Putting DOAC Doubts to Bed(Side): Preliminary Evidence of Comparable Functional Outcomes in Anticoagulated and Non-Anticoagulated Stroke Patients Using Point-of-Care ClotPro® Testing
Abstract
1. Introduction
1.1. The Challenge of Assessing DOAC Activity in Acute Stroke
1.2. The Role of Point-of-Care-Testing
2. Materials and Methods
2.1. Study Design and Setting
2.2. Point-of-Care-Testing for Anticoagulation Verification
2.3. Outcome Measures
2.4. Statistical Analysis
2.5. Ethics Approval
3. Results
3.1. Demographic and Clinical Characteristics
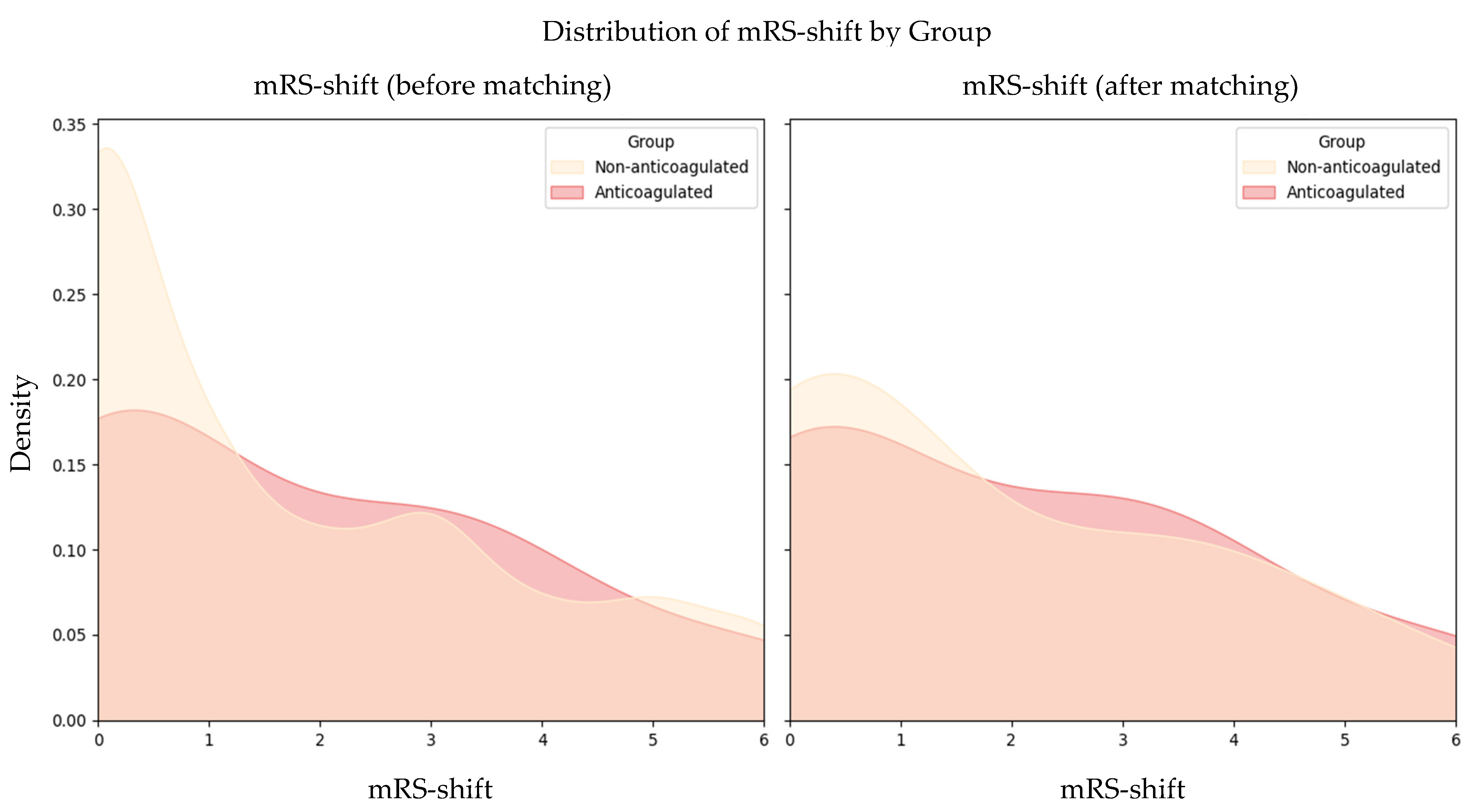
3.2. Functional Outcomes
3.3. Ordinary Least Squares Regression Analysis and Functional Outcome Prediction
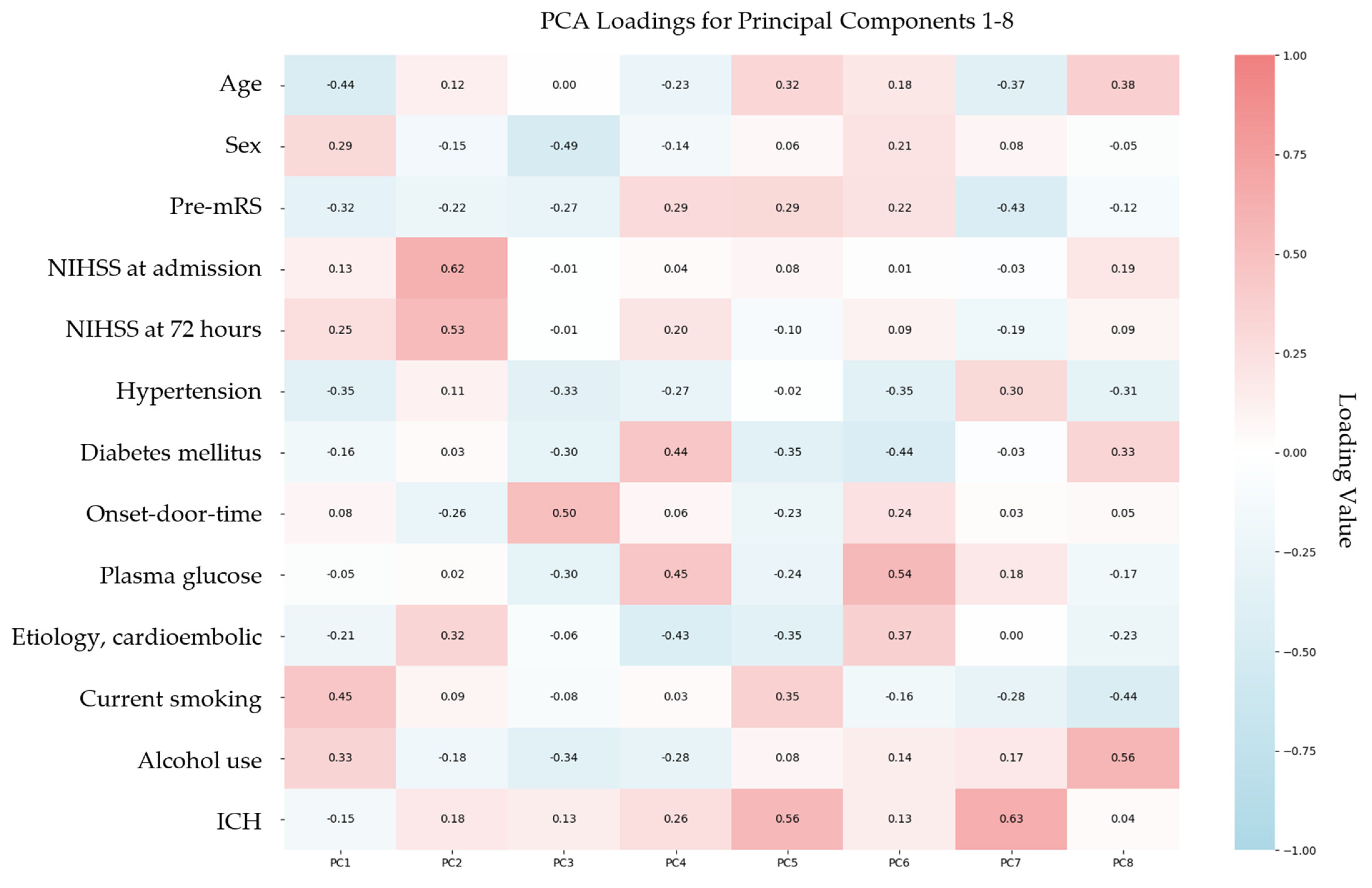
3.4. Principal Component Analysis and Functional Outcome Prediction
3.5. Regression Analysis Using Principal Component Analysis-Derived Components
4. Discussion
4.1. Interpretation of Findings
4.2. Mechanisms Underlying Observed Differences
4.3. Comparisons with Previous Research
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DOAC | direct oral anticoagulant |
| VKA | vitamin K antagonist |
| POCT | point-of-care testing |
| ECT | ecarin clotting time |
| RVV | Russell’s viper venom |
| mRS | modified Rankin Scale |
| NIHSS | National Institutes of Health Stroke Scale |
| OLS | ordinary least squares |
| PCA | principal component analysis |
| INR | international normalized ratio |
| TT | thrombin time |
| aPTT | activated partial thromboplastin time |
| PT | prothrombin time |
| TINL | Transzlációs Idegtudományi Nemzeti Laboratórium |
| CT | clotting time |
| SD | standard deviation |
| IQR | interquartile range |
| SMD | standardized mean difference |
| CI | confidence interval |
| IVT | intravenous thrombolysis |
| MT | mechanical thrombectomy |
| PC | principal component |
| ESOC | European Stroke Organisation Conference |
References
