Early Intrableb Features on Anterior Segment Swept-Source Optical Coherence Tomography Predict Surgical Success After Trabeculectomy in Uveitic and Neovascular Glaucoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Design
2.3. Surgical Technique
2.4. Definition of Surgical Success
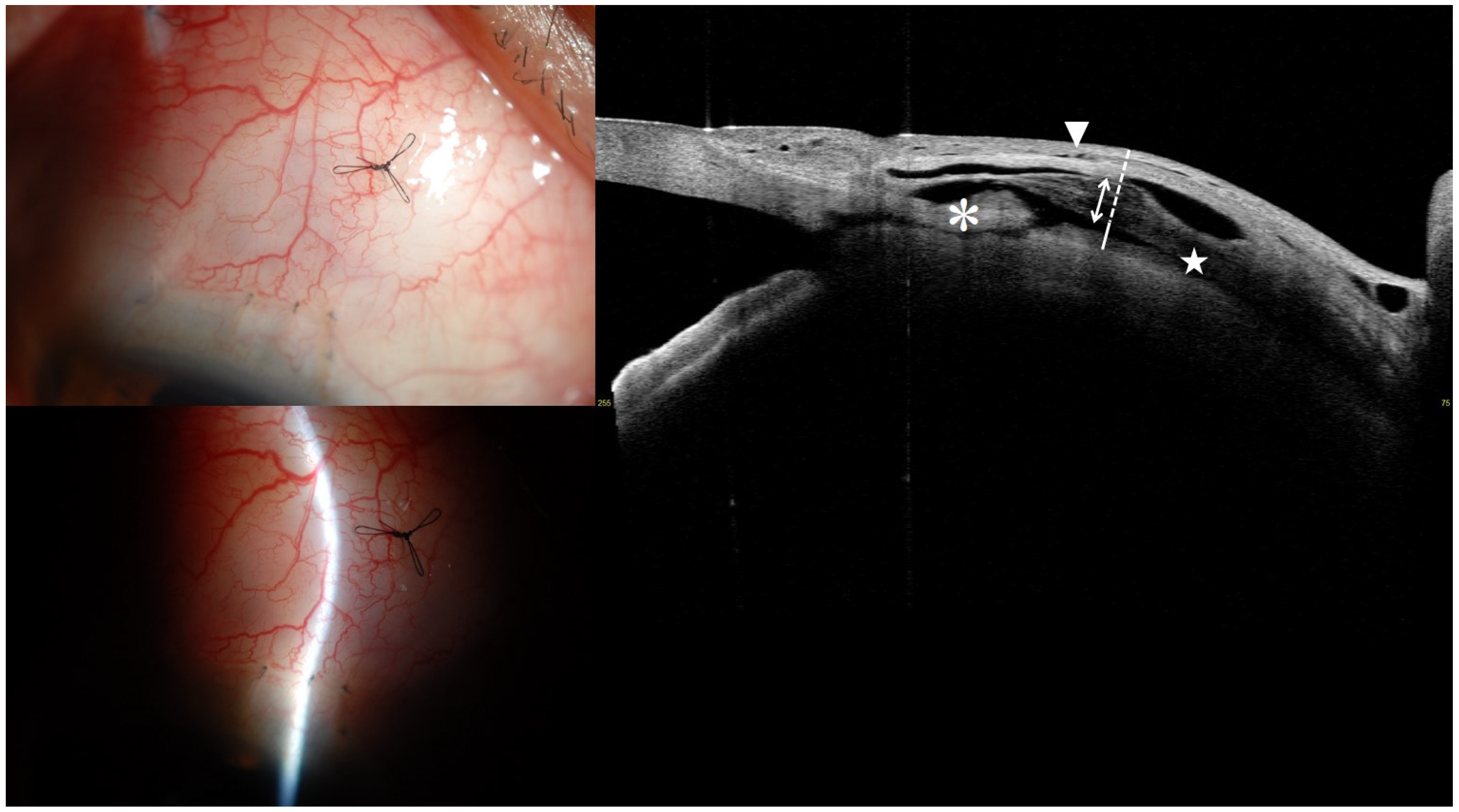
2.5. AS SS-OCT Imaging
2.6. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics in All Patients
3.2. Comparison of Anterior Segment Optical Coherence Tomography Parameters at 1 Month After Trabeculectomy
3.3. Logistic Regression Model of the Variables Associated with Successful IOP Control Postoperatively at 1 Year
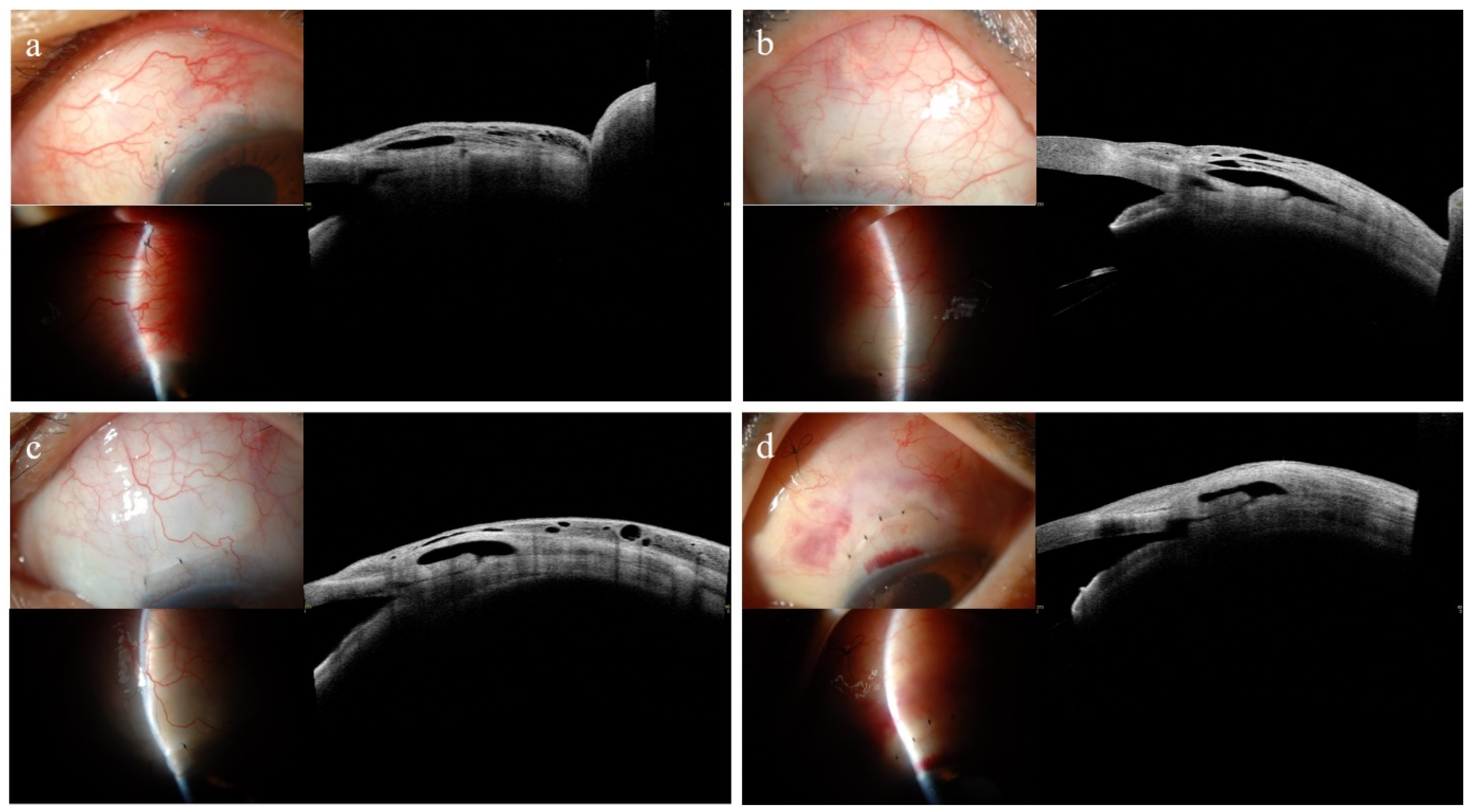
3.4. Representative Anterior Segment Optical Coherence Tomography Images at 1 Month
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barkana, Y.; Dorairaj, S. Re: Tham et al.: Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis (Ophthalmology 2014; 121: 2081-90). Ophthalmology 2015, 122, e40–e41. [Google Scholar] [CrossRef]
- Miglani, T.; Ullah, S. A Review of the Surgical Management of Neovascular Glaucoma. Curr. Surg. Rep. 2023, 11, 162–167. [Google Scholar] [CrossRef]
- Kesav, N.; Palestine, A.G.; Kahook, M.Y.; Pantcheva, M.B. Current Management of Uveitis-Associated Ocular Hypertension and Glaucoma. Surv. Ophthalmol. 2020, 65, 397–407. [Google Scholar] [CrossRef]
- Senthil, S.; Chary, R.; Ali, M.H.; Cherukuri, J.R.; Rani, P.K.; Krishnamurthy, R.; Choudhari, N.; Garudadri, C. Trabeculectomy for Neovascular Glaucoma in Proliferative Diabetic Retinopathy, Central Retinal Vein Occlusion, and Ocular Ischemic Syndrome: Surgical Outcomes and Prognostic Factors for Failure. Indian J. Ophthalmol. 2021, 69, 3341–3348. [Google Scholar] [CrossRef]
- Carreño, E.; Villarón, S.; Portero, A.; Herreras, J.M.; Maquet, J.A.; Calonge, M. Surgical Outcomes of Uveitic Glaucoma. J. Ophthalmic Inflamm. Infect. 2011, 1, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Maruyama, K.; Yokoyama, Y.; Tsuda, S.; Ryu, M.; Nakazawa, T. Characteristics of Uveitic Glaucoma and Evaluation of Its Surgical Treatment. Clin. Ophthalmol. 2014, 6, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Takihara, Y.; Inatani, M.; Fukushima, M.; Iwao, K.; Iwao, M.; Tanihara, H. Trabeculectomy with Mitomycin C for Neovascular Glaucoma: Prognostic Factors for Surgical Failure. Am. J. Ophthalmol. 2009, 147, 912–918.e1. [Google Scholar] [CrossRef]
