Long-Term Follow-Up of Left Atrial Appendage Exclusion: Results of the V-CLIP Multi-Center Post-Market Study
Abstract
1. Introduction
2. Methods
2.1. Patient Eligibility
2.2. Study Design
2.3. Primary and Secondary Endpoints
2.4. Sample Size Calculation
2.5. Statistical Analysis
2.6. Role of the Sponsor and Funding Source
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackshear, J.L.; Odell, J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 1996, 61, 755–759. [Google Scholar] [CrossRef]
- Whitlock, R.P.; Belley-Cote, E.P.; Paparella, D.; Healey, J.S.; Brady, K.; Sharma, M.; Reents, W.; Budera, P.; Baddour, A.J.; Fila, P.; et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N. Engl. J. Med. 2021, 384, 2081–2091. [Google Scholar] [CrossRef]
- Writing Committee Members; Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2023, 83, 109–279. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Wai, J.W.W.; Lee, S.H.; Yii, M.; Chaiyaroj, S.; Ruaengsri, C.; Ramanathan, T.; Ishii, Y.; Jeong, D.S.; Chang, J.; et al. 2023 APHRS expert consensus statements on surgery for AF. J. Arrhythmia 2023, 39, 841–852. [Google Scholar] [CrossRef]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.; Shamloo, A.S.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. J. Interv. Card. Electrophysiol. 2024, 67, 921–1072. [Google Scholar] [CrossRef] [PubMed]
- von Ballmoos, M.C.W.; Hui, D.S.; Mehaffey, J.H.; Malaisrie, S.C.; Vardas, P.N.; Gillinov, A.M.; Sundt, T.M.; Badhwar, V. The Society of Thoracic Surgeons 2023 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann. Thorac. Surg. 2024, 118, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Güner, A.; Kalçık, M.; Gündüz, S.; Gürsoy, M.O.; Güner, E.G.; Ulutaş, A.E.; Kalkan, S.; Onan, B.; Bayam, E.; Ertük, M.; et al. The relationship between incomplete surgical obliteration of the left atrial appendage and thromboembolic events after mitral valve surgery (from the ISOLATE Registry). J. Thromb. Thrombolysis 2021, 51, 1078–1089. [Google Scholar] [CrossRef]
- Aryana, A.; Singh, S.K.; Singh, S.M.; O’nEill, P.G.; Bowers, M.R.; Allen, S.L.; Lewandowski, S.L.; Vierra, E.C.; D’aVila, A. Association between incomplete surgical ligation of left atrial appendage and stroke and systemic embolization. Heart Rhythm. 2015, 12, 1431–1437. [Google Scholar] [CrossRef]
