A TAVI Programme Without an On-Site Cardiac Surgery Department: A Single-Center Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Study Selection, Data Abstraction, and Validity Assessment and Analysis
2.2. Study Endpoints
2.3. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eggebrecht, H.; Vaqueirzo, B.; Moris, C.; Bossone, E.; Lämmer, J.; Czerny, M.; Zierer, A.; Schröfel, H.; Kim, W.-K.; Walther, T.; et al. Incidence and outcomes of emergency cardiac surgery during transfemoral transcatheter aortic valve implantation (TAVI): Insights from the European Registry on Emergent Cardiac Surgery during TAVI (EuRECS-TAVI). Eur. Heart J. 2018, 39, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Lefevre, T.; Van Belle, E.; Eltchaninoff, H.; Iung, B.; Koning, R.; Motreff, P.; Leprince, P.; Verhoye, J.P.; Manigold, T.; et al. Temporal Trends in Transcatheter Aortic Valve Replacement in France: FRANCE 2 to FRANCE TAVI. J. Am. Coll. Cardiol. 2017, 70, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Asami, M.; Heg, D.; Lanz, J.; Praz, F.; Hagemeyer, D.; Brugger, N.; Gräni, C.; Huber, A.; Spirito, A.; et al. Impact of Left Ventricular Outflow Tract Calcification on Procedural Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2020, 13, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; Rai, D.; Tanveer Ud Din, M.; Khan, M.Z.; Ullah, W.; Usman Khan, M.; Thakkar, S.; Hussein, A.; Baibhav, B.; Rao, M.; et al. Same-Day Discharge After Transcatheter Aortic Valve Implantation: Insights from the Nationwide Readmission Database 2015. J. Am. Heart Assoc. 2022, 11, e024746. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; De Maria, G.L.; Joseph, J.; Fan, L.; Cahill, T.J.; Kotronias, R.A.; Burzotta, F.; Newton, J.D.; Kharbanda, R.; Prendergast, B.; et al. Impact of Complications During Transfemoral Transcatheter Aortic Valve Replacement: How Can They Be Avoided and Managed? J. Am. Heart Assoc. 2019, 8, e013801. [Google Scholar] [CrossRef] [PubMed]
- Langer, N.B.; Hamid, N.B.; Nazif, T.M.; Khalique, O.K.; Vahl, T.P.; White, J.; Terre, J.; Hastings, R.; Leung, D.; Hahn, R.T.; et al. Injuries to the Aorta, Aortic Annulus, and Left Ventricle During Transcatheter Aortic Valve Replacement: Management and Outcomes. Circ. Cardiovasc. Interv. 2017, 10, e004735. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Faour, A.; Rawlins, J.; Dawkins, S.; Appleby, C.E.; MacCarthy, P.; Byrne, J.; Trivedi, U.; Curzen, N.; Banning, A.P.; et al. ‘Valve for Life’: Tackling the deficit in transcatheter treatment of heart valve disease in the UK. Open Heart 2021, 8, e001547. [Google Scholar] [CrossRef] [PubMed]
- Elbaz-Greener, G.; Masih, S.; Fang, J.; Ko, D.T.; Lauck, S.B.; Webb, J.G.; Nallamothu, B.K.; Wijeysundera, H.C. Temporal trends and clinical consequences of wait times for transcatheter aortic valve replacement: A population-based study. Circulation 2018, 138, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [PubMed]
