Dynamic vs. Rigid: Transforming the Treatment Landscape for Multisegmental Lumbar Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection Criteria
- Age ≥ 45 years;
- Radiologically confirmed multisegmental lumbar degenerative disease involving at least seven motion segments, demonstrated on MRI and standing whole-spine radiographs;
- Presence of chronic low-back pain lasting > 6 months with or without associated neurogenic claudication or radicular symptoms;
- Failure of at least 6 months of conservative management, including physical therapy, nonsteroidal anti-inflammatory drugs (NSAIDs), epidural steroid injections, and activity modification;
- Demonstrable dynamic instability on flexion–extension X-rays or instability evidenced by CT (e.g., facet joint fluid, vacuum phenomena, subchondral sclerosis);
- Functional impairment with Oswestry Disability Index (ODI) ≥ 30% preoperatively;
- A minimum postoperative follow-up of 24 months.
- Flexion–extension radiographs demonstrating sagittal translation > 3 mm or segmental angulation > 10° between vertebrae, consistent with established instability criteria;
- CT findings suggestive of segmental instability, such as vacuum phenomena within the intervertebral disc or facet joints, subchondral sclerosis, and hypertrophy of ligamentum flavum;
- MRI indicators, including hyperintensity or effusion within facet joints and Modic changes at endplates adjacent to the degenerated disc.
2.2. Surgical Technique
2.3. Clinical and Radiological Follow Up
2.4. Statistical Analysis
3. Results