- Hyung, S.M.; Kim, S.K. Midd-Term Effects of Trabeculectomy with Mitomycin C in Neovascular Glaucoma Patients. Korean J. Ophthalmol. 2001, 15, 98–106. [Google Scholar] [CrossRef]
- Nakatake, S.; Yoshida, S.; Nakao, S.; Arita, R.; Yasuda, M.; Kita, T.; Enaida, H.; Ohshima, Y.; Ishibashi, T. Hyphema Is a Risk Factor for Failure of Trabeculectomy in Neovascular Glaucoma: A Retrospective Analysis. BMC Ophthalmol. 2014, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Iwao, K.; Inatani, M.; Seto, T.; Takihara, Y.; Ogata-Iwao, M.; Okinami, S.; Tanihara, H. Long-Term Outcomes and Prognostic Factors for Trabeculectomy with Mitomycin C in Eyes with Uveitic Glaucoma: A Retrospective Cohort Study. J. Glaucoma 2014, 23, 88–94. [Google Scholar] [CrossRef]
- Kaburaki, T.; Koshino, T.; Kawashima, H.; Numaga, J.; Tomidokoro, A.; Shirato, S.; Araie, M. Initial Trabeculectomy with Mitomycin C in Eyes with Uveitic Glaucoma with Inactive Uveitis. Eye 2009, 23, 1509–1517. [Google Scholar] [CrossRef][Green Version]
- Waibel, S.; Spoerl, E.; Furashova, O.; Pillunat, L.E.; Pillunat, K.R. Bleb Morphology After Mitomycin-C Augmented Trabeculectomy: Comparison Between Clinical Evaluation and Anterior Segment Optical Coherence Tomography. J. Glaucoma 2019, 28, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Skuta, G.L.; Parrish II, R.K. Wound Healing in Glaucoma Filtering Surgery. Surv. Ophthalmol. 1987, 32, 149–170. [Google Scholar] [CrossRef]
- Narita, A.; Morizane, Y.; Miyake, T.; Seguchi, J.; Baba, T.; Shiraga, F. Characteristics of Early Filtering Blebs That Predict Successful Trabeculectomy Identified via Three-Dimensional Anterior Segment Optical Coherence Tomography. Br. J. Ophthalmol. 2018, 102, 796–801. [Google Scholar] [CrossRef]
