P. aeruginosa and A. fumigatus infection [157,158]. Separate studies have found that the number of GT repeats in NOS1 promoter and G847T polymorphism in the NOS3 gene are positively associated with increased NO production and slower decline in lung function [159,160]. NO levels are reduced in CKD [169]. Experimental NOS inhibition is associated with systemic and glomerular hypertension, glomerular ischemia, glomerulosclerosis, tubulointerstitial injury, and proteinuria [161]. Meta-analysis of 13 studies suggests the presence of the two NOS3 gene polymorphisms, Glu298Asp polymorphisms 4 b/a and -786T > C that have been associated with an increased risk of ESKD in patients with CKD and ADPKD [162,163].
Glutathione S-transferase (GST) is an antioxidant enzyme that conjugates hydroperoxides with glutathione, thereby mitigating tissue damage. CF patients homozygous for glutathione S-transferase M1 (GSTM1) allele have worse chest radiographic scores and worse Shwachman–Kulczycki (SK) scores of CF disease severity [164]. Another polymorphism of the GSTM3*B allele contributes to clinical severity in CF [165]. A meta-analysis found an association between GSTM1, GSTT1, and GSTP1 genetic polymorphisms and the risk of ESKD [166]. Another study of 674 children identified the association of GSTM1 deletion with more rapid progression of pediatric CKD [167]. In conclusion, GWAS studies are needed to examine the role of specific CF genetic modifiers in CFKD risk.
ß-catenin has emerged as a key contributor to kidney fibrosis in the context of CFTR dysfunction. Zhang et al. showed a clear upregulation of the ß-catenin pathway in renal epithelial cells with CFTR knockdown and in the kidneys of F508del mice subjected to unilateral ureteral obstruction (UUO), a well-established model of kidney fibrosis [17]. Furthermore, knockdown of CFTR reduced the expression of tight junction proteins Occludin and ZO-1 in kidney tubular cells, reasoning that it could be a cause of increased leakage of low molecular weight proteins as seen in the urine of PwCF. This, along with enhanced epithelial-to-mesenchymal and fibrosis seen in CFTR mutant mice, may underlie the frequent kidney disease observed in CF.
5. Future Directions of Research on Kidney Function in PwCF
Elucidating the mechanisms of kidney injury is essential for its early detection and prevention. A critical question in CFKD is to what extent the increased prevalence of AKI and CKD results from extrarenal causes, including CF lung disease and nephrotoxic drug exposure, as opposed to intrinsic susceptibility to tubulointerstitial or glomerular injury resulting from renal CFTR dysfunction, decreased nephron endowment [170], pH sensitivity, or pro-fibrotic signaling. Although many of these pathways have been observed in human biospecimens, separating a primary defect from the response to injury is difficult. The recent development of excellent CF animal models [171,172,173] may allow for in vivo distinction of the effect of CFTR dysfunction on kidney health, as well as on the impact of CFKD on the aging population of PwCF in the era of HEMT. These models are increasingly responsive to clinically relevant CFTR modulators [174,175], helping to distinguish the response to nephrotoxic injury on or off therapy. For example, the humanized G551D CF rat model may prove a valuable tool for studying CF kidney disease, as it spontaneously develops lung disease [176], responds to the CFTR modulator Ivacaftor [174], and offers an appealing husbandry profile for reproduction and cost. Similarly, CFTR-knockout ferrets, which mimic human lung and pancreatic disease, offer a promising model to study CFTR-related kidney damage [177]. Additionally, machine learning presents a promising approach with the potential to identify PwCF at risk for kidney disease. Machine learning algorithms are capable of predicting the onset of CKD in asymptomatic individuals, improving risk stratification for progression or complications, and identifying distinct kidney phenotypes or subtypes by linking clinical features to underlying pathological mechanisms [178]. In the context of CF-related kidney disease, machine learning could be applied to integrate multi-dimensional datasets—including urinary and blood biomarkers, genotype (e.g., CFTR variants), comorbidities (such as CFRD), and medication exposure—to identify early signs of renal involvement and personalize treatment strategies. These tools could also help prioritize candidate biomarkers for clinical use and uncover patterns not readily apparent through conventional analysis. Incorporating machine learning into future CFKD studies may enhance our ability to deliver precision nephrology in this vulnerable population.
Author Contributions
Conceptualization, H.Y. and A.S.-U.; methodology, H.Y., A.S.-U., S.S.-G. and W.T.H.; investigation, H.Y. and A.S.-U.; writing—original draft preparation, H.Y. and A.S.-U.; writing—review and editing, H.Y., M.K., H.B.G., U.E., W.T.H., S.S.-G., M.G. and A.S.-U.; supervision, A.S.-U. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript was funded by the Cystic Fibrosis Foundation grant, CFF SWIATE24A0-MPI (to A.S.-U), SKOPEL24A0-MPI (to S.S.-G), and HARRIS24A0-MPI (to W.T.H.); National Institutes of Health grants R01HL144539, and P50DK096373-11 (to A.S.-U.); the American Diabetes Association grant #7-22-ICTSPM-19/GR100086 (to U.E.).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CF | Cystic Fibrosis |
| CFTR | CF Transmembrane Conductance Regulator |
| HEMT | Highly effective modulator therapy |
| PwCF | People with CF |
| CKD | Chronic kidney disease |
| CFKD | CF-related kidney disease |
| ESKD | End-stage kidney disease |
| AKI | Acute kidney injury |
| GFR | Acute kidney injury |
| eGFR | Estimate GFR |
| IDMS | Isotope dilution mass spectrometry |
| HPLC | High-performance liquid chromatography |
| CKiD | Chronic Kidney Disease in Children |
| CKD-EPI | Chronic kidney disease epidemiology collaboration |
| MDRD | Modification of diet in renal disease |
| UACR | Urine albumin to creatinine ratio |
| LMW | Low molecular weight |
| β2MG | Beta-2 microglobulin |
| RBP | Retinol-binding protein |
| ELISA | Enzyme-linked immunosorbent assay |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| LC-MS | Liquid chromatography-tandem mass spectrometry |
| UPEP | Urine protein electrophoresis |
| KDIGO | Kidney Disease Improving Global Outcomes |
| FeNa | Fractional excretion of sodium |
| FeUrea | Fractional excretion of urea |
| ATN | Acute tubular necrosis |
| RBC | Red blood cell |
| WBC | White blood cell |
| BUN | Blood urea nitrogen |
| SAP | Serum amyloid P component |
| IgA | Immunoglobulin A |
| UPCR | Urine protein to creatinine ratio |