- Dynesys group: Thread breakage occurred in 2/27 patients (7.4%);
- Rigid group: Instrument failure in 2/26 (7.7%), ASD in 6/26 (23.1%), pseudoarthrosis in 2/26 (7.7%), infection in 1/26 (3.8%), and 1 case (3.8%) of postoperative neurological deficit.
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, H.; Pang, Q.; Jiang, G. Medium-term effects of Dynesys dynamic stabilization versus posterior lumbar interbody fusion for treatment of multisegmental lumbar degenerative disease. J. Int. Med. Res. 2017, 45, 1562–1573. [Google Scholar] [CrossRef]
- Boden, S.D.; Davis, D.O.; Dina, T.S.; Patronas, N.J.; Wiesel, S.W. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J. Bone Jt. Surg. Am. 1990, 72, 403–408. [Google Scholar] [CrossRef]
- Yavin, D.; Casha, S.; Wiebe, S.; E Feasby, T.; Clark, C.; Isaacs, A.; Holroyd-Leduc, J.; Hurlbert, R.J.; Quan, H.; Nataraj, A.; et al. Lumbar Fusion for Degenerative Disease: A Systematic Review and Meta-Analysis. Neurosurgery 2017, 80, 701–715. [Google Scholar] [CrossRef]
- Lee, J.C.; Choi, S.W. Adjacent Segment Pathology after Lumbar Spinal Fusion. Asian Spine J. 2015, 9, 807–817. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.C.; Li, F.; Sun, T.-S.; Shan, J.-L.; Guan, K.; Zhao, G.-M.; Zhang, L.-Z. Long-Term Outcome of Dynesys Dynamic Stabilization for Lumbar Spinal Stenosis. Chin. Med. J. 2018, 131, 2537–2543. [Google Scholar] [CrossRef]