- Nakamura, M.; Naka, M.; Tatsumi, Y.; Nagai-Kusuhara, A.; Kanamori, A.; Yamada, Y.; Negi, A. Filtering Bleb Structure Associated with Long-Term Intraocular Pressure Control after Amniotic Membrane-Assisted Trabeculectomy. Curr. Eye Res. 2012, 37, 239–250. [Google Scholar] [CrossRef]
- Moon, S.; Kim, J.; Lee, J. Comparison of the Intrableb Characteristics of Anterior Segment Optical Coherence Tomography Imaging in Trabeculectomy According to Amniotic Membrane Transplantation. Ophthalmic Res. 2023, 66, 970–982. [Google Scholar] [CrossRef]
- Moon, S.; Lee, J. Distinctive Intrableb Structures of Functioning Blebs Following Trabeculectomy According to Amniotic Membrane Transplantation. Ophthalmic Res. 2025, 68, 1–12. [Google Scholar] [CrossRef]
- Sim, J.J.L.; Betzler, B.K.; Dorairaj, S.; Dada, T.; Ang, B.C.H. Trabeculectomy Bleb Characteristics in Relation to Bleb Success Using Anterior Segment Optical Coherence Tomography—A Systematic Review and Meta-Analysis. J. Glaucoma 2025. [Google Scholar] [CrossRef]
- Nahon-Estève, S.; Martel, A.; Maschi, C.; Baillif, S.; Lassalle, S.; Caujolle, J.-P. Swept-Source and Spectral-Domain OCT Imaging of Conjunctival Tumors. Ophthalmology 2021, 128, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, N.; Kamali, G.; Rahnavardi, M.; Mohammadi, B.; Nassiri, S.; Rahmani, L.; Nassiri, N. Ahmed Glaucoma Valve and Single-Plate Molteno Implants in Treatment of Refractory Glaucoma: A Comparative Study. Am. J. Ophthalmol. 2010, 149, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Cantor, L.B.; Mantravadi, A.; WuDunn, D.; Swamynathan, K.; Cortes, A. Morphologic Classification of Filtering Blebs after Glaucoma Filtration Surgery: The Indiana Bleb Appearance Grading Scale. J. Glaucoma 2003, 12, 266–271. [Google Scholar] [CrossRef]
- Romero, P.; Hirunpatravong, P.; Alizadeh, R.; Kim, E.-A.; Nouri-Mahdavi, K.; Morales, E.; Law, S.K.; Caprioli, J. Trabeculectomy With Mitomycin-C: Outcomes and Risk Factors for Failure in Primary Angle-Closure Glaucoma. J. Glaucoma 2018, 27, 101–107. [Google Scholar] [CrossRef]