- Murray, C.J.L. Findings from the Global Burden of Disease Study 2021. Lancet 2024, 403, 2259–2262. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the Efficacy and Safety of New Oral Anticoagulants with Warfarin in Patients with Atrial Fibrillation: A Meta-Analysis of Randomised Trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Gue, Y.; Zhang, J.; Chao, T.-F.; Calkins, H.; Potpara, T. Stroke Prevention in Atrial Fibrillation. Trends Cardiovasc. Med. 2022, 32, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Georg Haeusler, K.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K An-tagonist Oral Anticoagulants in Patients with Atrial Fibrillation: Executive Summary. EP Eur. 2018, 20, 1231–1242. [Google Scholar] [CrossRef]
- Douxfils, J.; Adcock, D.M.; Bates, S.M.; Favaloro, E.J.; Gouin-Thibault, I.; Guillermo, C.; Kawai, Y.; Lindhoff-Last, E.; Kitchen, S.; Gosselin, R.C. 2021 Update of the International Council for Standardization in Haematology Recommendations for La-boratory Measurement of Direct Oral Anticoagulants. Thromb. Haemost. 2021, 121, 1008–1020. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Jackevicius, C.A.; Tsadok, M.A.; Essebag, V.; Atzema, C.; Eisenberg, M.J.; Tu, J.V.; Lu, L.; Rahme, E.; Ho, P.M.; Turakhia, M.; et al. Early Non-Persistence with Dabigatran and Rivaroxaban in Patients with Atrial Fibrillation. Heart 2017, 103, 1331–1338. [Google Scholar] [CrossRef]
- Antovic, J.P.; Skeppholm, M.; Eintrei, J.; Boija, E.E.; Söderblom, L.; Norberg, E.-M.; Onelöv, L.; Rönquist-Nii, Y.; Pohanka, A.; Beck, O.; et al. Evaluation of Coagulation Assays versus LC-MS/MS for Determinations of Dabigatran Concentrations in Plasma. Eur. J. Clin. Pharmacol. 2013, 69, 1875–1881. [Google Scholar] [CrossRef]
- Hawes, E.M.; Deal, A.M.; Funk-Adcock, D.; Gosselin, R.; Jeanneret, C.; Cook, A.M.; Taylor, J.M.; Whinna, H.C.; Winkler, A.M.; Moll, S. Performance of Coagulation Tests in Patients on Therapeutic Doses of Dabigatran: A Cross-sectional Phar-macodynamic Study Based on Peak and Trough Plasma Levels. J. Thromb. Haemost. 