| UCaCR | Urine calcium to creatinine ratio |
| KIM-1 | Kidney injury molecule-1 |
| IL-18 | Interleukin-18 |
| TIMP-2 | Tissue inhibitor of metalloproteinases-2 |
| IGFBP7 | Insulin-like growth factor-binding protein 7 |
| FDA | Food and Drug Administration |
| SBP1 | Selenium-binding protein 1 |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| TNFα | Tumor necrosis factor α |
| NAG | N-acetyl-β-D-glucosaminidase |
| sFas | Soluble Fas |
| EVs | Extracellular vesicles |
| uEVs | Urinary extracellular vesicles |
| ATF3 | Activating transcription factor-3 |
| AQP2 | Aquaporin-2 |
| CD133 | Prominin-1 |
| WT-1 | Wilms tumor-1 |
| MUC1 | Mucin 1 |
| MGAM | Maltase-glucoamylase |
| TGFβ1 | Transforming growth factor β1 |
| HNF1β | Hepatocyte nuclear factor 1-beta |
| ADTKD | Autosomal dominant tubulointerstitial kidney disease |
| IgAN | Immunoglobulin A nephropathy |
| ACE | Angiotensin-converting enzyme |
| MBL2 | Mannose-binding lectin |
| AAT | α1-antitrypsin |
| ANCA | Antineutrophil cytoplasm antibodies |
| β2AR | Beta-2-adrenergic receptor |
| PC2 | Polycystin 2 |
| ADPKD | Autosomal dominant polycystic kidney disease |
| IL-10 | Interleukin-1 |
| NOS | Nitric oxide synthase |
| NO | Nitric oxide |
| GST | Glutathione S-transferase |
| CaCC | Calcium-activated chloride conductance |
| GSTM1 | Glutathione S-transferase M1 |
| SK | Shwachman–Kulczycki |
References
- Chen, Q.; Shen, Y.; Zheng, J. A review of cystic fibrosis: Basic and clinical aspects. Animal Model. Exp. Med. 2021, 4, 220–232. [Google Scholar] [CrossRef]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef]
- Martin, C.; Burnet, E.; Ronayette-Preira, A.; de Carli, P.; Martin, J.; Delmas, L.; Prieur, B.; Burgel, P.R. Patient perspectives following initiation of elexacaftor-tezacaftor-ivacaftor in people with cystic fibrosis and advanced lung disease. Respir. Med. Res. 2021, 80, 100829. [Google Scholar] [CrossRef] [PubMed]
- Schechter, M.S.; Stecenko, A.A. Chronic kidney disease: A new morbidity of cystic fibrosis or an old morbidity of diabetes mellitus? Am. J. Respir. Crit. Care Med. 2011, 184, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, M.; Graber, M.L. Characteristics of US Individuals With Cystic Fibrosis and ESRD: SA-PO324. J. Am. Soc. Nephrol. 2022, 33, 694. [Google Scholar] [CrossRef]
- Hart, M.; Kumar, M.; Goswami, H.B.; Harris, W.T.; Skopelja-Gardner, S.; Swiatecka-Urban, A. Cystic fibrosis-related kidney disease-emerging morbidity and disease modifier. Pediatr. Nephrol. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.C.H. Cystic Fibrosis Kidney Disease: 10 Tips for Clinicians. Front. Med. 2018, 5, 242. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Muirhead, C.; Lim, J.Y.; Lapidus, J.; MacDonald, K. Evaluation of the Risk for Acute Kidney Injury in Adult Cystic Fibrosis Patients Receiving Concomitant Vancomycin and Tobramycin. Cureus 2017, 9, e1912. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.S.; Silva, P.L.; Robba, C.; Battaglini, D.; Lopes-Pacheco, M.; Caruso-Neves, C.; Rocco, P.R.M. Advancements in understanding the mechanisms of lung-kidney crosstalk. Intensive Care Med. Exp. 2024, 12, 81. [Google Scholar] [CrossRef]
- Fu, Q.; Colgan, S.P.; Shelley, C.S. Hypoxia: The Force that Drives Chronic Kidney Disease. Clin. Med. Res. 2016, 14, 15–39. [Google Scholar] [CrossRef]
- Leung, K.C.; Tonelli, M.; James, M.T. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat. Rev. Nephrol. 2013, 9, 77–85. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Cheung, A.K.; Chang, T.I.; Cushman, W.C.; Furth, S.L.; Hou, F.F.; Ix, J.H.; Knoll, G.A.; Muntner, P.; Pecoits-Filho, R.; Sarnak, M.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, S1–S87. [Google Scholar] [CrossRef]
- Crawford, I.; Maloney, P.C.; Zeitlin, P.L.; Guggino, W.B.; Hyde, S.C.; Turley, H.; Gatter, K.C.; Harris, A.; Higgins, C.F. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc. Natl. Acad. Sci. USA 1991, 88, 9262–9266. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.M.; Carroll, T.P.; Morita, T.; Schwiebert, E.M.; Devuyst, O.; Wilson, P.D.; Lopes, A.G.; Stanton, B.A.; Dietz, H.C.; Cutting, G.R.; et al. Both the wild type and a functional isoform of CFTR are expressed in kidney. Am. J. Physiol. 1996, 270, F1038–F1048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Wang, Y.; Chen, J.J.; Zhang, X.H.; Dong, J.D.; Tsang, L.L.; Huang, X.R.; Cai, Z.; Lan, H.Y.; Jiang, X.H.; et al. Defective CFTR leads to aberrant β-catenin activation and kidney fibrosis. Sci. Rep. 2017, 7, 5233. [Google Scholar] [CrossRef] [PubMed]
- Quon, B.S.; Mayer-Hamblett, N.; Aitken, M.L.; Smyth, A.R.; Goss, C.H. Risk factors for chronic kidney disease in adults with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2011, 184, 1147–1152. [Google Scholar] [CrossRef]
- Berg, K.H.; Ryom, L.; Faurholt-Jepsen, D.; Pressler, T.; Katzenstein, T.L. Prevalence and characteristics of chronic kidney disease among Danish adults with cystic fibrosis. J. Cyst. Fibros. 2018, 17, 478–483. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working, G. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Matthew, D.B.; Qi, Z. Better nephrology for mice—And man. Kidney Int. 2010, 77, 487–489. [Google Scholar] [CrossRef]
- Doi, K.; Yuen, P.S.T.; Eisner, C.; Hu, X.; Leelahavanichkul, A.; Schnermann, J.; Star, R.A. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J. Am. Soc. Nephrol. 2009, 20, 1217–1221. [Google Scholar] [CrossRef]
- Toora, B.D.; Rajagopal, G. Measurement of creatinine by Jaffe’s reaction--determination of concentration of sodium hydroxide required for maximum color development in standard, urine and protein free filtrate of serum. Indian. J. Exp. Biol. 2002, 40, 352–354. [Google Scholar] [PubMed]
- Moss, G.A.; Bondar, R.J.; Buzzelli, D.M. Kinetic enzymatic method for determining serum creatinine. Clin. Chem. 1975, 21, 1422–1426. [Google Scholar] [CrossRef]
- Miller, W.G.; Myers, G.L.; Ashwood, E.R.; Killeen, A.A.; Wang, E.; Thienpont, L.M.; Siekmann, L. Creatinine measurement: State of the art in accuracy and interlaboratory harmonization. Arch. Pathol. Lab. Med. 2005, 129, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Yuen, P.S.T.; Dunn, S.R.; Miyaji, T.; Yasuda, H.; Sharma, K.; Star, R.A. A simplified method for HPLC determination of creatinine in mouse serum. Am. J. Physiol. Renal Physiol. 2004, 286, F1116–F1119. [Google Scholar] [CrossRef]
- Tian, Q.; Wu, W.; Liu, J.; Wu, Z.; Yao, W.; Ding, J.; Jiang, C. Dimensional heterostructures of 1D CdS/2D ZnIn(2)S(4) composited with 2D graphene: Designed synthesis and superior photocatalytic performance. Dalton Trans. 2017, 46, 2770–2777. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507821/ (accessed on 2 June 2025).