- Shaarawy, T.; Grehn, F. Guidelines on Design and Reporting of Glaucoma Surgical Trials; Kugler Publications: Amsterdam, The Netherlands, 2009; ISBN 90-6299-219-6. [Google Scholar]
- Jung, K.I.; Lim, S.A.; Park, H.-Y.L.; Park, C.K. Visualization of Blebs Using Anterior-Segment Optical Coherence Tomography after Glaucoma Drainage Implant Surgery. Ophthalmology 2013, 120, 978–983. [Google Scholar] [CrossRef]
- Narita, A.; Morizane, Y.; Miyake, T.; Seguchi, J.; Baba, T.; Shiraga, F. Characteristics of Successful Filtering Blebs at 1 Year after Trabeculectomy Using Swept-Source Three-Dimensional Anterior Segment Optical Coherence Tomography. Jpn. J. Ophthalmol. 2017, 61, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Theelen, T.; Wesseling, P.; Keunen, J.E.; Klevering, B.J. A Pilot Study on Slit Lamp-Adapted Optical Coherence Tomography Imaging of Trabeculectomy Filtering Blebs. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 877–882. [Google Scholar] [CrossRef]
- Nakano, N.; Hangai, M.; Nakanishi, H.; Inoue, R.; Unoki, N.; Hirose, F.; Ojima, T.; Yoshimura, N. Early Trabeculectomy Bleb Walls on Anterior-Segment Optical Coherence Tomography. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Strzalkowska, A.; Strzalkowski, P.; Al Yousef, Y.; Grehn, F.; Hillenkamp, J.; Loewen, N.A. Exact Matching of Trabectome-Mediated Ab Interno Trabeculectomy to Conventional Trabeculectomy with Mitomycin C Followed for 2 Years. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 963–970. [Google Scholar] [CrossRef]
- Addicks, E.M.; Quigley, H.A.; Green, W.R.; Robin, A.L. Histologic Characteristics of Filtering Blebs in Glaucomatous Eyes. Arch. Ophthalmol. 1983, 101, 795–798. [Google Scholar] [CrossRef]
- Ciancaglini, M.; Carpineto, P.; Agnifili, L.; Nubile, M.; Lanzini, M.; Fasanella, V.; Mastropasqua, L. Filtering Bleb Functionality: A Clinical, Anterior Segment Optical Coherence Tomography and In Vivo Confocal Microscopy Study. J. Glaucoma 2008, 17, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Broadway, D.C.; Chang, L.P. Trabeculectomy, Risk Factors for Failure and the Preoperative State of the Conjunctiva. J. Glaucoma 2001, 10, 237–249. [Google Scholar] [CrossRef]