- Zhou, L.P.; Zhang, R.J.; Wang, J.Q.; Zhang, H.-Q.; Shang, J.; Gao, Y.; Jia, C.-Y.; Ding, J.-Y.; Zhang, L.; Shen, C.-L. Medium and long-term radiographic and clinical outcomes of Dynesys dynamic stabilization versus instrumented fusion for degenerative lumbar spine diseases. BMC Surg. 2023, 23, 46. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.J.; Lai, C.Y.; Chiu, L.T.; Huang, W.-S.; Hsiao, P.-H.; Chang, C.-C.; Lin, C.-J.; Lo, Y.-S.; Chen, Y.-J.; Chen, H.-T. Adjacent segment disease following Dynesys stabilization for lumbar disorders: A case series of mid- and long-term follow-ups. World J. Clin. Cases 2021, 9, 10850–10860. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.W.; Yen, C.Y.; Wu, C.H.; Kao, F.C.; Kao, Y.H.; Tu, Y.K. Radiographic and clinical results of posterior dynamic stabilization for the treatment of multisegment degenerative disc disease with a minimum follow-up of 3 years. Arch. Orthop. Trauma Surg. 2012, 132, 583–589. [Google Scholar] [CrossRef]
- Di Silvestre, M.; Lolli, F.; Bakaloudis, G.; Parisini, P. Dynamic Stabilization for Degenerative Lumbar Scoliosis in Elderly Patients. Spine 2010, 35, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.C.; Tsai, H.W.; Huang, W.C.; Wu, J.-C.; Chen, Y.-C.; Shih, Y.-H.; Chen, H.-C.; Wu, C.-L.; Cheng, H. Screw loosening in the Dynesys stabilization system: Radiographic evidence and effect on outcomes. Neurosurg. Focus 2010, 28, E10. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, S.B.; Jahng, T.A.; Chung, C.K.; Kim, H.J. Clinical Experience of the Dynamic Stabilization System for the Degenerative Spine Disease. J. Korean Neurosurg. Soc. 2008, 43, 221. [Google Scholar] [CrossRef]