- Mizdrak, M.; Kumrić, M.; Kurir, T.T.; Božić, J. Emerging Biomarkers for Early Detection of Chronic Kidney Disease. J. Pers. Med. 2022, 12, 548. [Google Scholar] [CrossRef]
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am. J. Kidney Dis. 2022, 79, 268–288.E1. [Google Scholar] [CrossRef]
- Gottlieb, E.R.; Estiverne, C.; Tolan, N.V.; Melanson, S.E.F.; Mendu, M.L. Estimated GFR With Cystatin C and Creatinine in Clinical Practice: A Retrospective Cohort Study. Kidney Med. 2023, 5, 100600. [Google Scholar] [CrossRef]
- Inker, L.A.; Tighiouart, H.; Adingwupu, O.M.; Ng, D.K.; Estrella, M.M.; Maahs, D.; Yang, W.; Froissart, M.; Mauer, M.; Kalil, R.; et al. Performance of GFR Estimating Equations in Young Adults. Am. J. Kidney Dis. 2024, 83, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.B.; Muñoz, A.; Ng, D.K.; Warady, B.A.; Furth, S.L.; Schwartz, G.J. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021, 99, 948–956. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Lente, F.L. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Munoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Lente, F.V.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Beringer, P.M.; Hidayat, L.; Heed, A.; Zheng, L.; Owens, H.; Benitez, D.; Rao, A.P. GFR estimates using cystatin C are superior to serum creatinine in adult patients with cystic fibrosis. J. Cyst. Fibros. 2009, 8, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Halacova, M.; Kotaska, K.; Kukacka, J.; Vavrova, V.; Kuzelova, M.; Ticha, J.; Prusa, R. Serum cystatin C level for better assessment of glomerular filtration rate in cystic fibrosis patients treated by amikacin. J. Clin. Pharm. Ther. 2008, 33, 409–417. [Google Scholar] [CrossRef]
- Wallace, A.; Price, A.; Fleischer, E.; Khoury, M.; Filler, G. Estimation of GFR in Patients With Cystic Fibrosis: A Cross-Sectional Study. Can. J. Kidney Health Dis. 2020, 7, 2054358119899312. [Google Scholar] [CrossRef]
- Sung, K.C.; Ryu, S.; Lee, J.Y.; Lee, S.H.; Cheong, E.; Hyun, Y.Y.; Lee, K.B.; Kim, H.; Byrne, C.D. Urine Albumin/Creatinine Ratio Below 30 mg/g is a Predictor of Incident Hypertension and Cardiovascular Mortality. J. Am. Heart Assoc. 2016, 5, e003245. [Google Scholar] [CrossRef]
- Chaumont, A.; De Winter, F.; Dumont, X.; Haufroid, V.; Bernard, A. The threshold level of urinary cadmium associated with increased urinary excretion of retinol-binding protein and beta 2-microglobulin: A re-assessment in a large cohort of nickel-cadmium battery workers. Occup. Environ. Med. 2011, 68, 257–264. [Google Scholar] [CrossRef]
- Tate, J.; Caldwell, G.; Daly, J.; Gillis, D.; Jenkins, M.; Jovanovich, S.; Martin, H.; Steele, R.; Wienholt, L.; Mollee, P.; et al. Recommendations for standardized reporting of protein electrophoresis in Australia and New Zealand. Ann. Clin. Biochem. 2012, 49, 242–256. [Google Scholar] [CrossRef]
- Telser, A.; Farbman, A.I.; Chacko, C. A low-molecular-weight soluble protein from bovine lingual epithelium. II. Purification and characterization. J. Investig. Dermatol. 1982, 79, 286–292. [Google Scholar] [CrossRef]
- Zhang, J.; Lou, X.; Shen, H.; Zellmer, L.; Sun, Y.; Liu, S.; Xu, N.; Liao, D.J. Isoforms of wild type proteins often appear as low molecular weight bands on SDS-PAGE. Biotechnol. J. 2014, 9, 1044–1054. [Google Scholar] [CrossRef]
- Hamadah, A.; Gharaibeh, K. Fractional Excretion of Sodium and Urea are Useful Tools in the Evaluation of AKI: PRO. Kidney360 2023, 4, e725–e727. [Google Scholar] [CrossRef] [PubMed]
- Wołyniec, W.; Kasprowicz, K.; Rita-Tkachenko, P.; Renke, M.; Ratkowski, W. Biochemical Markers of Renal Hypoperfusion, Hemoconcentration, and Proteinuria after Extreme Physical Exercise. Medicina 2019, 55, 154. [Google Scholar] [CrossRef] [PubMed]
- Rosner, G.M.; Goswami, H.B.; Sessions, K.; Mendyka, L.K.; Kerin, B.; Vlasac, I.; Mellinger, D.; Gwilt, L.; Hampton, T.H.; Graber, M.; et al. Lung-kidney axis in cystic fibrosis: Early urinary markers of kidney injury correlate with neutrophil activation and worse lung function. J. Cyst. Fibros. 2025, 24, 613–620. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Downes, K.J.; Goldstein, S.L. Biomarkers in Kidney Disease; Springer: Dordrecht, The Netherlands, 2016; pp. 689–718. [Google Scholar]
- Scurati-Manzoni, E.; Fossali, E.F.; Agostoni, C.; Riva, E.; Simonetti, G.D.; Zanolari-Calderari, M.; Bianchetti, M.G.; Lava, S.A.G. Electrolyte abnormalities in cystic fibrosis: Systematic review of the literature. Pediatr. Nephrol. 2014, 29, 1015–1023. [Google Scholar] [CrossRef]
- Real de Asúa, D.; Costa, R.; Galván, J.M.; Filigheddu, M.T.; Trujillo, D.; Cadiñanos, J. Systemic AA amyloidosis: Epidemiology, diagnosis, and management. Clin. Epidemiol. 2014, 6, 369–377. [Google Scholar] [CrossRef]
- Allinovi, M.; Trivioli, G.; Gaudio, C.; L’Imperio, V.; Rauf, M.U.; Gillmore, J.D. The evolving spectrum of kidney amyloidosis: Advances in diagnosis, typing and treatment. Nephrol. Dial. Transplant. 2025, gfaf042. [Google Scholar] [CrossRef]
- Moresco, R.N.; Speeckaert, M.M.; Delanghe, J.R. Diagnosis and monitoring of IgA nephropathy: The role of biomarkers as an alternative to renal biopsy. Autoimmun. Rev. 2015, 14, 847–853. [Google Scholar] [CrossRef]
- Dabla, P.K. Renal function in diabetic nephropathy. World J. Diabetes 2010, 1, 48–56. [Google Scholar] [CrossRef]
- Perazella, M.A.; Markowitz, G.S. Drug-induced acute interstitial nephritis. Nat. Rev. Nephrol. 2010, 6, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Joyce, E.; Glasner, P.; Ranganathan, S.; Swiatecka-Urban, A. Tubulointerstitial nephritis: Diagnosis, treatment, and monitoring. Pediatr. Nephrol. 2017, 32, 577–587. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.C.; Dietzen, D.J.; Bennett, M.J.; Haymond, S. (Eds.) Biochemical and Molecular Basis of Pediatric Disease, 5th ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 167–228. [Google Scholar]