- Broadway, D.C.; Bates, A.K.; Lightman, S.L.; Grierson, I.; Hitchings, R.A. The Importance of Cellular Changes in the Conjunctiva of Patients with Uveitic Glaucoma Undergoing Trabeculectomy. Eye 1993, 7, 495–501. [Google Scholar] [CrossRef] [PubMed]

| Entire | Uveitic Glaucoma | Neovascular Glaucoma | |
|---|---|---|---|
| Number of eyes (patients) | 22 (22) | 11 (11) | 11 (11) |
| Age at trabeculectomy, years | 68.62 ± 7.41 | 71.13 ± 5.34 | 66.11 ± 8.54 |
| Sex, female | 7 (31.8) | 3 (27.3) | 4 (36.4) |
| Eye laterality, right | 13 (59.1) | 6 (54.5) | 7 (63.6) |
| Diagnosis | |||
| Uveitic glaucoma | 11 (50.0) | VZV (1), CMV(1), Behcet disease (1) Not definitely established (8) | PDR (9), CRVO (2) |
| Neovascular glaucoma | 11 (50.0) | ||
| Anterior chamber angle status, open | 12 (54.55) | 8 (72.7) | 4 (36.4) |
| IOP at AS-OCT test, mmHg | 9.87 ± 2.57 (6–15) | 9.82 ± 2.75 (6–15) | 9.91 ± 2.51 (6–15) |
| Preoperative visual acuity, logMAR | 1.46 ± 0.96 (3.0–0) | 0.89 ± 0.65 (2.0–0) | 2.04 ± 0.90 (3.0–0.5) |
| Preoperative IOP, mmHg | 32.86 ± 12.54 (15–70) | 31.45 ± 8.99 (17–45) | 34.27 ± 15.65 (15–70) |
| Number of glaucoma medication | 3.86 ± 0.56 (3–5) | 4.00 ± 0.63 (3–5) | 3.73 ± 0.47 (3–4) |
| History of glaucoma medication, year | 1.12 ± 1.47 (0.01–5.02) | 1.35 ± 1.82 (0.01–5.02) | 0.91 ± 1.11 (0.02–3.41) |
| Preoperative lens status, phakic | 10 (45.5) | 7 (63.63) | 3 (27.3) |
| Central corneal thickness, μm | 547.41 ± 34.61 | 553.27 ± 37.21 | 541.55 ± 32.49 |
| Axial length, mm | 24.27 ± 2.13 | 25.12 ± 2.67 | 23.42 ± 0.87 |
| Spherical equivalent, diopters | −1.16 ± 1.95 | −1.58 ± 1.96 | −0.74 ± 1.94 |
| Cup to disk ratio | 0.81 ± 0.14 | 0.81 ± 0.18 | 0.80 ± 0.10 |
| Visual field parameter | |||
| Visual Field Index, % | 35.55 ± 31.57 | 28.27 ± 28.39 | 42.82 ± 34.21 |
| Mean deviation, dB | −21.73 ± 7.36 | −22.97 ± 6.74 | −20.49 ± 8.05 |
| Pattern standard deviation, dB | 7.39 ± 3.06 | 7.56 ± 3.39 | 7.22 ± 2.85 |
| Successful IOP control at 1 year, % | 16 (72.7) | 9 (81.8) | 7 (63.6) |
| Subsequent bleb management | |||
| Bleb needling | 8 (36.4) | 2 (18.2) | 6 (54.5) |