- Putzier, M.; Schneider, S.V.; Funk, J.F.; Tohtz, S.W.; Perka, C. The Surgical Treatment of the Lumbar Disc Prolapse. Spine 2005, 30, E109–E114. [Google Scholar] [CrossRef]
- Schaeren, S.; Broger, I.; Jeanneret, B. Minimum Four-Year Follow-up of Spinal Stenosis with Degenerative Spondylolisthesis Treated with Decompression and Dynamic Stabilization. Spine 2008, 33, E636–E642. [Google Scholar] [CrossRef]
- Stoll, T.M.; Dubois, G.; Schwarzenbach, O. The dynamic neutralization system for the spine: A multi-center study of a novel non-fusion system. Eur. Spine J. 2002, 11 (Suppl. 2), S170–S178. [Google Scholar] [CrossRef]
- Pham, M.H.; Mehta, V.A.; Patel, N.N.; Jakoi, A.M.; Hsieh, P.C.; Liu, J.C.; Wang, J.C.; Acosta, F.L. Complications associated with the Dynesys dynamic stabilization system: A comprehensive review of the literature. Neurosurg. Focus 2016, 40, E2. [Google Scholar] [CrossRef] [PubMed]
- Akgun, M.Y.; Ucar, E.A.; Gedik, C.C.; Gunerbuyuk, C.; Hekimoglu, M.; Cerezci, O.; Oktenoglu, T.; Sasani, M.; Ates, O.; Ozer, A.F. Use of Dynamic Spinal Instruments (Dynesys) in Adult Spinal Deformities According to Silva–Lenke and Berjano–Lamartina Classifications. Diagnostics 2024, 14, 549. [Google Scholar] [CrossRef]
- Fei, H.; Xu, J.; Wang, S.; Xie, Y.; Ji, F.; Xu, Y. Comparison between posterior dynamic stabilization and posterior lumbar interbody fusion in the treatment of degenerative disc disease: A prospective cohort study. J. Orthop. Surg. Res. 2015, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Berven, S.H.; Fortin, M.; Weber, M.H. Adjacent Segment Degeneration Versus Disease After Lumbar Spine Fusion