- Kim, W.K.; Schäfer, U.; Tchetche, D.; Nef, H.; Arnold, M.; Avanzas, P.; Rudolph, T.; Scholtz, S.; Barbanti, M.; Kempfert, J. Incidence and outcome of peri-procedural transcatheter heart valve embolization and migration: The TRAVEL registry (TranscatheteR HeArt Valve EmboLization and Migration). Eur. Heart J. 2019, 40, 3156–3165. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, H.; Schmermund, A.; Kahlert, P.; Erbel, R.; Voigtländer, T.; Mehta, R.H. Emergent cardiac surgery during transcatheter aortic valve implantation (TAVI): A weighted meta-analysis of 9251 patients from 46 studies. EuroIntervention 2013, 8, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.-A.; et al. Valve Academic Research Consortium (VARC)-Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document (VARC-2). Eur. J. Cardiothorac. Surg. 2012, 42, S45–S60. [Google Scholar] [CrossRef] [PubMed]
- Mylotte, D.; Head, S.J.; Kappetein, A.P.; Piazza, N. TAVI at institutions without cardiovascular surgery departments: Why? EuroIntervention 2014, 10, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, H.; Mehta, R.H.; Kahlert, P.; Schymik, G.; Lefèvre, T.; Lange, R.; Macaya, C.; Mandinov, L.; Wendler, O.; Thomas, M.; et al. Emergent cardiac surgery during transcatheter aortic valve implantation (TAVI): Insights from the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry. EuroIntervention 2014, 10, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Griese, D.P.; Reents, W.; Kerber, S.; Diegeler, A.; Babin-Ebell, J. Emergency cardiac surgery during transfemoral and transapical transcatheter aortic valve implantation: Incidence, reasons, management, and outcome of 411 patients from a single center. Catheter. Cardiovasc. Interv. 2013, 82, E726–E733. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, H.; Bestehorn, M.; Haude, M.; Schmermund, A.; Bestehorn, K.; Voigtländer, T.; Kuck, K.-H.; Mehta, R.H. Outcomes of transfemoral transcatheter aortic valve implantation at hospitals with and without on-site cardiac surgery department: Insights from the prospective German aortic valve replacement quality assurance registry (AQUA) in 17 919 patients. Eur. Heart J. 2016, 37, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Compagnone, M.; Dall’Ara, G.; Grotti, S.; Santarelli, A.; Balducelli, M.; Savini, C.; Tarantino, F.F.; Galvani, M. Transcatheter Aortic Valve Replacement Without On-Site Cardiac Surgery: Ready for Prime Time? JACC Cardiovasc. Interv. 2023, 16, 3026–3030. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.A.; Lauck, S.B.; Cairns, J.A.; Humphries, K.H.; Cook, R.; Welsh, R.; Leipsic, J.; Genereux, P.; Moss, R.; Jue, J.; et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) Clinical pathway facilitates safe next-day discharge home at low-, medium-, and high-volume transfemoral transcatheter aortic valve replacement centers: The 3M TAVR Study. JACC Cardiovasc. Interv. 