| 1 | 4 | 2 | 2 |
| ≥2 | 4 | 0 | 4 |
| Additional glaucoma surgery | 3 (13.6) | 0 | 3 (Trab1, AGV2) |
| Successful, n = 16 Eyes | Unsuccessful, n = 6 Eyes | p-Value * | |
|---|---|---|---|
| Bleb height | >0.999 | ||
| H0: flat bleb | |||
| H1: low bleb | |||
| H2: medium bleb | 16 (100%) | 6 (100%) | |
| H3: high bleb | |||
| Horizontal extent | 0.094 | ||
| E0: <1 clock hours | |||
| E1: ≥1 <2 clock hours | 1 (16.7%) | ||
| E2: ≥2 <4 clock hours | 10 (62.5%) | 5 (83.3%) | |
| E3: ≥4 clock hours | 6 (37.5%) | ||
| Vascularity | >0.999 | ||
| V0: avascular white | |||
| V1: avascular cystic | |||
| V2: mild vascularity | 15 (93.8%) | 6 (100%) | |
| V3: moderate vascularity | 1 (6.3%) | ||
| V4: extensive vascularity | |||
| Seidel test | >0.999 | ||
| S0: no leak | 16 (100%) | 6 (100%) | |
| S1: multiple pinpoint leaks | |||
| S2: streaming leak |
| Intrableb Parameters | Entire, n = 22 | Successful, n = 16 | Unsuccessful, n = 6 | p-Value * |
|---|---|---|---|---|
| Bleb height, μm | 1523.57 ± 181.06 | 1555.38 ± 178.58 | 1438.75 ± 173.61 | 0.178 |
| Bleb wall thickness, μm | 813.02 ± 401.35 | 898.47 ± 330.74 | 585.17 ± 512.55 | 0.033 |
| Striping layer thickness, μm | 376.14 ± 338.67 | 431.81 ± 349.96 | 227.67 ± 279.19 | 0.115 |
| Striping/Bleb wall ratio | 0.42 ± 0.24 | 0.44 ± 0.20 | 0.37 ± 0.35 | 0.407 |
| Bleb wall reflectivity | 100.36 ± 25.60 | 91.53 ± 18.90 | 123.88 ± 27.71 | 0.021 |
| Fluid-filled space area, mm2 | 2.81 ± 1.50 | 2.70 ± 1.27 | 3.10 ± 2.11 | 0.541 |
| Fluid-filled space height, μm | 710.55 ± 331.50 | 656.91 ± 275.26 | 853.58 ± 447.76 | 0.083 |
| Microcyst formation | 16 (72.7) | 14 (87.5) | 2 (33.3) | 0.025 |
| Univariate | Multivariate Model | |||
|---|---|---|---|---|
| Prognostic Factor | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Sex (reference male) | 0.333 (0.031, 3.579) | 0.361 | ||
| Age | 0.902 (0.788, 1.034) | 0.139 | ||
| Glaucoma type (reference uveitic) | 2.571 (0.361, 18.326) | 0.346 | ||
| Preoperative lens status (reference phakic) | 0.778 (0.119, 5.110) | 0.793 | ||
| Number of preoperative glaucoma medication | 1.908 (0.317, 11.474) | 0.480 | ||