- Healey, J.S.; Crystal, E.; Lamy, A.; Teoh, K.; Semelhago, L.; Hohnloser, S.H.; Cybulsky, I.; Abouzahr, L.; Sawchuck, C.; Carroll, S.; et al. Left Atrial Appendage Occlusion Study (LAAOS): Results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am. Heart J. 2005, 150, 288–293. [Google Scholar] [CrossRef]
- Lin, B.; Jaros, B.D.; Grossi, E.A.; Saric, M.; Garshick, M.S.; Donnino, R. Prevalence and Risk Factors of Incomplete Surgical Closure of the Left Atrial Appendage on Follow-up Transesophageal Echocardiogram. J. Atr. Fibrillation 2020, 13, 2357. [Google Scholar] [CrossRef]
- Cullen, M.W.; Stulak, J.M.; Li, Z.; Powell, B.D.; White, R.D.; Ammash, N.M.; Nkomo, V.T. Left Atrial Appendage Patency at Cardioversion After Surgical Left Atrial Appendage Intervention. Ann. Thorac. Surg. 2016, 101, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Kanderian, A.S.; Gillinov, A.M.; Pettersson, G.B.; Blackstone, E.; Klein, A.L. Success of surgical left atrial appendage closure: Assessment by transesophageal echocardiography. J. Am. Coll. Cardiol. 2008, 52, 924–929. [Google Scholar] [CrossRef]
- Katz, E.S.; Tsiamtsiouris, T.; Applebaum, R.M.; Schwartzbard, A.; Tunick, P.A.; Kronzon, I. Surgical left atrial appendage ligation is frequently incomplete: A transesophageal echocardiograhic study. J. Am. Coll. Cardiol. 2000, 36, 468–471. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, M.N.; Pérez-David, E.; Quiles, J.; Peralta, J.; García-Rojas, I.; Bermejo, J.; Moreno, M.; Silva, J. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: A transesophageal echocardiographic study. J. Am. Coll. Cardiol. 2003, 42, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Vassallo, P.; Kruse, J.; Malaisrie, S.C.; Rigolin, V.; Andrei, A.-C.; McCarthy, P. A randomized, prospective pilot comparison of 3 atrial appendage elimination techniques: Internal ligation, stapled excision, and surgical excision. J. Thorac. Cardiovasc. Surg. 2016, 152, 1075–1080. [Google Scholar] [CrossRef]
- Ailawadi, G.; Gerdisch, M.W.; Harvey, R.L.; Hooker, R.L.; Damiano, R.J.; Salamon, T.; Mack, M.J. Exclusion of the left atrial appendage with a novel device: Early results of a multicenter trial. J. Thorac. Cardiovasc. Surg. 2011, 142, 1002–1009.e1. [Google Scholar] [CrossRef]
- Toale, C.; Fitzmaurice, G.J.; Eaton, D.; Lyne, J.; Redmond, K.C. Outcomes of left atrial appendage occlusion using the AtriClip device: A systematic review. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 655–662. [Google Scholar] [CrossRef]