2013, 11, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, S.; Tafur, A.; Grant, R.; Esmon, N.; Mauer, K.; Marlar, R.A. Comparison of Methods to Determine Rivaroxaban Anti-Factor Xa Activity. Thromb. Res. 2015, 135, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Skeppholm, M.; Al-Aieshy, F.; Berndtsson, M.; Al-Khalili, F.; Rönquist-Nii, Y.; Söderblom, L.; Östlund, A.Y.; Pohanka, A.; Antovic, J.; Malmström, R.E. Clinical Evaluation of Laboratory Methods to Monitor Apixaban Treatment in Patients with Atrial Fibrillation. Thromb. Res. 2015, 136, 148–153. [Google Scholar] [CrossRef]
- Samama, M.M.; Mendell, J.; Guinet, C.; Le Flem, L.; Kunitada, S. In Vitro Study of the Anticoagulant Effects of Edoxaban and Its Effect on Thrombin Generation in Comparison to Fondaparinux. Thromb. Res. 2012, 129, e77–e82. [Google Scholar] [CrossRef]
- Chin, P.K.L.; Patterson, D.M.; Zhang, M.; Jensen, B.P.; Wright, D.F.B.; Barclay, M.L.; Begg, E.J. Coagulation Assays and Plasma Fibrinogen Concentrations in Real-world Patients with Atrial Fibrillation Treated with Dabigatran. Br. J. Clin. Pharmacol. 2014, 78, 630–638. [Google Scholar] [CrossRef]
- Dager, W.E.; Gosselin, R.C.; Kitchen, S.; Dwyre, D. Dabigatran Effects on the International Normalized Ratio, Activated Partial Thromboplastin Time, Thrombin Time, and Fibrinogen: A Multicenter, In Vitro Study. Ann. Pharmacother. 2012, 46, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Barrett, Y.C.; Wang, Z.; Frost, C.; Shenker, A. Clinical Laboratory Measurement of Direct Factor Xa Inhibitors: Anti-Xa Assay Is Preferable to Prothrombin Time Assay. Thromb. Haemost. 2010, 104, 1263–1271. [Google Scholar] [CrossRef]
- Morishima, Y.; Kamisato, C. Laboratory Measurements of the Oral Direct Factor Xa Inhibitor Edoxaban. Am. J. Clin. Pathol. 2015, 143, 241–247. [Google Scholar] [CrossRef]
- Shaw, J.R.; Li, N.; Nixon, J.; Moffat, K.A.; Spyropoulos, A.C.; Schulman, S.; Douketis, J.D. Coagulation Assays and Direct Oral Anticoagulant Levels among Patients Having an Elective Surgery or Procedure. J. Thromb. Haemost. 2022, 20, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Davenport, R.; Manson, J.; De’Ath, H.; Platton, S.; Coates, A.; Allard, S.; Hart, D.; Pearse, R.; Pasi, K.J.; MacCallum, P.; et al. Functional Definition and Characterization of Acute Traumatic Coagulopathy. Crit. Care Med. 2011, 39, 2652–2658. [Google Scholar] [CrossRef]