- Fan, H.; Hui, L.; Yan, X.; Hou, W.; Bai, E.; Wang, L.; Yu, X. Serum 25 hydroxyvitamin D levels and affecting factors among preconception fertile women. BMC Women’s Health 2020, 20, 146. [Google Scholar] [CrossRef]
- Meher, D.; Agarwal, V.; Das, S.; Choudhury, A.; Sahoo, D.; Sahu, S.K.; Prusty, B.; Das, B. Idiopathic Hypercalciuria: A Comprehensive Review of Clinical Insights and Management Strategies. Cureus 2025, 17, e81778. [Google Scholar] [CrossRef]
- Kronenberg, H.M.; Polonsky, K.S.; Larsen, P.R.; Melmed, S. (Eds.) Williams Textbook of Endocrinology, 13th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1365–1384. [Google Scholar]
- Alge, J.L.; Arthur, J.M. Biomarkers of AKI: A review of mechanistic relevance and potential therapeutic implications. Clin. J. Am. Soc. Nephrol. 2015, 10, 147–155. [Google Scholar] [CrossRef]
- van Meer, L.; Moerland, M.; Cohen, A.F.; Burggraaf, J. Urinary kidney biomarkers for early detection of nephrotoxicity in clinical drug development. Br. J. Clin. Pharmacol. 2014, 77, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A specific and sensitive biomarker of kidney injury. Scand. J. Clin. Lab. Investig. Suppl. 2008, 241, 78–83. [Google Scholar] [CrossRef]
- Bonventre, J.V. Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol. Dial. Transplant. 2009, 24, 3265–3268. [Google Scholar] [CrossRef]
- Lin, X.; Yuan, J.; Zhao, Y.; Zha, Y. Urine interleukin-18 in prediction of acute kidney injury: A systemic review and meta-analysis. J. Nephrol. 2015, 28, 7–16. [Google Scholar] [CrossRef]
- Shang, W.; Wang, Z. The Update of NGAL in Acute Kidney Injury. Curr. Protein Pept. Sci. 2017, 18, 1211–1217. [Google Scholar] [CrossRef]
- Huang, F.; Zeng, Y.; Lv, L.; Chen, Y.; Yan, Y.; Luo, L.; Pan, R.; Jiang, J.; Wei, X. Predictive value of urinary cell cycle arrest biomarkers for all cause-acute kidney injury: A meta-analysis. Sci. Rep. 2023, 13, 6037. [Google Scholar] [CrossRef] [PubMed]
- Duru, O.K.; Vargas, R.B.; Kermah, D.; Nissenson, A.R.; Norris, K.C. High prevalence of stage 3 chronic kidney disease in older adults despite normal serum creatinine. J. Gen. Intern. Med. 2009, 24, 86–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Waikar, S.S.; Bonventre, J.V. Creatinine kinetics and the definition of acute kidney injury. J. Am. Soc. Nephrol. 2009, 20, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Nakayama, M.; Sakoh, T.; Yoshitomi, R.; Fukui, A.; Katafuchi, E.; Tsuda, S.; Nakano, T.; Tsuruya, K.; Kitazono, T. Blood urea nitrogen is independently associated with renal outcomes in Japanese patients with stage 3-5 chronic kidney disease: A prospective observational study. BMC Nephrol. 2019, 20, 115. [Google Scholar] [CrossRef]
- Laville, S.M.; Couturier, A.; Lambert, O.; Metzger, M.; Mansencal, N.; Jacquelinet, C.; Laville, M.; Frimat, L.; Fouque, D.; Combe, C.; et al. Urea levels and cardiovascular disease in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2022, 38, 184–192. [Google Scholar] [CrossRef]
- Benoit, S.W.; Ciccia, E.A.; Devarajan, P. Cystatin C as a biomarker of chronic kidney disease: Latest developments. Expert. Rev. Mol. Diagn. 2020, 20, 1019–1026. [Google Scholar] [CrossRef]
- Menon, V.; Shlipak, M.G.; Wang, X.; Coresh, J.; Greene, T.; Stevens, L.; Kusek, J.W.; Beck, G.J.; Collins, A.J.; Levey, A.S.; et al. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann. Intern. Med. 2007, 147, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mula-Abed, W.A.; Al Rasadi, K.; Al-Riyami, D. Estimated Glomerular Filtration Rate (eGFR): A Serum Creatinine-Based Test for the Detection of Chronic Kidney Disease and its Impact on Clinical Practice. Oman Med. J. 2012, 27, 108–113. [Google Scholar] [CrossRef]
- Gorriz, J.L.; Alberto, M.-C. Proteinuria: Detection and role in native renal disease progression. Transplant. Rev. 2012, 26, 3–13. [Google Scholar] [CrossRef]
- Song, J.; Yu, J.; Prayogo, G.W.; Cao, W.; Wu, Y.; Jia, Z.; Zhang, A. Understanding kidney injury molecule 1: A novel immune factor in kidney pathophysiology. Am. J. Transl. Res. 2019, 11, 1219–1229. [Google Scholar] [PubMed] [PubMed Central]
- Sedighi, O.; Abediankenari, S.; Omranifar, B. Association between plasma Beta-2 microglobulin level and cardiac performance in patients with chronic kidney disease. Nephrourol. Mon. 2015, 7, e23563. [Google Scholar] [CrossRef]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.-H.; Roumelioti, M.-E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering Beta-2 Microglobulin As a Biomarker across the Spectrum of Kidney Diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef]
- Bolignano, D.; Lacquaniti, A.; Coppolino, G.; Donato, V.; Campo, S.; Fazio, M.R.; Nicocia, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 337–344. [Google Scholar] [CrossRef]
- Porazko, T.; Kuźniar, J.; Kusztal, M.; Kuźniar, T.J.; Weyde, W.; Kuriata-Kordek, M.; Klinger, M. IL-18 is involved in vascular injury in end-stage renal disease patients. Nephrol. Dial. Transplant. 2009, 24, 589–596. [Google Scholar] [CrossRef]
- Yong, K.; Ooi, E.M.; Dogra, G.; Mannion, M.; Boudville, N.; Chan, D.; Lim, E.M.; Lim, W.H. Elevated interleukin-12 and interleukin-18 in chronic kidney disease are not associated with arterial stiffness. Cytokine 2013, 64, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.T.; Ahmed, F.A.; Hamm, L.L.; Teran, F.J.; Chen, C.-S.; Liu, Y.; Shah, K.; Rifai, N.; Batuman, V.; Simon, E.E.; et al. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol. 2015, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Ramseyer, V.D.; Garvin, J.L. Tumor necrosis factor-α: Regulation of renal function and blood pressure. Am. J. Physiol. Renal Physiol. 2013, 304, F1231–F1242. [Google Scholar] [CrossRef]