- Gerdisch, M.W.; Garrett, H.E.; Mumtaz, M.A.; Grehan, J.F.; Castillo-Sang, M.; Miller, J.S.; Zorn, G.L.; Gall, S.A.; Johnkoski, J.A.; Ramlawi, B. Prophylactic Left Atrial Appendage Exclusion in Cardiac Surgery Patients With Elevated CHA(2)DS(2)-VASc Score: Results of the Randomized ATLAS Trial. Innovations 2022, 17, 463–470. [Google Scholar] [CrossRef]
- Emmert, M.Y.; Puippe, G.; Baumüller, S.; Alkadhi, H.; Landmesser, U.; Plass, A.; Bettex, D.; Scherman, J.; Grünenfelder, J.; Genoni, M.; et al. Safe, effective and durable epicardial left atrial appendage clip occlusion in patients with atrial fibrillation undergoing cardiac surgery: First long-term results from a prospective device trial. Eur. J. Cardiothorac. Surg. 2014, 45, 126–131. [Google Scholar] [CrossRef]
- Salzberg, S.P.; Plass, A.; Emmert, M.Y.; Desbiolles, L.; Alkadhi, H.; Grünenfelder, J.; Genoni, M. Left atrial appendage clip occlusion: Early clinical results. J. Thorac. Cardiovasc. Surg. 2010, 139, 1269–1274. [Google Scholar] [CrossRef]
- Kiankhooy, A.; Liem, B.; Dunnington, G.H.; Pierce, C.; Eisenberg, S.J.; Burk, S.; Kaiser, D.W.; Lyons, T.; Huber, D. Left Atrial Appendage Ligation Using the AtriClip Device: Single-Center Study of Device Safety and Efficacy. Innovations 2022, 17, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Doshi, S.K.; Sadhu, A.; Horton, R.; Osorio, J.; Ellis, C.; Stone, J., Jr.; Shah, M.; Dukkipati, S.R.; Adler, S.; et al. Primary Outcome Evaluation of a Next-Generation Left Atrial Appendage Closure Device: Results From the PINNACLE FLX Trial. Circulation 2021, 143, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Kamohara, K.; Fukamachi, K.; Ootaki, Y.; Akiyama, M.; Cingoz, F.; Ootaki, C.; Vince, D.G.; Popović, Z.B.; Kopcak, M.W.; Dessoffy, R.; et al. Evaluation of a novel device for left atrial appendage exclusion: The second-generation atrial exclusion device. J. Thorac. Cardiovasc. Surg. 2006, 132, 340–346. [Google Scholar] [CrossRef]
- Kamohara, K.; Fukamachi, K.; Ootaki, Y.; Akiyama, M.; Zahr, F.; Kopcak, M.W.; Dessoffy, R.; Popović, Z.B.; Daimon, M.; Cosgrove, D.M.; et al. A novel device for left atrial appendage exclusion. J. Thorac. Cardiovasc. Surg. 2005, 130, 1639–1644. [Google Scholar] [CrossRef]