for Degenerative Pathology. Clin. Spine Surg. A Spine Publ. 2016, 29, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Özer, A.F.; Başak, A.T.; Özbek, M.A.; Hekimoğlu, M.; Aydın, A.L.; Ateş, Ö.; Günerbüyük, C.; Akgül, T.; Sasani, M.; Öktenoğlu, T. Lumbar Dynamic Stabilization with 2-Stage Surgery: Early Results. Int. J. Spine Surg. 2022, 16, 638–645. [Google Scholar] [CrossRef]
- Hu, A.; Sun, C.; Liang, Y.; Wang, H.; Li, X.; Dong, J. Multi-segmental lumbar spinal stenosis treated with Dynesys stabilization versus lumbar fusion in elderly patients: A retrospective study with a minimum of 5 years’ follow-up. Arch. Orthop. Trauma Surg. 2019, 139, 1361–1368. [Google Scholar] [CrossRef]
- Lee, C.H.; Jahng, T.A.; Hyun, S.J.; Kim, C.H.; Park, S.-B.; Kim, K.-J.; Chung, C.K.; Kim, H.-J.; Lee, S.-E. Dynamic stabilization using the Dynesys system versus posterior lumbar interbody fusion for the treatment of degenerative lumbar spinal disease: A clinical and radiological outcomes-based meta-analysis. Neurosurg. Focus 2016, 40, E7. [Google Scholar] [CrossRef]
- Nockels, R.P. Dynamic Stabilization in the Surgical Management of Painful Lumbar Spinal Disorders. Spine 2005, 30, S68–S72. [Google Scholar] [CrossRef]
- Schnake, K.J.; Schaeren, S.; Jeanneret, B. Dynamic Stabilization in Addition to Decompression for Lumbar Spinal Stenosis with Degenerative Spondylolisthesis. Spine 2006, 31, 442–449. [Google Scholar] [CrossRef]