- Merrelaar, A.E.; Bögl, M.S.; Buchtele, N.; Merrelaar, M.; Herkner, H.; Schoergenhofer, C.; Harenberg, J.; Douxfils, J.; Siriez, R.; Jilma, B.; et al. Performance of a Qualitative Point-of-Care Strip Test to Detect DOAC Exposure at the Emergency Department: A Cohort-Type Cross-Sectional Diagnostic Accuracy Study. Thromb. Haemost. 2022, 122, 1723–1731. [Google Scholar] [CrossRef]
- Harenberg, J.; Beyer-Westendorf, J.; Crowther, M.; Douxfils, J.; Elalamy, I.; Verhamme, P.; Bauersachs, R.; Hetjens, S.; Weiss, C. Accuracy of a Rapid Diagnostic Test for the Presence of Direct Oral Factor Xa or Thrombin Inhibitors in Urine—A Multi-center Trial. Thromb. Haemost. 2020, 120, 132–140. [Google Scholar] [CrossRef]
- Margetić, S.; Ćelap, I.; Huzjan, A.L.; Puretić, M.B.; Goreta, S.Š.; Glojnarić, A.Č.; Brkljačić, D.D.; Mioč, P.; Harenberg, J.; Het-jens, S.; et al. DOAC Dipstick Testing Can Reliably Exclude the Presence of Clinically Relevant DOAC Concentrations in Circulation. Thromb. Haemost. 2022, 122, 1542–1548. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Lippi, G. The Pointy End of Point-of-Care Testing for Direct Oral Anticoagulants. Thromb. Haemost. 2020, 120, 011–013. [Google Scholar] [CrossRef] [PubMed]
- Seyve, L.; Richarme, C.; Polack, B.; Marlu, R. Impact of Four Direct Oral Anticoagulants on Rotational Thromboelastometry (ROTEM). Int. J. Lab. Hematol. 2018, 40, 84–93. [Google Scholar] [CrossRef]
- Solbeck, S.; Jensen, A.S.; Maschmann, C.; Stensballe, J.; Ostrowski, S.R.; Johansson, P.I. The Anticoagulant Effect of Thera-peutic Levels of Dabigatran in Atrial Fibrillation Evaluated by Thrombelastography (TEG®), Hemoclot Thrombin Inhibitor (HTI) Assay and Ecarin Clotting Time (ECT). Scand. J. Clin. Lab. Investig. 2018, 78, 25–30. [Google Scholar] [CrossRef]
- Langanay, L.; Gonzalez Sanchez, R.; Hamroun, A.; Dauchet, L.; Amouyel, P.; Dallongeville, J.; Meirhaeghe, A.; Gauthier, V. Ischemic Stroke Subtypes: Risk Factors, Treatments, and 1-Month Prognosis—The Lille, France Stroke Registry. J. Stroke Cerebrovasc. Dis. 2024, 33, 107761. [Google Scholar] [CrossRef]