- Azukaitis, K.; Ju, W.; Kirchner, M.; Nair, V.; Smith, M.; Fang, Z.; Thurn-Valsassina, D.; Bayazit, A.; Niemirska, A.; Canpolat, N.; et al. Low levels of urinary epidermal growth factor predict chronic kidney disease progression in children. Kidney Int. 2019, 96, 214–221. [Google Scholar] [CrossRef]
- Menez, S.; Wen, Y.; Xu, L.; Moledina, D.G.; Thiessen-Philbrook, H.; Hu, D.; Obeid, W.; Bhatraju, P.K.; Ikizler, T.A.; Siew, E.D.; et al. The ASSESS-AKI Study found urinary epidermal growth factor is associated with reduced risk of major adverse kidney events. Kidney Int. 2023, 104, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, A.J.; Uges, J.W.F.; Heijerman, H.G.M.; Arets, B.G.M.; Nuijsink, M.; van der Wiel-Kooij, E.C.; van Maarseveen, E.M.; van Zanten, G.A.; Pullens, B.; Touw, D.J.; et al. Pharmacokinetics and safety of tobramycin nebulization with the I-neb and PARI-LC Plus in children with cystic fibrosis: A randomized, crossover study. Br. J. Clin. Pharmacol. 2019, 85, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Guy, E.L.; Bosomworth, M.; Denton, M.; Conway, S.P.; Brownlee, K.G.; Lee, T.W.R. Serum tobramycin levels following delivery of tobramycin (Tobi) via eFlow advanced nebuliser in children with cystic fibrosis. J. Cyst. Fibros. 2010, 9, 292–295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hart, A.; Cesar, F.; Zelnick, L.R.; O’Connor, N.; Bailey, Z.; Lo, J.; Van Ness, K.; Stanaway, I.B.; Bammler, T.K.; MacDonald, J.W.; et al. Identification of prognostic biomarkers for antibiotic associated nephrotoxicity in cystic fibrosis. J. Cyst. Fibros. 2024, 23, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Bussolati, B. Extracellular vesicles in kidney disease. Nat. Rev. Nephrol. 2022, 18, 499–513. [Google Scholar] [CrossRef]
- Quesenberry, P.J.; Aliotta, J.; Deregibus, M.C.; Camussi, G. Role of extracellular RNA-carrying vesicles in cell differentiation and reprogramming. Stem Cell Res. Ther. 2015, 6, 153. [Google Scholar] [CrossRef]
- Yavuz, H.; Weder, M.M.; Erdbrügger, U. Extracellular Vesicles in Acute Kidney Injury. Nephron 2023, 147, 48–51. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Wu, Q.; Poulsen, S.B.; Murali, S.K.; Grimm, P.R.; Su, X.-T.; Delpire, E.; Welling, P.A.; Ellison, D.H.; Fenton, R.A. Large-Scale Proteomic Assessment of Urinary Extracellular Vesicles Highlights Their Reliability in Reflecting Protein Changes in the Kidney. J. Am. Soc. Nephrol. 2021, 32, 2195–2209. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Erdbrugger, U.; Hoorn, E.J.; Le, T.H.; Blijdorp, C.J.; Burger, D. Extracellular Vesicles in Kidney Diseases: Moving Forward. Kidney360 2023, 4, 245–257. [Google Scholar] [CrossRef]
- Alvarez, S.; Suazo, C.; Boltansky, A.; Ursu, M.; Carvajal, D.; Innocenti, G.; Vukusich, A.; Hurtado, M.; Villanueva, S.; Carreño, J.E.; et al. Urinary exosomes as a source of kidney dysfunction biomarker in renal transplantation. Transplant. Proc. 2013, 45, 3719–3723. [Google Scholar] [CrossRef]
- Panich, T.; Chancharoenthana, W.; Somparn, P.; Issara-Amphorn, J.; Hirankarn, N.; Leelahavanichkul, A. Urinary exosomal activating transcriptional factor 3 as the early diagnostic biomarker for sepsis-induced acute kidney injury. BMC Nephrol. 2017, 18, 10. [Google Scholar] [CrossRef]
- Svenningsen, P.; Maslauskiene, R.; Palarasah, Y.; Bumblyte, I.A.; Tepel, M. Urinary Extracellular Vesicles for Non-Invasive Quantification of Principal Cell Damage in Kidney Transplant Recipients. Biomolecules 2024, 14, 1124. [Google Scholar] [CrossRef]
- Miller, D.; Eagle-Hemming, B.; Sheikh, S.; Joel-David, L.; Adebayo, A.; Lai, F.Y.; Roman, M.; Kumar, T.; Aujla, H.; Murphy, G.J.; et al. Urinary extracellular vesicles and micro-RNA as markers of acute kidney injury after cardiac surgery. Sci. Rep. 2022, 12, 10402. [Google Scholar] [CrossRef]
- Dimuccio, V.; Peruzzi, L.; Brizzi, M.F.; Cocchi, E.; Fop, F.; Boido, A.; Gili, M.; Gallo, S.; Biancone, L.; Camussi, G.; et al. Acute and chronic glomerular damage is associated with reduced CD133 expression in urinary extracellular vesicles. Am. J. Physiol. Renal Physiol. 2020, 318, F486–F495. [Google Scholar] [CrossRef]
- Ranghino, A.; Dimuccio, V.; Papadimitriou, E.; Bussolati, B. Extracellular vesicles in the urine: Markers and mediators of tissue damage and regeneration. Clin. Kidney J. 2015, 8, 23–30. [Google Scholar] [CrossRef]
- Santelli, A.; Sun, I.O.; Eirin, A.; Abumoawad, A.M.; Woollard, J.R.; Lerman, A.; Textor, S.C.; Puranik, A.S.; Lerman, L.O. Senescent Kidney Cells in Hypertensive Patients Release Urinary Extracellular Vesicles. J. Am. Heart Assoc. 2019, 8, e012584. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, K.; Ueda, K.; Sekiguchi, M.; Nakano, E.; Nishimura, T.; Kajiho, Y.; Kanda, S.; Miura, K.; Hattori, M.; Hashimoto, J.; et al. Urinary extracellular vesicles signature for diagnosis of kidney disease. iScience 2022, 25, 105416. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Gallins, P.J.; Pace, R.G.; Dang, H.; Aksit, M.A.; Blue, E.E.; Buckingham, K.J.; Collaco, J.M.; Faino, A.V.; Gordon, W.W.; et al. Genetic Modifiers of Cystic Fibrosis Lung Disease Severity: Whole-Genome Analysis of 7,840 Patients. Am. J. Respir. Crit. Care Med. 2023, 207, 1324–1333. [Google Scholar] [CrossRef]
- Sepahzad, A.; Morris-Rosendahl, D.J.; Davies, J.C. Cystic Fibrosis Lung Disease Modifiers and Their Relevance in the New Era of Precision Medicine. Genes 2021, 12, 562. [Google Scholar] [CrossRef]
- Leggatt, G.P.; Seaby, E.G.; Veighey, K.; Gast, C.; Gilbert, R.D.; Ennis, S. A Role for Genetic Modifiers in Tubulointerstitial Kidney Diseases. Genes 2023, 14, 1582. [Google Scholar] [CrossRef]