| Axial length | 0.935 (0.572, 1.530) | 0.790 | ||
| Preoperative visual acuity (logMAR) | 1.234 (0.457, 3.330) | 0.679 | ||
| Preoperative mean deviation | 1.060 (0.931, 1.206) | 0.380 | ||
| Preoperative IOP | 0.935 (0.841, 1.039) | 0.210 | ||
| Postoperative IOP at AS-OCT test (1 month) | 1.443 (0.954, 2.183) | 0.083 | ||
| Intrableb parameters at 1 month | ||||
| Bleb height | 0.996 (0.991, 1.002) | 0.184 | ||
| Bleb wall thickness | 0.997 (0.994, 1.001) | 0.121 | ||
| Striping layer thickness | 0.997 (0.992, 1.002) | 0.229 | ||
| Striping/Bleb wall ratio | 0.292 (0.005, 18.241) | 0.559 | ||
| Bleb wall reflectivity | 1.072 (1.006, 1.141) | 0.032 | 1.072 (1.006, 1.141) | 0.032 |
| Fluid-filled space area | 1.214 (0.627, 2.351) | 0.566 | ||
| Fluid-filled space height | 1.002 (0.999, 1.006) | 0.224 | ||
| Microcyst formation | 0.071 (0.008, 0.680) | 0.022 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.; Lee, S.; Lee, J. Early Intrableb Features on Anterior Segment Swept-Source Optical Coherence Tomography Predict Surgical Success After Trabeculectomy in Uveitic and Neovascular Glaucoma. J. Clin. Med. 2025, 14, 5499. https://doi.org/10.3390/jcm14155499
Moon S, Lee S, Lee J. Early Intrableb Features on Anterior Segment Swept-Source Optical Coherence Tomography Predict Surgical Success After Trabeculectomy in Uveitic and Neovascular Glaucoma. Journal of Clinical Medicine. 2025; 14(15):5499. https://doi.org/10.3390/jcm14155499
Chicago/Turabian StyleMoon, Sangwoo, Seungmin Lee, and Jiwoong Lee. 2025. "Early Intrableb Features on Anterior Segment Swept-Source Optical Coherence Tomography Predict Surgical Success After Trabeculectomy in Uveitic and Neovascular Glaucoma" Journal of Clinical Medicine 14, no. 15: 5499. https://doi.org/10.3390/jcm14155499
APA StyleMoon, S., Lee, S., & Lee, J. (2025). Early Intrableb Features on Anterior Segment Swept-Source Optical Coherence Tomography Predict Surgical Success After Trabeculectomy in Uveitic and Neovascular Glaucoma. Journal of Clinical Medicine, 14(15), 5499. https://doi.org/10.3390/jcm14155499