- Tu, H.T.H.; Campbell, B.C.V.; Christensen, S.; Desmond, P.M.; De Silva, D.A.; Parsons, M.W.; Churilov, L.; Lansberg, M.G.; Mlynash, M.; Olivot, J.-M.; et al. Worse Stroke Outcome in Atrial Fibrillation Is Explained by More Severe Hypoperfusion, Infarct Growth, and Hemorrhagic Transformation. Int. J. Stroke 2015, 10, 534–540. [Google Scholar] [CrossRef]
- Kim, Y.D.; Hong, H.J.; Cha, M.J.; Nam, C.M.; Nam, H.S.; Heo, J.H. Determinants of Infarction Patterns in Cardioembolic Stroke. Eur. Neurol. 2011, 66, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Seiffge, D.J.; Hooff, R.-J.; Nolte, C.H.; Béjot, Y.; Turc, G.; Ikenberg, B.; Berge, E.; Persike, M.; Dequatre-Ponchelle, N.; Strbian, D.; et al. Recanalization Therapies in Acute Ischemic Stroke Patients. Circulation 2015, 132, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Küpper, C.; Feil, K.; Wollenweber, F.A.; Tiedt, S.; Herzberg, M.; Dorn, F.; Liebig, T.; Dieterich, M.; Kellert, L. Endovascular Stroke Treatment in Orally Anticoagulated Patients: An Analysis from the German Stroke Registry-Endovascular Treatment. J. Neurol. 2021, 268, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Groene, P.; Wagner, D.; Kammerer, T.; Kellert, L.; Giebl, A.; Massberg, S.; Schäfer, S.T. Viscoelastometry for Detecting Oral Anticoagulants. Thromb. J. 2021, 19, 18. [Google Scholar] [CrossRef]
- Yoshii, R.; Sawa, T.; Kawajiri, H.; Amaya, F.; Tanaka, K.A.; Ogawa, S. A Comparison of the ClotPro System with Rotational Thromboelastometry in Cardiac Surgery: A Prospective Observational Study. Sci. Rep. 2022, 12, 17269. [Google Scholar] [CrossRef] [PubMed]
- Infanger, L.; Dibiasi, C.; Schaden, E.; Ulbing, S.; Wiegele, M.; Lacom, C.; Gratz, J. Comparison of the New Viscoelastic Co-agulation Analyzer ClotPro® with ROTEM® Delta and Conventional Coagulation Tests in Critically Ill Patients with COVID-19. Front. Med. 2021, 8, 777145. [Google Scholar] [CrossRef]