2019, 12, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; van Mourik, M.S.; Spence, M.S.; Iacovelli, F.; Martinelli, G.L.; Muir, D.F.; Saia, F.; Bortone, A.S.; Densem, C.G.; van der Kley, F.; et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: The multicentre European FAST-TAVI trial. EuroIntervention 2019, 15, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Van Wiechen, M.P.; de Ronde-Tillmans, M.J.; Van Mieghem, N.M. Referring hospital involvement in early discharge post transcatheter aortic valve implantation: The TAVI (R-) EXPRES program. Mini-Invasive Surg. 2022, 6, 1. [Google Scholar] [CrossRef]

| Variable | N = 149 |
| Age (y) (mean ± SD) | 80.5 ± 6.4 |
| Female, no. (%) | 75 (50.3%) |
| Body-mass index (mean ± SD) | 28 ± 7.1 |
| Time from diagnosis to TAVI (mean ± SD) | 55 days ± 9 |
| STS score (mean ± SD) | 4.36 ± 2.96 |
| EuroSCORE II score (mean ± SD) | 3.16 ± 2.27 |
| NYHA class III/IV, no. (%) | 68 (45.6%) |
| Previous myocardial infarction, no. (%) (N = 131) | 15 (11.4%) |
| Coronary artery disease, no. (%) | 83 (55.7%) |
| Prior PCI, no. (%) | 50 (33.5%) |
| Peripheral vascular disease (%) | 26 (17.45%) |
| Previous valvular surgery, no. (%) | 9 (6%) |
| Hypertension, no. (%) | 125 (84%) |
| Previous stroke, no. (%) | 20 (13.4%) |
| Diabetes Mellitus, no. (%) | 68 (45.6%) |
| Hyperlipidemia, no. (%) | 130 (87.2%) |
| Atrial fibrillation, no. (%) | 38 (25.5%) |
| COPD, no. (%) | 25 (16.8%) |
| CKD (eGFR < 60 mL/min/1.73 m2), no. (%) | 41 (27.5%) |
| Anemia (Hg < 10 mg/dL), no. | 76 (51%) |
| Permanent pacemaker, no. (%) (N = 134) | 10 (7.4%) |
| Echocardiographic data | |
| Aortic valve area (cm2) | 0.75 ± 0.15 |
| Max aortic valve gradient (mm Hg) (mean ± SD) | 75 ± 23.3 |
| Mean aortic gradient (mm Hg) (mean ± SD) | 46.9 ± 15.5 |
| Peak aortic valve velocity (m/s) (mean ± SD) | 4.59 ± 0.55 |
| LVEF, % (mean ± SD) | 57.8 ± 9.9 |
| Moderate to severe mitral regurgitation | 8 (5.4%) |
| Moderate to severe tricuspid regurgitation | 1 (0.7%) |
| CT analysis | |
| Calcium, score (mean ± SD), annular | 2065 ± 610 |
| Annular Perimeter (mean ± SD) | 76.85 ± 6.57 |
| Variables | N = 149 |
| Prosthesis type | no. (%) |
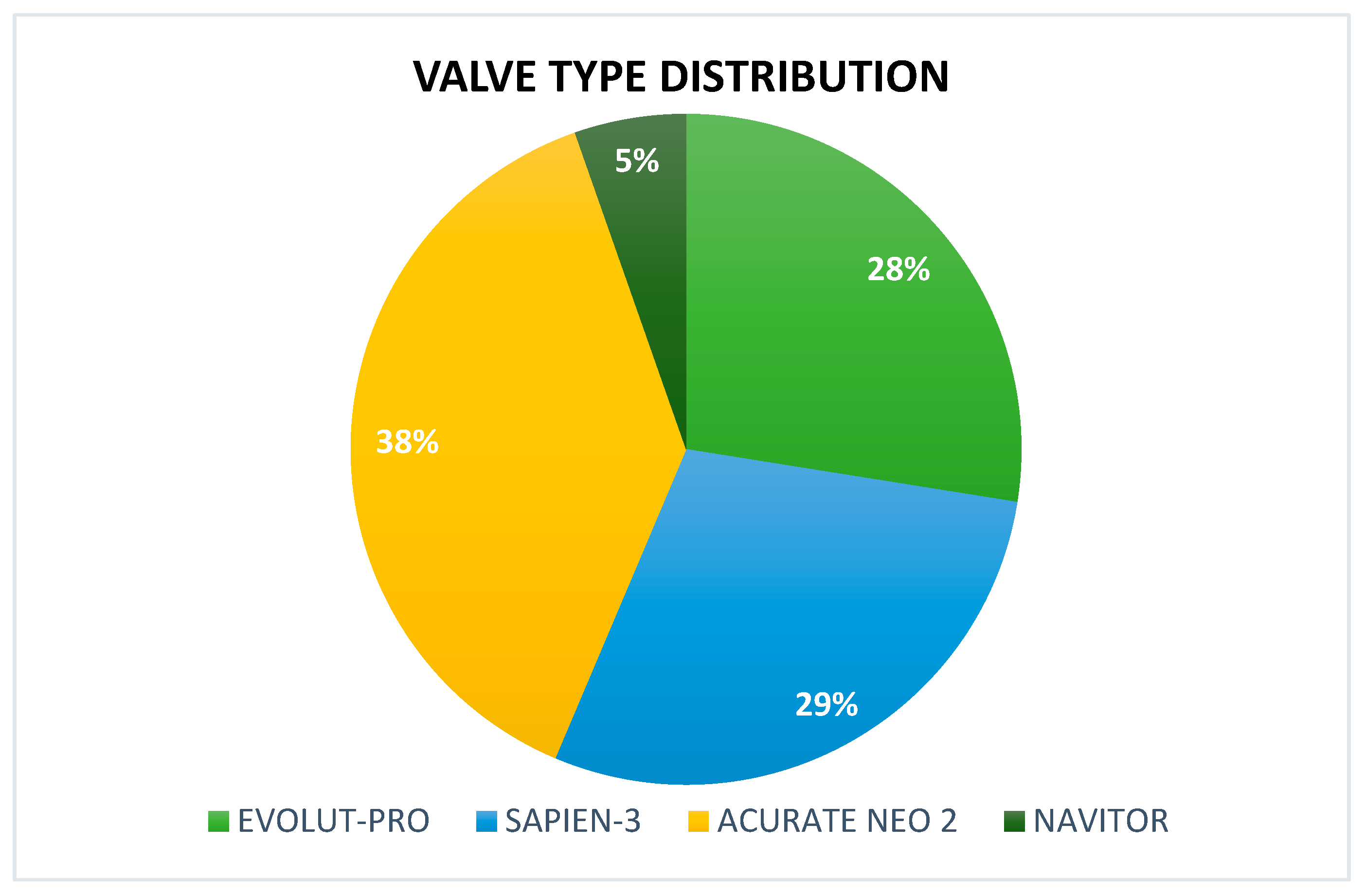
| Evolut-PRO | 41 (27.5%) |
| Edwards SAPIEN 3 | 43 (28.8%) |
| ACURATE neo2 | 57 (38.2%) |
| Navitor | 8 (5.4%) |
| Valve Platform (N) | Size Distribution (%) |
| Evolut-PRO (n = 41) | S (40%), M (50%), L (10%) |
| Edwards SAPIEN 3 (n = 43) | 23 mm (30%), 26 mm (45%), 29 mm (25%) |
| ACURATE neo2 (n = 57) | 26 mm (20%), 29 mm (55%), 34 mm (25%) |
| Navitor (n = 8) | 23 mm (37.5%), 25 mm (37.5%), 27 mm (25.0%) |
| Valve in bioprosthetic valve (%) | 7 (4.7%) |