- Deltas, C.; Papagregoriou, G.; Louka, S.F.; Malatras, A.; Flinter, F.; Gale, D.P.; Gear, S.; Gross, O.; Hoefele, J.; Lennon, R.; et al. Genetic Modifiers of Mendelian Monogenic Collagen IV Nephropathies in Humans and Mice. Genes 2023, 14(9), 1686. [Google Scholar] [CrossRef]
- Trojan, T.; Alejandre Alcazar, M.A.; Fink, G.; Thomassen, J.C.; Maessenhausen, M.V.; Rietschel, E.; Schneider, P.M.; van Koningsbruggen-Rietschel, S. The effect of TGF-beta(1) polymorphisms on pulmonary disease progression in patients with cystic fibrosis. BMC Pulm. Med. 2022, 22, 183. [Google Scholar] [CrossRef]
- Kabir, F.L.; Ambalavanan, N.; Liu, G.; Li, P.; Solomon, G.M.; Lal, C.V.; Mazur, M.; Halloran, B.; Szul, T.; Gerthoffer, W.T.; et al. MicroRNA-145 Antagonism Reverses TGF-beta Inhibition of F508del CFTR Correction in Airway Epithelia. Am. J. Respir. Crit. Care Med. 2018, 197, 632–643. [Google Scholar] [CrossRef]
- Sun, H.; Harris, W.T.; Kortyka, S.; Kotha, K.; Ostmann, A.J.; Rezayat, A.; Sridharan, A.; Sanders, Y.; Naren, A.P.; Clancy, J.P. Tgf-beta downregulation of distinct chloride channels in cystic fibrosis-affected epithelia. PLoS ONE 2014, 9, e106842. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.; Zhang, Y.; Shao, A.; Avdulov, S.; Herrera, J.; Aboudehen, K.; Pontoglio, M.; Igarashi, P. Mechanism of Fibrosis in HNF1B-Related Autosomal Dominant Tubulointerstitial Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 2493–2509. [Google Scholar] [CrossRef] [PubMed]
- Vuong, M.T.; Lundberg, S.; Gunnarsson, I.; Wramner, L.; Seddighzadeh, M.; Hahn-Zoric, M.; Fernström, A.; Hanson, L.Å.; Do, L.T.; Jacobson, S.H.; et al. Genetic variation in the transforming growth factor-beta1 gene is associated with susceptibility to IgA nephropathy. Nephrol. Dial. Transplant. 2009, 24, 3061–3067. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Raman, A.; Daniel, E.; Metcalf, J.; Reif, G.; Pierucci-Alves, F.; Wallace, D.P. Overexpression of TGF-beta1 induces renal fibrosis and accelerates the decline in kidney function in polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2020, 319, F1135–F1148. [Google Scholar] [CrossRef]
- Tang, P.C.-T.; Chan, A.S.-W.; Zhang, C.-B.; García Córdoba, C.A.; Zhang, Y.-Y.; To, K.-F.; Leung, K.-T.; Lan, H.-Y.; Tang, P.M.-K. TGF-beta1 Signaling: Immune Dynamics of Chronic Kidney Diseases. Front. Med. 2021, 8, 628519. [Google Scholar] [CrossRef] [PubMed]
- Marson, F.A.; Bertuzzo, C.S.; Hortencio, T.D.R.; Ribeiro, J.D.; Bonadia, L.C.; Ribeiro, A.F. The ACE gene D/I polymorphism as a modulator of severity of cystic fibrosis. BMC Pulm. Med. 2012, 12, 41. [Google Scholar] [CrossRef]
- Bezzerri, V.; Gentili, V.; Api, M.; Finotti, A.; Papi, C.; Tamanini, A.; Boni, C.; Baldisseri, E.; Olioso, D.; Duca, M.; et al. SARS-CoV-2 viral entry and replication is impaired in Cystic Fibrosis airways due to ACE2 downregulation. Nat. Commun. 2023, 14, 132. [Google Scholar] [CrossRef]
- Baldassarri, M.; Zguro, K.; Tomati, V.; Pastorino, C.; Fava, F.; Croci, S.; Bruttini, M.; Picchiotti, N.; Furini, S.; Pedemonte, N.; et al. Gain- and Loss-of-Function CFTR Alleles Are Associated with COVID-19 Clinical Outcomes. Cells 2022, 11, 4096. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Singh, N.P.; Kar, P.; Husain, S.A.; Kapoor, S.; Pollipalli, S.K.; Kumar, A.; Garg, N. Does angiotensin-converting enzyme-1 (ACE-1) gene polymorphism lead to chronic kidney disease among hypertensive patients? Ren. Fail. 2016, 38, 765–769. [Google Scholar] [CrossRef]
- Tanaka, R.; Iijima, K.; Murakami, R.; Koide, M.; Nakamura, H.; Yoshikawa, N. ACE gene polymorphism in childhood IgA nephropathy: Association with clinicopathologic findings. Am. J. Kidney Dis. 1998, 31, 774–779. [Google Scholar] [CrossRef]
- Garred, P.; Pressler, T.; Madsen, H.O.; Frederiksen, B.; Svejgaard, A.; Høiby, N.; Schwartz, M.; Koch, C. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J. Clin. Investig. 1999, 104, 431–437. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, M.; Giménez, E.; Lora, D.; Aguado, J.M.; Pascual, M.; Manuel, O. Impact of MBL2 gene polymorphisms on the risk of infection in solid organ transplant recipients: A systematic review and meta-analysis. Am. J. Transplant. 2019, 19, 1072–1085. [Google Scholar] [CrossRef]
- Sanchez-Dominguez, C.N.; Reyes-Lopez, M.A.; Bustamante, A.; Cerda-Flores, R.M.; Villalobos-Torres, M.d.C.; Gallardo-Blanco, H.L.; Rojas-Martinez, A.; Martinez-Rodriguez, H.G.; Barrera-Saldaña, H.A.; Ortiz-Lopez, R. The tumor necrosis factor alpha (-308 A/G) polymorphism is associated with cystic fibrosis in Mexican patients. PLoS ONE 2014, 9, e90945. [Google Scholar] [CrossRef]
- Guo, W.Y.; Zhu, L.; Meng, S.-J.; Shi, S.-F.; Liu, L.-J.; Lv, J.-C.; Zhang, H. Mannose-Binding Lectin Levels Could Predict Prognosis in IgA Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Barratt, J.; Lafayette, R.A.; Zhang, H.; Tesar, V.; Rovin, B.H.; Tumlin, J.A.; Reich, H.N.; Floege, J. IgA nephropathy: The lectin pathway and implications for targeted therapy. Kidney Int. 2023, 104, 254–264. [Google Scholar] [CrossRef]
- Cai, K.; Ma, Y.; Wang, J.; Nie, W.; Guo, J.; Zhang, M.; Yang, Y.; Chen, J.; Han, F. Mannose-binding lectin activation is associated with the progression of diabetic nephropathy in type 2 diabetes mellitus patients. Ann. Transl. Med. 2020, 8, 1399. [Google Scholar] [CrossRef]
- McElvaney, N.G. Alpha-1 Antitrypsin Therapy in Cystic Fibrosis and the Lung Disease Associated with Alpha-1 Antitrypsin Deficiency. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 2), S191–S196. Available online: https://pubmed.ncbi.nlm.nih.gov/27115956/ (accessed on 2 June 2025).