- Oberladstätter, D.; Voelckel, W.; Schlimp, C.; Zipperle, J.; Ziegler, B.; Grottke, O.; Schöchl, H. A Prospective Observational Study of the Rapid Detection of Clinically-relevant Plasma Direct Oral Anticoagulant Levels Following Acute Traumatic In-jury. Anaesthesia 2021, 76, 373–380. [Google Scholar] [CrossRef]
- Sedghi, A.; Heubner, L.; Klimova, A.; Tiebel, O.; Pietsch, J.; Mirus, M.; Barlinn, K.; Minx, T.; Beyer-Westendorf, J.; Puetz, V.; et al. Point-of-Care Assessment of Direct Oral Anticoagulation in Acute Ischemic Stroke: Protocol for a Prospective Observa-tional Diagnostic Accuracy Study. Thromb. Haemost. 2022, 122, 1954–1962. [Google Scholar] [CrossRef]
- Sedghi, A.; Meyer-Bender, E.; Heubner, L.; Pietsch, J.; Tiebel, O.; Barlinn, K.; Beyer-Westendorf, J.; Puetz, V.; Spieth, P.; Siepmann, T. Diagnostic Accuracy of Point-of-Care Viscoelastic Assessment of Direct Oral Anticoagulation in Acute Ischemic Stroke: Interim Results from a Prospective Validation Study. Eur. Stroke. J. 2025, 10 (Suppl. 2), 3–789. [Google Scholar] [CrossRef]
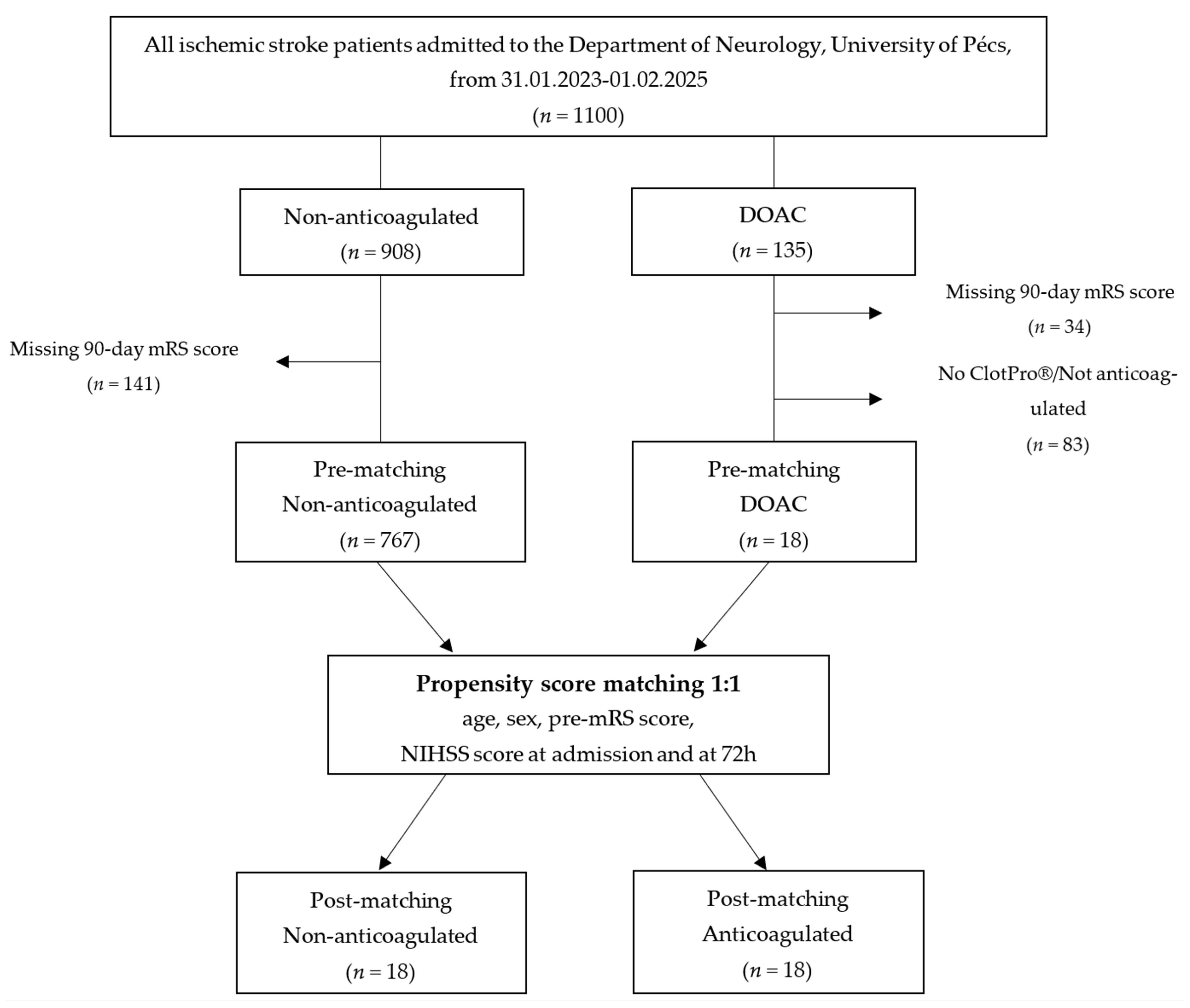
| Non-Anticoagulated (n = 767) | DOAC (n = 19) | p-Value | Matched Non-Anticoagulated (n = 18) | Matched DOAC (n = 18) | p-Value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (years), mean ± SD | 69.40 ± 12.51 | 79.74 ± 9.40 | <0.001 * | 78.00 ± 8.17 | 79.72 ± 9.67 | 0.568 |
| Sex, male, n (%) | 378.0 (49.3%) | 11.0 (57.9%) | 0.494 | 10 (55.6%) | 11 (61.1%) | 1.000 |
| Clinical Characteristics | ||||||
| Pre-stroke mRS score, mean ± SD | 0.61 ± 1.15 | 1.21 ± 1.27 | 0.057 | 1.11 ± 1.23 | 1.28 ± 1.27 | 0.692 |
| NIHSS score at admission, mean ± SD | 6.83 ± 6.17 | 7.11 ± 6.24 | 0.851 | 7.06 ± 6.31 | 7.28 ± 6.37 | 0.917 |
| NIHSS score at 72 h, mean ± SD | 5.44 ± 8.17 | 5.37 ± 9.77 | 0.975 | 5.06 ± 9.67 | 5.61 ± 9.99 | 0.866 |
| ICH, n (%) | 37 (4.8%) | 0 (0.0%) | 0.326 | 1 (5.6%) | 0 (0.0%) | 0.310 |
| Etiology, cardioembolic, n (%) | 206 (26.9%) | 12 (63.2%) | <0.001 * | 4 (22.2%) | 12 (66.7%) | 0.007 * |
| Onset-to-door time (min), median [IQR] | 310 (102–832) | 105 (68–232) | <0.001 * | 408 (130–584) | 110 (72–221) | <0.001 * |