| Closure device (valve access only) (%) | |
| Prostar/Proglide | 21 (14.1%) |
| Prostar/Proglide + Angioseal | 86 (57.7%) |
| Manta | 42 (28.2%) |
| Intra-procedural complications | |
| Conversion to surgery | 0 (0%) |
| Emergent surgery | 0 (0%) |
| Need for a second valve | 1 (0.67%) |
| Coronary obstruction | 0 (0%) |
| Cardiac tamponade | 0 (0%) |
| Annular rupture | 0 (0%) |
| Valve migration/embolization | 1 (0.67%) |
| In-hospital outcomes | N = 149 |
| In-hospital death | 0 (0%) |
| Peri-procedural MI | 0 (0%) |
| Stroke | 0 (0%) |
| Major vascular complication | 1 (0.67%) |
| Major bleeding (Type 2 ≥ BARC 3) | 4 (2.68%) |
| Permanent pacemaker implantation | 7(4.7%) |
| Acute renal failure | 5 (3.36%) |
| Hemodialysis | 0 (0%) |
| Time to discharge (days, mean ± SD) | 2.1 ± 1.7 |
| Echocardiographic findings—in-hospital post-procedural evaluation | |
| Ejection fraction, % (mean ± SD) | 60 ± 8 |
| mean aortic valve gradient, mm Hg (mean ± SD) | 11.25 ± 6.2 |
| Moderate or severe AR | 0 (0%) |
| 30-day outcomes | |
| Mortality | 1/149 (0.67%) |
| Myocardial Infarction | 2/149 (1.34%) |
| Stroke | 2/149 (1.34%) |
| Cardiovascular hospitalization | 7/149 (4.7%) |
| HALT | 1 (0.67%) |
| Endocarditis | 1 (0.67%) |
| Acute coronary syndrome | 2 (1.34%) |
| Heart failure | 3 (2%) |
| Non-cardiovascular hospitalization | 14 (9.39%) |
| Mortality—1 year | |
| Total | 6/149 (4.0%) |
| Non-cardiac | 5/149 (3.36%) |
| Cardiac | 1/149 (0.67%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barashi, R.; Gabarin, M.; Arow, Z.; Hilu, R.; Losin, I.; Novikov, I.; Abd El Hai, K.; Arnson, Y.; Neuman, Y.; Pesis, K.; et al. A TAVI Programme Without an On-Site Cardiac Surgery Department: A Single-Center Retrospective Study. J. Clin. Med. 2025, 14, 5449. https://doi.org/10.3390/jcm14155449
Barashi R, Gabarin M, Arow Z, Hilu R, Losin I, Novikov I, Abd El Hai K, Arnson Y, Neuman Y, Pesis K, et al. A TAVI Programme Without an On-Site Cardiac Surgery Department: A Single-Center Retrospective Study. Journal of Clinical Medicine. 2025; 14(15):5449. https://doi.org/10.3390/jcm14155449
Chicago/Turabian StyleBarashi, Rami, Mustafa Gabarin, Ziad Arow, Ranin Hilu, Ilya Losin, Ivan Novikov, Karam Abd El Hai, Yoav Arnson, Yoram Neuman, Koby Pesis, and et al. 2025. "A TAVI Programme Without an On-Site Cardiac Surgery Department: A Single-Center Retrospective Study" Journal of Clinical Medicine 14, no. 15: 5449. https://doi.org/10.3390/jcm14155449
APA StyleBarashi, R., Gabarin, M., Arow, Z., Hilu, R., Losin, I., Novikov, I., Abd El Hai, K., Arnson, Y., Neuman, Y., Pesis, K., Jebara, Z., Pereg, D., Koifman, E., Assali, A., & Vaknin-Assa, H. (2025). A TAVI Programme Without an On-Site Cardiac Surgery Department: A Single-Center Retrospective Study. Journal of Clinical Medicine, 14(15), 5449. https://doi.org/10.3390/jcm14155449