- Salzberg, S.P.; Gillinov, A.M.; Anyanwu, A.; Castillo, J.; Filsoufi, F.; Adams, D.H. Surgical left atrial appendage occlusion: Evaluation of a novel device with magnetic resonance imaging. Eur. J. Cardiothorac. Surg. 2008, 34, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Starck, C.T.; Steffel, J.; Emmert, M.Y.; Plass, A.; Mahapatra, S.; Falk, V.; Salzberg, S.P. Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 416–418. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Sanchez, J.; Mohanty, S.; Horton, R.; Gallinghouse, G.J.; Bailey, S.M.; Zagrodzky, J.D.; Santangeli, P.; et al. Left atrial appendage: An underrecognized trigger site of atrial fibrillation. Circulation 2010, 122, 109–118. [Google Scholar] [CrossRef]
- Caliskan, E.; Eberhard, M.; Falk, V.; Alkadhi, H.; Emmert, M.Y. Incidence and characteristics of left atrial appendage stumps after device-enabled epicardial closure. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 663–669. [Google Scholar] [CrossRef]
- Ellis, C.R.; Aznaurov, S.G.; Patel, N.J.; Williams, J.R.; Sandler, K.L.; Hoff, S.J.; Ball, S.K.; Whalen, S.P.; Carr, J.J. Angiographic efficacy of the AtriClip left atrial appendage exclusion device placed by minimally invasive thoracoscopic approach. JACC Clin. Electrophysiol. 2017, 3, 1356–1365. [Google Scholar] [CrossRef]
- Ahmed, A.; Pothineni, N.V.K.; Singh, V.; Bawa, D.; Darden, D.; Kabra, R.; Singh, A.; Memon, S.; Romeya, A.; Van Meeteren, J.; et al. Long-Term Imaging and Clinical Outcomes of Surgical Left Atrial Appendage Occlusion With AtriClip. Am. J. Cardiol. 2023, 201, 193–199. [Google Scholar] [CrossRef]
- Gaudino, M.; Benesch, C.; Bakaeen, F.; DeAnda, A.; Fremes, S.E.; Glance, L.; Messé, S.R.; Pandey, A.; Rong, L.Q.; On behalf of the American Heart Association Council on Cardiovascular Surgery; et al. Considerations for Reduction of Risk of Perioperative Stroke in Adult Patients Undergoing Cardiac and Thoracic Aortic Operations: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e193–e209. [Google Scholar] [CrossRef]
- Kim, K.M.; Arghami, A.; Habib, R.; Daneshmand, M.A.; Parsons, N.; Elhalabi, Z.; Krohn, C.; Thourani, V.; Bowdish, M.E. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2022 Update on Outcomes and Research. Ann. Thorac. Surg. 2023, 115, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Ellis, C.R.; Nielsen-Kudsk, J.E.; Thaler, D.; Gupta, N.; Koulogiannis, K.; Anderson, J.A.; Gage, R.; Lakkireddy, D. Peridevice Leak After Transcatheter Left Atrial Appendage Occlusion: An Analysis of the Amulet IDE Trial. JACC Cardiovasc. Interv. 2022, 15, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Dukkipati, S.R.; Holmes, D.R., Jr.; Doshi, S.K.; Kar, S.; Singh, S.M.; Gibson, D.; Price, M.J.; Natale, A.; Mansour, M.; Sievert, H.; et al. Impact of Peridevice Leak on 5-Year Outcomes After Left Atrial Appendage Closure. J. Am. Coll. Cardiol. 2022, 80, 469–483. [Google Scholar] [CrossRef]