| Plasma glucose (mmol/L), mean ± SD | 7.74 ± 2.99 | 7.14 ± 2.21 | 0.381 | 7.41 ± 162 | 7.23 ± 2.23 | 0.993 |
| Medical history, n (%) | ||||||
| Hypertension | 612 (79.8%) | 19 (100%) | 0.020 * | 14 (77.8%) | 18 (100.0%) | 0.104 |
| Diabetes mellitus | 258 (33.6%) | 11 (57.9%) | 0.047 * | 3 (16.7%) | 10 (55.6%) | 0.035 * |
| Current smoking | 245 (31.9%) | 0 (0.0%) | 0.002 * | 3 (16.7%) | 0 (0.0%) | 0.229 |
| Alcohol use | 332 (43.3%) | 9 (47.4%) | 0.816 | 13 (72.2%) | 9 (50.0%) | 0.305 |
| Recanalization therapy, n (%) | ||||||
| IVT | 214 (27.9%) | 0 (0.0%) | 0.007 * | 6 (33.3%) | 0 (0.0%) | 0.007 * |
| MT | 132 (17.2%) | 3 (15.8%) | 0.870 | 3 (16.7%) | 3 (16.7%) | 1.000 |
| IVT + MT | 65 (8.5%) | 0 (0.0%) | 0.186 | 0 (0.0%) | 0 (0.0%) | 1.000 |
| SMD Before Matching | SMD After Matching | |
|---|---|---|
| Age | 0.935 | 0.192 |
| Sex | 0.171 | 0.110 |
| Pre-stroke mRS score | 0.493 | 0.133 |
| NIHSS score at admission | 0.044 | 0.035 |
| NIHSS score at 72 h | 0.008 | 0.056 |
| Predictor | Coefficient | p-Value | 95% CI |
|---|---|---|---|
| Anticoagulation status | 0.4905 | 0.599 | −1.433 to 2.414 |
| Age | 0.1188 | 0.015 * | 0.026 to 0.211 |
| Sex | −0.4080 | 0.629 | −2.154 to 1.338 |
| Pre-stroke mRS score | −0.1236 | 0.707 | −0.804 to 0.557 |
| NIHSS score at admission | −0.0083 | 0.942 | −0.245 to 0.229 |
| NIHSS score at 72 h | 0.1930 | 0.004 * | 0.070 to 0.316 |
| ICH | −1.7137 | 0.439 | −6.267 to 2.839 |
| Etiology, cardioembolic | −1.1696 | 0.289 | −3.419 to 1.080 |
| Onset-to-door time | 0.0004 | 0.583 | −0.001 to 0.002 |
| Plasma glucose | 0.0944 | 0.682 | −0.381 to 0.570 |
| Hypertension | 0.6411 | 0.624 | −2.059 to 3.341 |
| Diabetes mellitus | −0.7698 | 0.423 | −2.740 to 1.201 |
| Current smoking | −0.6960 | 0.599 | −3.427 to 2.035 |
| Alcohol use | −0.0914 | 0.915 | −1.872 to 1.689 |
| IVT | 0.3097 | 0.826 | −2.601 to 3.220 |
| MT | 0.6762 | 0.449 | −1.161 to 2.513 |
| Principal Component | Explained Variance (%) | Cumulative Variance (%) |
|---|---|---|
| PC1 | 18.27 | 18.27 |
| PC2 | 16.64 | 34.90 |
| PC3 | 14.35 | 49.26 |
| PC4 | 11.52 | 60.78 |
| PC5 | 9.89 | 70.67 |
| PC6 | 8.25 | 78.93 |