- Karadag, M.K.; Akgun, M.Y.; Basak, A.T.; Ates, O.; Tepebasili, M.A.; Gunerbuyuk, C.; Oktenoglu, T.; Sasani, M.; Ozer, A.F. Clinical and radiological analysis of the effects of three different lumbar transpedicular dynamic stabilization system on disc degeneration and regeneration. Front. Surg. 2023, 10, 1297790. [Google Scholar] [CrossRef] [PubMed]
- Gunerbuyuk, C.; Akgun, M.Y.; Ozer, A.F. Is The Modular Dynamic System as Effective as Classical Dynamic Systems in Long Segment Dynamic Thoracolumbar Stabilization? Turk. Neurosurg. 2024, 34, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Ozer, A.F.; Akgun, M.Y.; Ucar, E.A.; Hekimoglu, M.; Basak, A.T.; Gunerbuyuk, C.; Toklu, S.; Oktenoglu, T.; Sasani, M.; Akgul, T.; et al. Can Dynamic Spinal Stabilization Be an Alternative to Fusion Surgery in Adult Spinal Deformity Cases? Int. J. Spine Surg. 2024, 18, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.L. Fundamentals of Implant Dentistry: Prosthodontic Principles. Beumer J III, Faulkner RF, Shah KC, Moy PK. Hanover Park, Ill: Quintessence Publishing, 2015. J. Oral Implantol. 2015, 41, 343. [Google Scholar] [CrossRef]
- Rowan, M.; Lee, D.; Pi-Anfruns, J.; Shiffler, P.; Aghaloo, T.; Moy, P.K. Mechanical Versus Biological Stability of Immediate and Delayed Implant Placement Using Resonance Frequency Analysis. J. Oral Maxillofac. Surg. 2015, 73, 253–257. [Google Scholar] [CrossRef]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef]
- Hekimoglu, M.; Akgun, M.Y.; Ozer, H.; Basak, A.T.; Ucar, E.A.; Oktenoglu, T.; Ates, O.; Ozer, A.F. Two-Stage Lumbar Dynamic Stabilization Surgery: A Comprehensive Analysis of Screw Loosening Rates and Functional Outcomes Compared to Single-Stage Approach in Osteopenic and Osteoporotic Patients. Diagnostics 2024, 14, 1505. [Google Scholar] [CrossRef]