| Characteristic | Result |
|---|---|
| Age, years | N = 155 |
| Mean | 65.8 |
| Median | 66.0 |
| Range | 41, 85 |
| Sex, % (n) | N = 155 |
| Male | 78.1 (121) |
| Female | 21.9 (34) |
| Race, % (n) | N = 155 |
| White | 94.2 (146) |
| Black | 3.2 (5) |
| Asian | 1.3 (2) |
| Other | 1.3 (2) |
| Ethnicity, % (n) | N = 155 |
| Not Hispanic/Latino | 99.4 (154) |
| Hispanic/Latino | 0.6 (1) |
| BMI, kg/m2 | N = 152 |
| Mean | 30.0 |
| Median | 29.0 |
| Range | 18.7, 51.4 |
| Atrial arrhythmia, % (n) | N = 155 |
| Paroxysmal AF | 22.6 (35) |
| Persistent AF | 16.8 (26) |
| Longstanding persistent AF | 11.6 (18) |
| Not reported 1 | 49 (76) |
| NYHA Classification, % (n) | N = 155 |
| I | 9.7 (15) |
| II | 20.0 (31) |
| III | 9.7 (15) |
| IV | 1.3 (2) |
| No heart failure | 12.9 (20) |
| Not assessed 1 | 46.5 (72) |
| CHA2DS2-VASc, % (n) | N = 155 |
| Mean | 2.7 |
| Median | 3 |
| 0 | 3.2 (5) |
| 1 | 18.1 (28) |
| 2 | 23.2 (36) |
| 3 | 27.1 (42) |
| 4 | 13.5 (21) |
| 5 | 6.5 (10) |
| 6 | 3.2 (5) |
| 7 | 0.6 (1) |
| Not done | 4.5 (7) |
| HAS-BLED, % (n) | N = 155 |
| Mean | 2.0 |
| Median | 2 |
| 0 | 6.5 (10) |
| 1 | 14.2 (22) |
| 2 | 25.8 (40) |
| 3 | 17.4 (27) |
| 4 | 6.5 (10) |
| 5 | 1.3 (2) |
| 6 | 0.0 (0) |
| 7 | 0.0 (0) |
| 8 | 0.0 (0) |
| 9 | 0.0 (0) |
| Not done | 28.4 (44) |
| Surgical Approach (N = 155) | % (n) |
|---|---|
| Sternotomy | 58.1 (90) |
| Minimally Invasive, Right Mini-Thoracotomy | 20.6 (32) |
| Minimally Invasive, Left-Sided Thoracoscopy | 21.3 (33) |
| Concomitant Surgical Procedure (N = 155) | % (n) |
|---|---|
| Coronary artery bypass graft | 35.5 (55) |
| AF ablation/surgery | 34.8 (54) |
| Mitral valve | 32.9 (51) |
| Aortic valve | 17.4 (27) |
| Tricuspid valve | 12.9 (20) |
| Convergent hybrid MAZE (various) | 11.0 (17) |
| MAZE (various) | 5.8 (9) |
| Patent foramen ovale closure | 4.5 (7) |
| Aortic surgery | 3.9 (6) |
| Atrial septal defect repair | 1.3 (2) |
| Aortic aneurysmectomy | 0.6 (1) |
| Excision of atrial myxoma | 0.6 (1) |
| Extended septal myectomy | 0.6 (1) |
| Extended septal myectomy, ascending aorta replacement | 0.6 (1) |
| Left atrial appendage closure ꭞ | 0.6 (1) |
| Partial atrial ventricular canal closure | 0.6 (1) |
| Pulmonary vein isolation | 0.6 (1) |
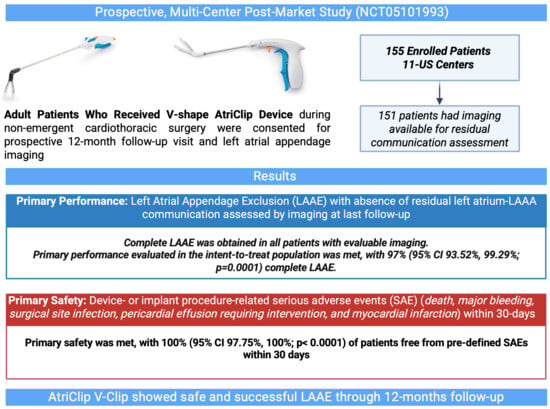
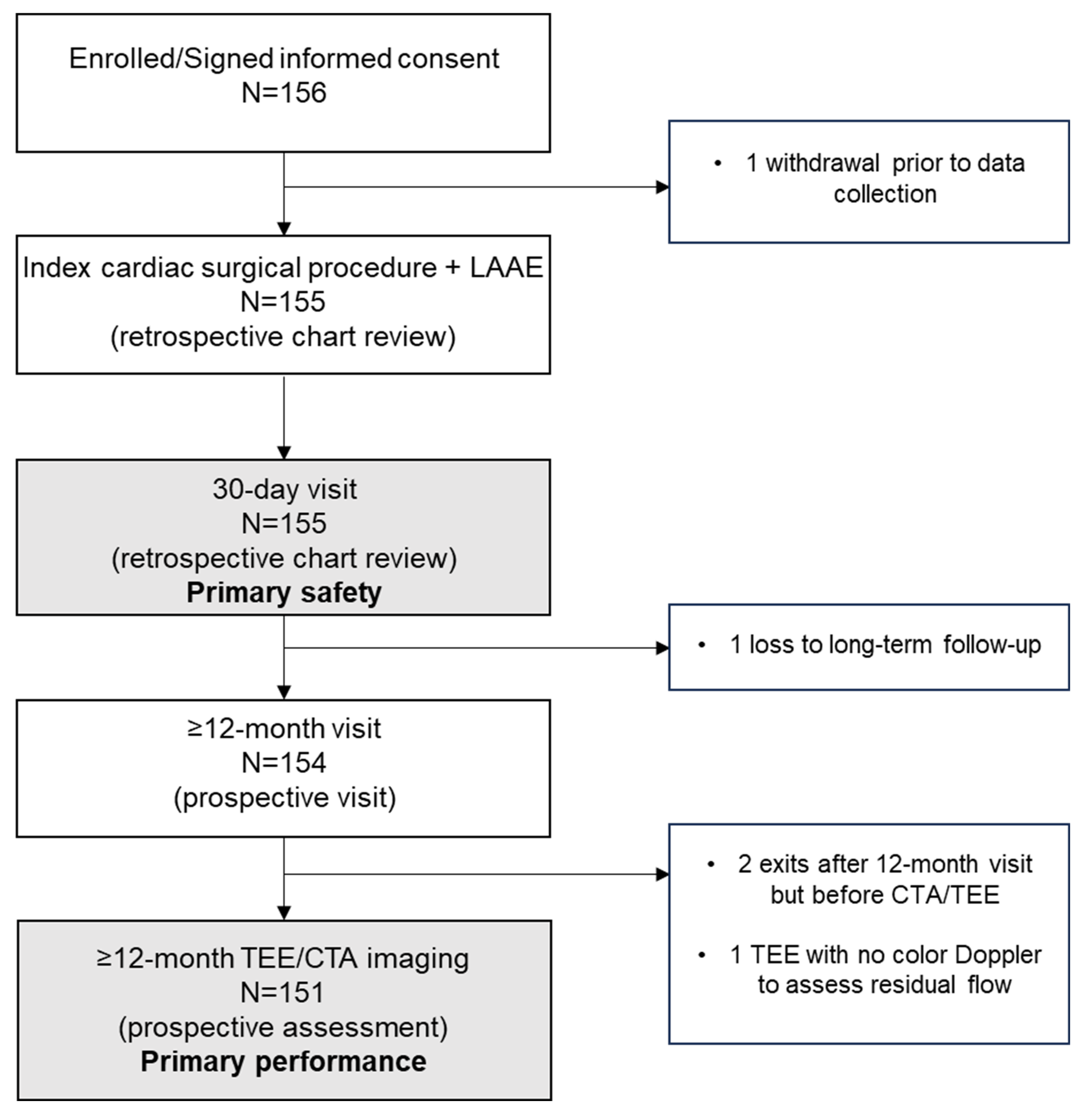