| PC7 | 5.98 | 84.90 |
| PC8 | 5.02 | 89.92 |
| Predictor | Coefficient | p-Value | 95% CI |
|---|---|---|---|
| Anticoagulation status | −0.3264 | 0.703 | −2.076 to 1.423 |
| PC1 | −0.0392 | 0.841 | −0.439 to 0.360 |
| PC2 | 0.9276 | <0.001 * | 0.495 to 1.360 |
| PC3 | 0.1233 | 0.561 | −0.309 to 0.556 |
| PC4 | 0.2432 | 0.269 | −0.201 to 0.688 |
| PC5 | 0.0934 | 0.708 | −0.415 to 0.602 |
| PC6 | 0.1789 | 0.517 | −0.384 to 0.742 |
| PC7 | −0.5224 | 0.106 | −1.166 to 0.121 |
| PC8 | 0.5545 | 0.100 | −0.114 to 1.223 |
| IVT | −1.1450 | 0.306 | −3.407 to 1.117 |
| MT | −0.4734 | 0.540 | −2.046 to 1.099 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seetge, J.; Cséke, B.; Karádi, Z.N.; Bosnyák, E.; Jozifek, E.J.; Szapáry, L. Putting DOAC Doubts to Bed(Side): Preliminary Evidence of Comparable Functional Outcomes in Anticoagulated and Non-Anticoagulated Stroke Patients Using Point-of-Care ClotPro® Testing. J. Clin. Med. 2025, 14, 5476. https://doi.org/10.3390/jcm14155476
Seetge J, Cséke B, Karádi ZN, Bosnyák E, Jozifek EJ, Szapáry L. Putting DOAC Doubts to Bed(Side): Preliminary Evidence of Comparable Functional Outcomes in Anticoagulated and Non-Anticoagulated Stroke Patients Using Point-of-Care ClotPro® Testing. Journal of Clinical Medicine. 2025; 14(15):5476. https://doi.org/10.3390/jcm14155476
Chicago/Turabian StyleSeetge, Jessica, Balázs Cséke, Zsófia Nozomi Karádi, Edit Bosnyák, Eszter Johanna Jozifek, and László Szapáry. 2025. "Putting DOAC Doubts to Bed(Side): Preliminary Evidence of Comparable Functional Outcomes in Anticoagulated and Non-Anticoagulated Stroke Patients Using Point-of-Care ClotPro® Testing" Journal of Clinical Medicine 14, no. 15: 5476. https://doi.org/10.3390/jcm14155476
APA StyleSeetge, J., Cséke, B., Karádi, Z. N., Bosnyák, E., Jozifek, E. J., & Szapáry, L. (2025). Putting DOAC Doubts to Bed(Side): Preliminary Evidence of Comparable Functional Outcomes in Anticoagulated and Non-Anticoagulated Stroke Patients Using Point-of-Care ClotPro® Testing. Journal of Clinical Medicine, 14(15), 5476. https://doi.org/10.3390/jcm14155476