- Tan, L.-X.; Du, X.-K.; Tang, R.-M.; Rong, L.-M.; Zhang, L.-M. Effect of spinal-pelvic sagittal balance on the clinical outcomes after lumbar fusion surgery. BMC Surg. 2023, 23, 334. [Google Scholar] [CrossRef] [PubMed]
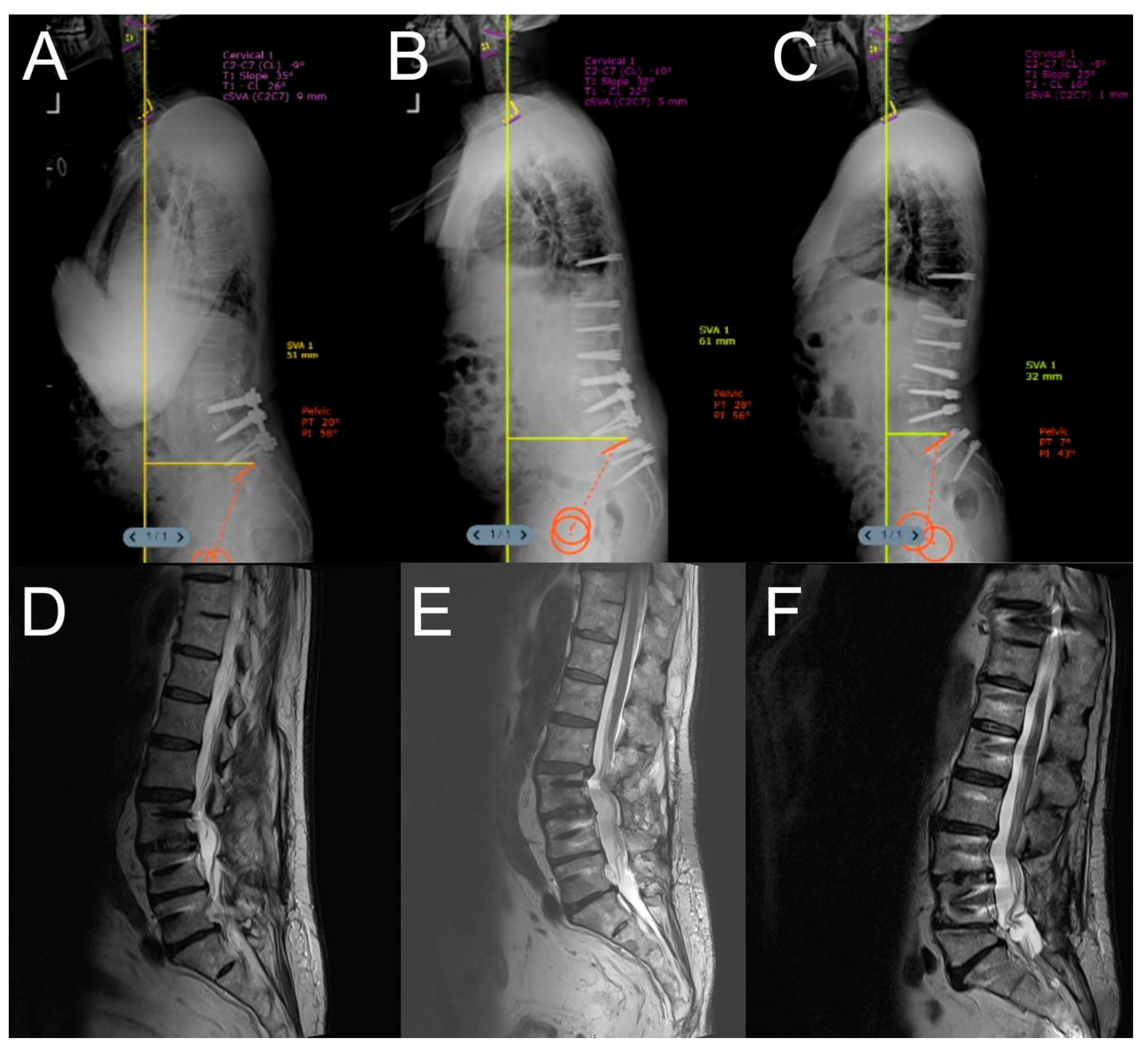
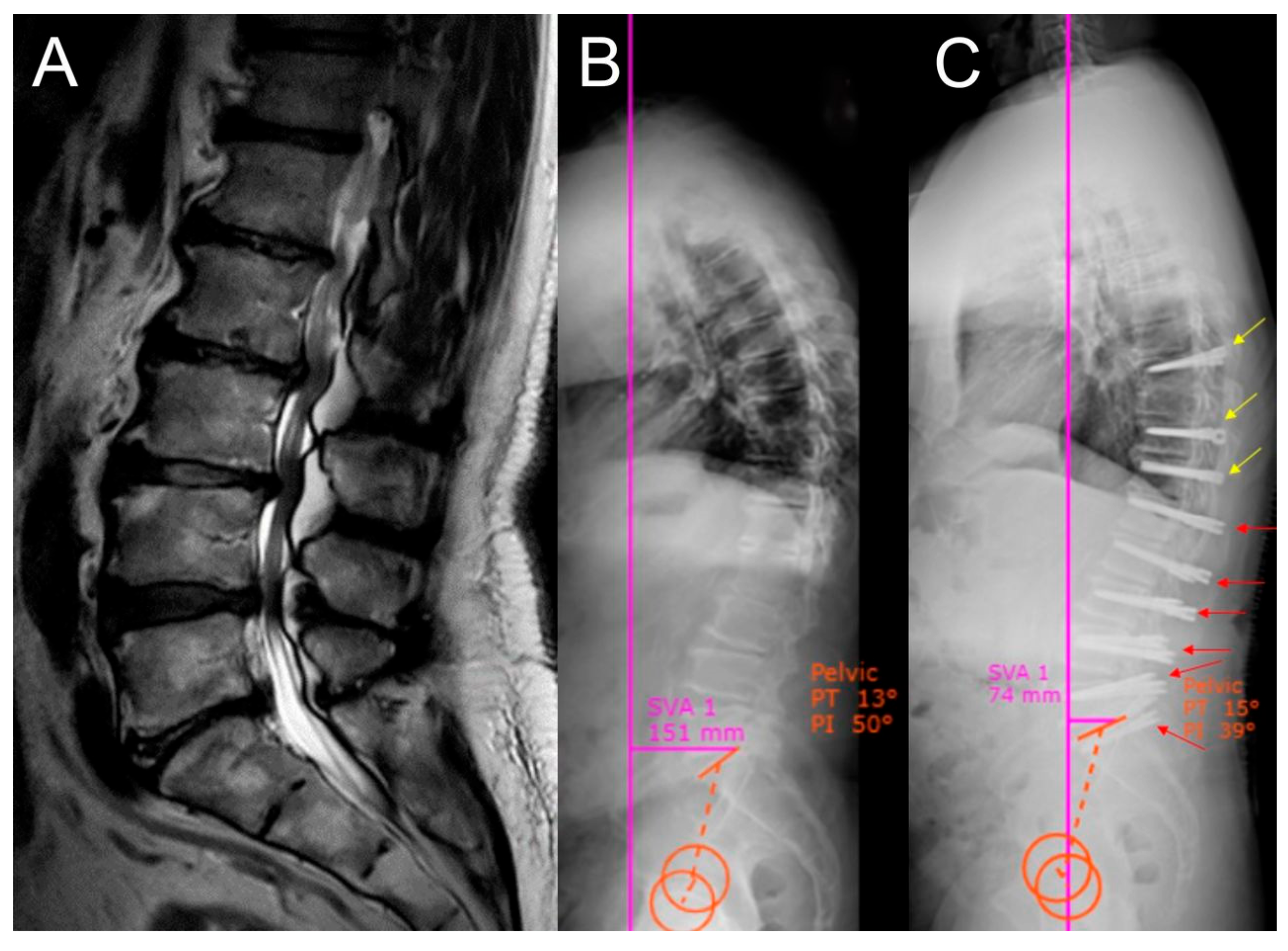
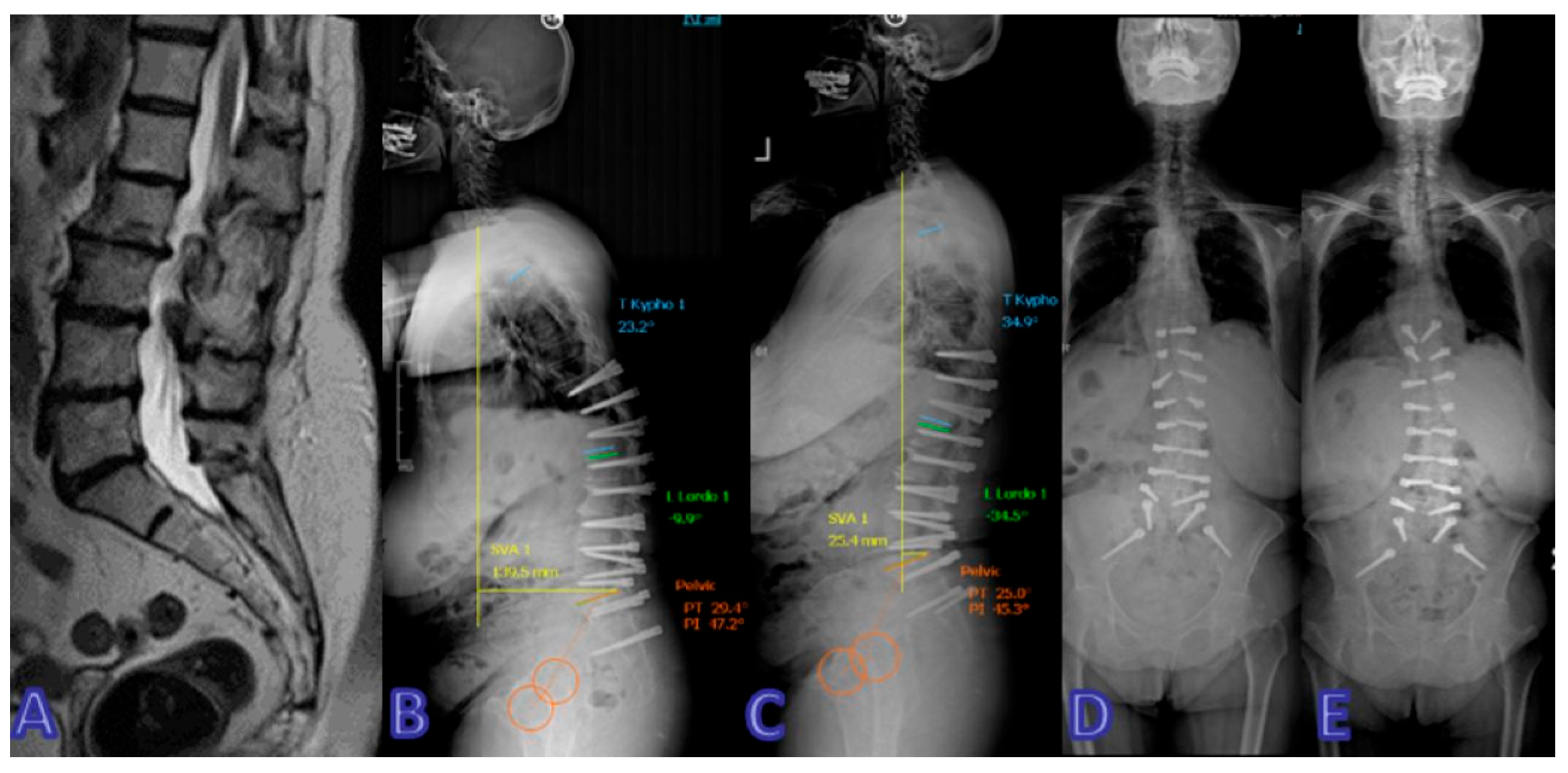
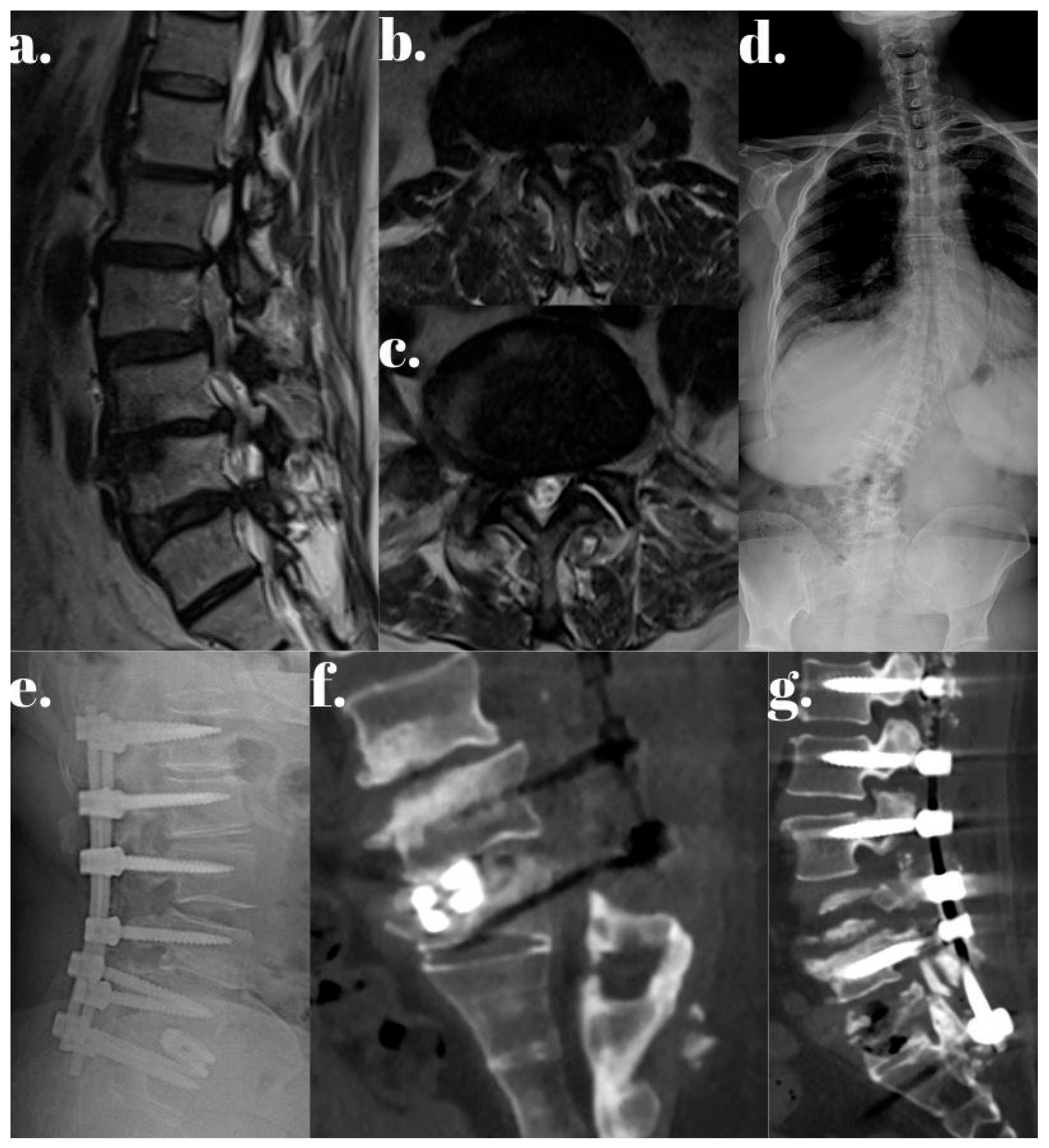

| Dynamic Stabilization Group (50.94%) | Rigid Stabilization Group (49.05%) | p-Value | |
|---|---|---|---|
| Gender (male/female) | 19/8 | 12/14 | |
| Age (years) | 64.26 ± 15.48 | 60.24 ± 15.52 | 0.367 |
| Follow up (months) | 30.25 | 43.41 | <0.05 |
| Surgical levels | |||
| 7 | 7 | 6 | |
| 8 | 6 | 7 | |
| 9 | 6 | 6 | |
| 10 | 8 | 7 |
| Time Point | Dynesys Group (Mean ± SD) | Rigid Stabilization Group (Mean ± SD) | p-Value |
|---|---|---|---|
| Preoperative | 7.4 ± 1.2 | 7.5 ± 1.3 | 0.772 |
| 3 Months Postop | 3.1 ± 2.0 | 3.0 ± 1.9 | 0.853 |
| 6 Months Postop | 2.6 ± 1.8 | 2.7 ± 1.7 | 0.836 |
| 12 Months Postop | 2.2 ± 1.5 | 2.4 ± 1.6 | 0.641 |
| 24 Months Postop | 1.9 ± 1.4 | 2.1 ± 1.5 | 0.618 |
| Time Point | Dynesys Group (Mean ± SD) | Rigid Stabilization Group (Mean ± SD) | p-Value |
|---|---|---|---|
| Preoperative | 45 ± 10 | 46 ± 11 | 0.731 |
| 3 Months Postop | 25 ± 15 | 26 ± 14 | 0.803 |
| 6 Months Postop | 22 ± 14 | 23 ± 13 | 0.788 |
| 12 Months Postop | 18 ± 12 | 19 ± 12 | 0.763 |
| 24 Months Postop | 16 ± 11 | 17 ± 12 | 0.753 |