- Brand, P.; Schulte, M.; Wencker, M.; Herpich, C.H.; Klein, G.; Hanna, K.; Meyer, T. Lung deposition of inhaled alpha1-proteinase inhibitor in cystic fibrosis and alpha1-antitrypsin deficiency. Eur. Respir. J. 2009, 34, 354–360. [Google Scholar] [CrossRef]
- Henry, M.T.; Cave, S.; Rendall, J.; O’Connor, C.M.; Morgan, K.; FitzGerald, M.X.; Kalsheker, N. An alpha1-antitrypsin enhancer polymorphism is a genetic modifier of pulmonary outcome in cystic fibrosis. Eur. J. Hum. Genet. 2001, 9, 273–278. [Google Scholar] [CrossRef]
- Frangolias, D.D.; Ruan, J.; Wilcox, P.J.; Davidson, A.G.F.; Wong, L.T.K.; Berthiaume, Y.; Hennessey, R.; Freitag, A.; Pedder, L.; Corey, M.; et al. Alpha 1-antitrypsin deficiency alleles in cystic fibrosis lung disease. Am. J. Respir. Cell Mol. Biol. 2003, 29, 390–396. [Google Scholar] [CrossRef]
- Mahadeva, R.; Westerbeek, R.C.; Perry, D.J.; Lovegrove, U.; Whitehouse, D.B.; Carroll, N.R.; Ross-Russell, A.I.; Webb, K.; Bilton, D.A.; Lomas, D.A. Alpha1-antitrypsin deficiency alleles and the Taq-I G-->A allele in cystic fibrosis lung disease. Eur. Respir. J. 1998, 11, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Doring, G.; Krogh-Johansen, H.; Weidinger, S.; Hoiby, N. Allotypes of alpha 1-antitrypsin in patients with cystic fibrosis, homozygous and heterozygous for deltaF508. Pediatr. Pulmonol. 1994, 18, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Braun, A.; Roscher, A.A. Analysis of the two common alpha-1-antitrypsin deficiency alleles PiMS and PiMZ as modifiers of Pseudomonas aeruginosa susceptibility in cystic fibrosis. Clin. Genet. 2002, 62, 325–327. [Google Scholar] [CrossRef]
- Zager, R.A.; Johnson, A.C.; Frostad, K.B. Rapid renal alpha-1 antitrypsin gene induction in experimental and clinical acute kidney injury. PLoS ONE 2014, 9, e98380. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Tian, J.; Xiao, Z.; Luo, Z.; Lin, T.; Zheng, S.; Ai, J. Serum alpha 1-antitrypsin predicts severe acute kidney injury after cardiac surgery. J. Thorac. Dis. 2019, 11, 5053–5062. [Google Scholar] [CrossRef]
- Esnault, V.L.; Testa, A.; Audrain, M.; Roge, C.; Hamidou, M.; Barrier, J.H.; Sesboue, R.; Martin, J.P.; Lesavre, P. Alpha 1-antitrypsin genetic polymorphism in ANCA-positive systemic vasculitis. Kidney Int. 1993, 43, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.F.; Ellis, P.; Farrugia, D.; Turner, A.M. Nephrotic syndrome secondary to alpha-1 antitrypsin deficiency. BMJ Case Rep. 2021, 14, e240288. [Google Scholar] [CrossRef]
- Bennett, W.D. Effect of beta-adrenergic agonists on mucociliary clearance. J. Allergy Clin. Immunol. 2002, 110, S291–S297. [Google Scholar] [CrossRef]
- Steagall, W.K.; Barrow, B.J.; Glasgow, C.G.; Mendoza, J.W.; Ehrmantraut, M.; Lin, J.P.; Insel, P.A.; Moss, J. Beta-2-adrenergic receptor polymorphisms in cystic fibrosis. Pharmacogenet Genom. 2007, 17, 425–430. [Google Scholar] [CrossRef]
- Vijftigschild, L.A.W.; Berkers, G.; Dekkers, J.F.; Zomer-van Ommen, D.D.; Matthes, E.; Kruisselbrink, E.; Vonk, A.; Hensen, C.E.; Heida-Michel, S.; Geerdink, M.; et al. beta2-Adrenergic receptor agonists activate CFTR in intestinal organoids and subjects with cystic fibrosis. Eur. Respir. J. 2016, 48, 768–779. [Google Scholar] [CrossRef]
- Arif, E.; Nihalani, D. Beta2-adrenergic receptor in kidney biology: A current prospective. Nephrology 2019, 24, 497–503. [Google Scholar] [CrossRef]
- Kamiar, A.; Yousefi, K.; Dunkley, J.C.; Webster, K.A.; Shehadeh, L.A. beta(2)-Adrenergic receptor agonism as a therapeutic strategy for kidney disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R575–R587. [Google Scholar] [CrossRef] [PubMed]
- Yarden, J.; Radojkovic, D.; De Boeck, K.; Macek Jr, M.; Zemkova, D.; Vavrova, V.; Vlietinck, R.; Cassiman, J.-J.; Cuppens, H. Association of tumour necrosis factor alpha variants with the CF pulmonary phenotype. Thorax 2005, 60, 320–325. [Google Scholar] [CrossRef]
- Allen, R.D. Polymorphism of the human TNF-alpha promoter--random variation or functional diversity? Mol. Immunol. 1999, 36, 1017–1027. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Perianayagam, M.C.; Tighiouart, H.; Liangos, O.; Bonventre, J.V.; Jaber, B.L. Tumor necrosis factor alpha promoter polymorphism and severity of acute kidney injury. Nephron Clin. Pract. 2013, 123, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jaber, B.L.; Liangos, O.; Pereira, B.J.; Balakrishnan, V.S. Polymorphism of immunomodulatory cytokine genes: Implications in acute renal failure. Blood Purif. 2004, 22, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Magenheimer, B.S.; Xia, S.; Johnson, T.; Wallace, D.P.; Calvet, J.P.; Li, R. A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat. Med. 2008, 14, 863–868. [Google Scholar] [CrossRef]
- Pirson, Y. Does TNF-alpha enhance cystogenesis in ADPKD? Nephrol. Dial. Transplant. 2008, 23, 3773–3775. [Google Scholar] [CrossRef]
- Tesse, R.; Cardinale, F.; Santostasi, T.; Polizzi, A.; Mappa, L.; Manca, A.; De Robertis, F.; Silecchia, O.; Armenio, L. Association of interleukin-10 gene haplotypes with Pseudomonas aeruginosa airway colonization in cystic fibrosis. J. Cyst. Fibros. 2008, 7, 329–332. [Google Scholar] [CrossRef]
- Brouard, J.; Knauer, N.; Boelle, P.-Y.; Corvol, H.; Henrion-Caude, A.; Flamant, C.; Bremont, F.; Delaisi, B.; Duhamel, J.-F.; Marguet, C.; et al. Influence of interleukin-10 on Aspergillus fumigatus infection in patients with cystic fibrosis. J. Infect. Dis. 2005, 191, 1988–1991. [Google Scholar] [CrossRef]
- Sinuani, I.; Beberashvili, I.; Averbukh, Z.; Sandbank, J. Role of IL-10 in the progression of kidney disease. World J. Transplant. 2013, 3, 91–98. [Google Scholar] [CrossRef]
- Mu, H.; Zheng, Q.; Hao, L. IL-10 -1082 A/G polymorphism is related with the risk and clinical characteristics of acute kidney injury: A case-control study. BMC Nephrol. 2021, 22, 212. [Google Scholar] [CrossRef] [PubMed]
- Bantis, C.; Heering, P.J.; Aker, S.; Klein-Vehne, N.; Grabensee, B.; Ivens, K. Association of interleukin-10 gene G-1082A polymorphism with the progression of primary glomerulonephritis. Kidney Int. 2004, 66, 288–294. [Google Scholar] [CrossRef]