| Endpoint | % (95% Confidence Interval); n/N | p-Value a |
|---|---|---|
| Primary Safety: Freedom from primary SAE within 30 days of the implant procedure b | 100 (97.75, 100); 155/155 | <0.0001 |
| Primary Performance (ITT population): LAA exclusion with no residual communication between the LA and LAA c | 97.42 (93.52, 99.29); 151/155 | 0.0001 |
| Primary Performance (mITT population): LAA exclusion with no residual communication between the LA and LAA | 100 (97.59, 100); 151/151 | 0.0001 |
| Sub-Analysis | Performance Endpoint Rate, % (95% Confidence Interval); n/N | p-Value a |
|---|---|---|
| Open sternotomy | 100 (95.89, 100); 88/88 | 0.0001 |
| Minimally Invasive Surgery | 100 (94.3, 100); 63/63 | 0.0001 |
| Left access | 100 (89.4, 100); 33/33 | 0.0001 |
| Right access | 100 (88.43, 100); 30/30 | 0.0012 |
| Endpoints | % (n/N) |
|---|---|
| Secondary Performance: Residual LAA neck ≤ 10 mm at last follow-up visit a | 88.8 (135/152) |
| Secondary Safety: Device- or procedure-related SAEs through last (12-month) follow-up visit b | 3.22 (5/155) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zias, E.; Phillips, K.G.; Gerdisch, M.; Johnson, S.; El-Eshmawi, A.; Saum, K.; Moront, M.; Kasten, M.; Singh, C.; Bhatia, G.; et al. Long-Term Follow-Up of Left Atrial Appendage Exclusion: Results of the V-CLIP Multi-Center Post-Market Study. J. Clin. Med. 2025, 14, 5473. https://doi.org/10.3390/jcm14155473
Zias E, Phillips KG, Gerdisch M, Johnson S, El-Eshmawi A, Saum K, Moront M, Kasten M, Singh C, Bhatia G, et al. Long-Term Follow-Up of Left Atrial Appendage Exclusion: Results of the V-CLIP Multi-Center Post-Market Study. Journal of Clinical Medicine. 2025; 14(15):5473. https://doi.org/10.3390/jcm14155473
Chicago/Turabian StyleZias, Elias, Katherine G. Phillips, Marc Gerdisch, Scott Johnson, Ahmed El-Eshmawi, Kenneth Saum, Michael Moront, Michael Kasten, Chanderdeep Singh, Gautam Bhatia, and et al. 2025. "Long-Term Follow-Up of Left Atrial Appendage Exclusion: Results of the V-CLIP Multi-Center Post-Market Study" Journal of Clinical Medicine 14, no. 15: 5473. https://doi.org/10.3390/jcm14155473
APA StyleZias, E., Phillips, K. G., Gerdisch, M., Johnson, S., El-Eshmawi, A., Saum, K., Moront, M., Kasten, M., Singh, C., Bhatia, G., Takayama, H., & Damiano, R. (2025). Long-Term Follow-Up of Left Atrial Appendage Exclusion: Results of the V-CLIP Multi-Center Post-Market Study. Journal of Clinical Medicine, 14(15), 5473. https://doi.org/10.3390/jcm14155473