| Parameter | Dynesys Group (n = 27) | Rigid Stabilization Group (n = 26) | p-Value |
|---|---|---|---|
| Total Surgery Time (min) | 180 ± 35 (combined stages) | 230 ± 40 | 0.002 |
| Blood Loss (mL) | 350 ± 80 (combined) | 520 ± 110 | 0.001 |
| Hospital Stay (days) | 5.8 ± 1.2 (cumulative) | 7.3 ± 1.5 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunerbuyuk, C.; Akgun, M.Y.; Durmus, N.; Ucar, E.A.; Orak, H.I.; Oktenoglu, T.; Ates, O.; Akgul, T.; Ozer, A.f. Dynamic vs. Rigid: Transforming the Treatment Landscape for Multisegmental Lumbar Degeneration. J. Clin. Med. 2025, 14, 5472. https://doi.org/10.3390/jcm14155472
Gunerbuyuk C, Akgun MY, Durmus N, Ucar EA, Orak HI, Oktenoglu T, Ates O, Akgul T, Ozer Af. Dynamic vs. Rigid: Transforming the Treatment Landscape for Multisegmental Lumbar Degeneration. Journal of Clinical Medicine. 2025; 14(15):5472. https://doi.org/10.3390/jcm14155472
Chicago/Turabian StyleGunerbuyuk, Caner, Mehmet Yigit Akgun, Nazenin Durmus, Ege Anil Ucar, Helin Ilkay Orak, Tunc Oktenoglu, Ozkan Ates, Turgut Akgul, and Ali fahir Ozer. 2025. "Dynamic vs. Rigid: Transforming the Treatment Landscape for Multisegmental Lumbar Degeneration" Journal of Clinical Medicine 14, no. 15: 5472. https://doi.org/10.3390/jcm14155472
APA StyleGunerbuyuk, C., Akgun, M. Y., Durmus, N., Ucar, E. A., Orak, H. I., Oktenoglu, T., Ates, O., Akgul, T., & Ozer, A. f. (2025). Dynamic vs. Rigid: Transforming the Treatment Landscape for Multisegmental Lumbar Degeneration. Journal of Clinical Medicine, 14(15), 5472. https://doi.org/10.3390/jcm14155472