- Wooldridge, J.L.; Deutsch, G.H.; Sontag, M.K.; Osberg, I.; Chase, D.R.; Silkoff, P.E.; Wagener, J.S.; Abman, S.H.; Accurso, F.J. NO pathway in CF and non-CF children. Pediatr. Pulmonol. 2004, 37, 338–350. [Google Scholar] [CrossRef]
- Zheng, S.; Xu, W.; Bose, S.; Banerjee, A.K.; Haque, S.J.; Erzurum, S.C. Impaired nitric oxide synthase-2 signaling pathway in cystic fibrosis airway epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 287, L374–L381. [Google Scholar] [CrossRef]
- Grasemann, H.; Storm van’s Gravesande, K.; Gärtig, S.; Kirsch, M.; Büscher, R.; Drazen, J.M.; Ratjen, F. Nasal nitric oxide levels in cystic fibrosis patients are associated with a neuronal NO synthase (NOS1) gene polymorphism. Nitric Oxide 2002, 6, 236–241. [Google Scholar] [CrossRef]
- Grasemann, H.; Knauer, N.; Büscher, R.; Hübner, K.; Drazen, J.M.; Ratjen, F. Airway nitric oxide levels in cystic fibrosis patients are related to a polymorphism in the neuronal nitric oxide synthase gene. Am. J. Respir. Crit. Care Med. 2000, 162, 2172–2176. [Google Scholar] [CrossRef]
- Texereau, J.; Marullo, S.; Hubert, D.; Coste, J.; Dusser, D.J.; Dall’Ava-Santucci, J.; Dinh-Xuan, A.T. Nitric oxide synthase 1 as a potential modifier gene of decline in lung function in patients with cystic fibrosis. Thorax 2004, 59, 156–158. [Google Scholar] [CrossRef]
- Grasemann, H.; Gravesande, K.S.V.; Büscher, R.; Knauer, N.; Silverman, E.S.; Palmer, L.J.; Drazen, J.M.; Ratjen, F. Endothelial nitric oxide synthase variants in cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 2003, 167, 390–394. [Google Scholar] [CrossRef]
- Baylis, C. Nitric oxide synthase derangements and hypertension in kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.M.; Esteban Zubero, E.; Alatorre Jiménez, M.A.; Alonso Barragan, S.A.; López García, C.A.; Gómez Ramos, J.J.; Santoscoy Gutierrez, J.F.; González Castillo, Z. NOS3 Polymorphisms and Chronic Kidney Disease. J. Bras. Nefrol. 2018, 40, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Padhi, U.N.; Mulkalwar, M.; Saikrishna, L.; Verma, H.K.; Bhaskar, L. NOS3 gene intron 4 a/b polymorphism is associated with ESRD in autosomal dominant polycystic kidney disease patients. J. Bras. Nefrol. 2022, 44, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.; Thomson, A.H. Contribution of genetic factors other than CFTR to disease severity in cystic fibrosis. Thorax 1998, 53, 1018–1021. [Google Scholar] [CrossRef][Green Version]
- Flamant, C.; Henrion-Caude, A.; Boëlle, P.-Y.; Brémont, F.; Brouard, J.; Delaisi, B.; Duhamel, J.-F.; Marguet, C.; Roussey, M.; Miesch, M.-C.; et al. Glutathione-S-transferase M1, M3, P1 and T1 polymorphisms and severity of lung disease in children with cystic fibrosis. Pharmacogenetics 2004, 14, 295–301. [Google Scholar] [CrossRef]
- Nomani, H.; Hagh-Nazari, L.; Aidy, A.; Vaisi-Raygani, A.; Kiani, A.; Rahimi, Z.; Bahrehmand, F.; Shakiba, E.; Mozaffari, H.R.; Tavilani, H.; et al. Association between GSTM1, GSTT1, and GSTP1 variants and the risk of end stage renal disease. Ren. Fail. 2016, 38, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.V.; Reidy, K.J.; Le, T.H.; David, V.; Winkler, C.; Xu, Y.; Warady, B.; Furth, S.; Kaskel, F.; Melamed, M.L.; et al. Association of GSTM1 Deletion With Progression of CKD in Children: Findings From the Chronic Kidney Disease in Children (CKiD) Study. Am. J. Kidney Dis. 2022, 80, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, D.; Zhang, W.; Xing, Y.; Guo, Y.; Wang, F.; Jia, J.; Yan, T.; Liu, Y.; Lin, S. ACE Inhibitor Benefit to Kidney and Cardiovascular Outcomes for Patients with Non-Dialysis Chronic Kidney Disease Stages 3-5: A Network Meta-Analysis of Randomised Clinical Trials. Drugs 2020, 80, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C. Nitric oxide deficiency in chronic kidney disease. Am. J. Physiol. Renal Physiol. 2008, 294, F1–F9. [Google Scholar] [CrossRef]
- Phua, Y.L.; Chen, K.H.; Hemker, S.L.; Marrone, A.K.; Bodnar, A.J.; Liu, X.; Clugston, A.; Kostka, D.; Butterworth, M.B.; Ho, J. Loss of miR-17~92 results in dysregulation of Cftr in nephron progenitors. Am. J. Physiol. Renal Physiol. 2019, 316, F993–F1005. [Google Scholar] [CrossRef]
- McHugh, D.R.; Steele, M.S.; Valerio, D.M.; Miron, A.; Mann, R.J.; LePage, D.F.; Conlon, R.A.; Cotton, C.U.; Drumm, M.L.; Hodges, C.A. A G542X cystic fibrosis mouse model for examining nonsense mutation directed therapies. PLoS ONE 2018, 13, e0199573. [Google Scholar] [CrossRef]
- Hodges, C.A.; Grady, B.R.; Mishra, K.; Cotton, C.U.; Drumm, M.L. Cystic fibrosis growth retardation is not correlated with loss of Cftr in the intestinal epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G528–G536. [Google Scholar] [CrossRef]
- van Heeckeren, A.M.; Schluchter, M.D.; Drumm, M.L.; Davis, P.B. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L944–L952. [Google Scholar] [CrossRef]
- Birket, S.E.; Davis, J.M.; Fernandez-Petty, C.M.; Henderson, A.G.; Oden, A.M.; Tang, L.; Wen, H.; Hong, J.; Fu, L.; Chambers, A.; et al. Ivacaftor Reverses Airway Mucus Abnormalities in a Rat Model Harboring a Humanized G551D-CFTR. Am. J. Respir. Crit. Care Med. 2020, 202, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yi, Y.; Yan, Z.; Rosen, B.H.; Liang, B.; Winter, M.C.; Apak Evans, T.I.; Rotti, P.G.; Yang, Y.; Gray, J.S.; et al. In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci. Transl. Med. 2019, 11, eaau7531. [Google Scholar] [CrossRef] [PubMed]
- Birket, S.E.; Davis, J.M.; Fernandez, C.M.; Tuggle, K.L.; Oden, A.M.; Chu, K.K.; Tearney, G.J.; Fanucchi, M.V.; Sorscher, E.J.; Rowe, S.M.; et al. Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight 2018, 3, e97199. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sui, H.; Fisher, J.T.; Yan, Z.; Liu, X.; Cho, H.-J.; Joo, N.S.; Zhang, Y.; Zhou, W.; Yi, Y.; et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J. Clin. Investig. 2010, 120, 3149–3160. [Google Scholar] [CrossRef] [PubMed]
- Delrue, C.; De Bruyne, S.; Speeckaert, M.M. Application of Machine Learning in Chronic Kidney Disease: Current Status and Future Prospects. Biomedicines 2024, 12, 568. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).