Standard Percutaneous Transluminal Angioplasty Versus Intravascular Lithotripsy to Facilitate Trans-Femoral Transcatheter Aortic Valve Implantation in Patients with Aortic Stenosis and Severe Peripheral Arterial Disease
Abstract
1. Introduction
2. Methods
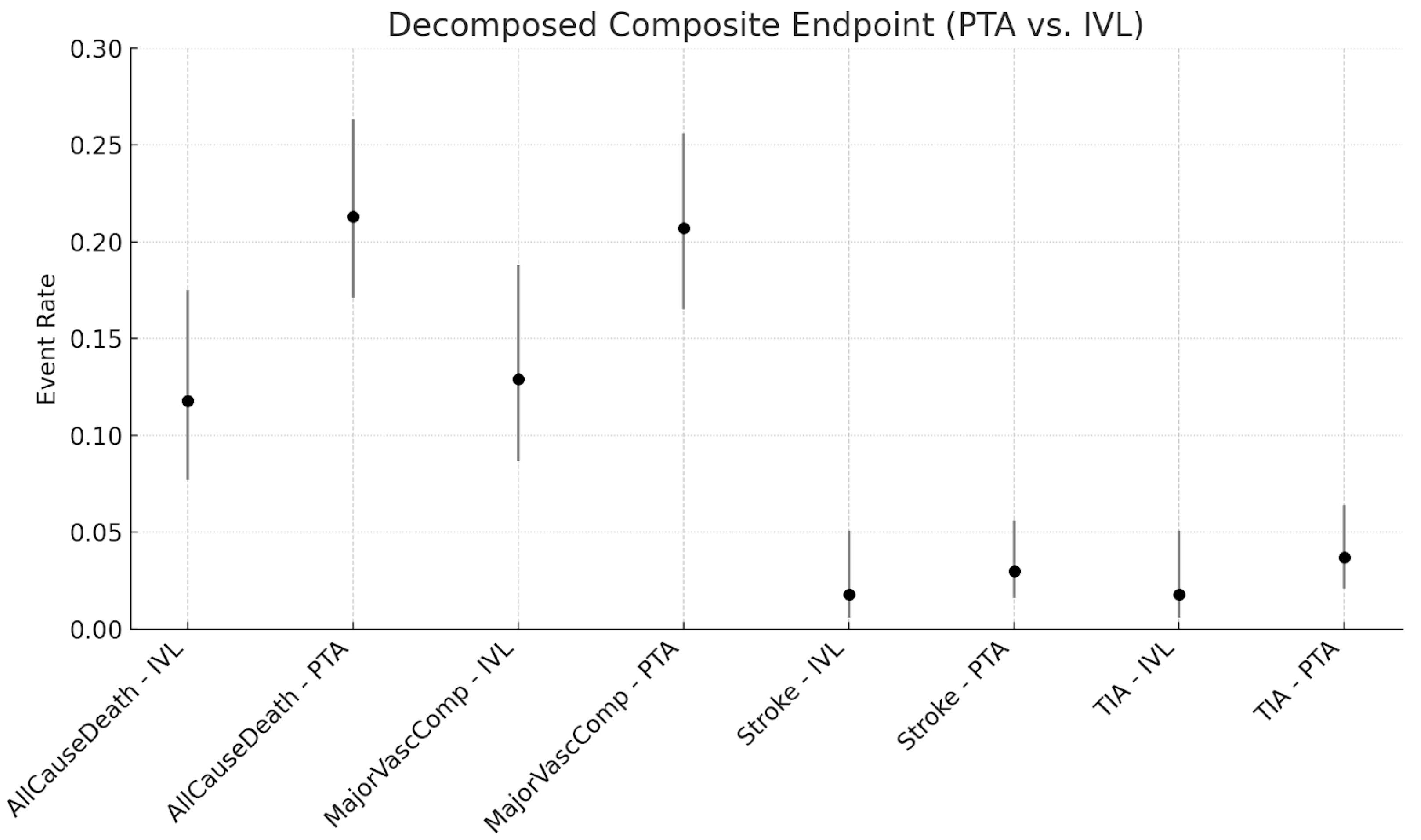
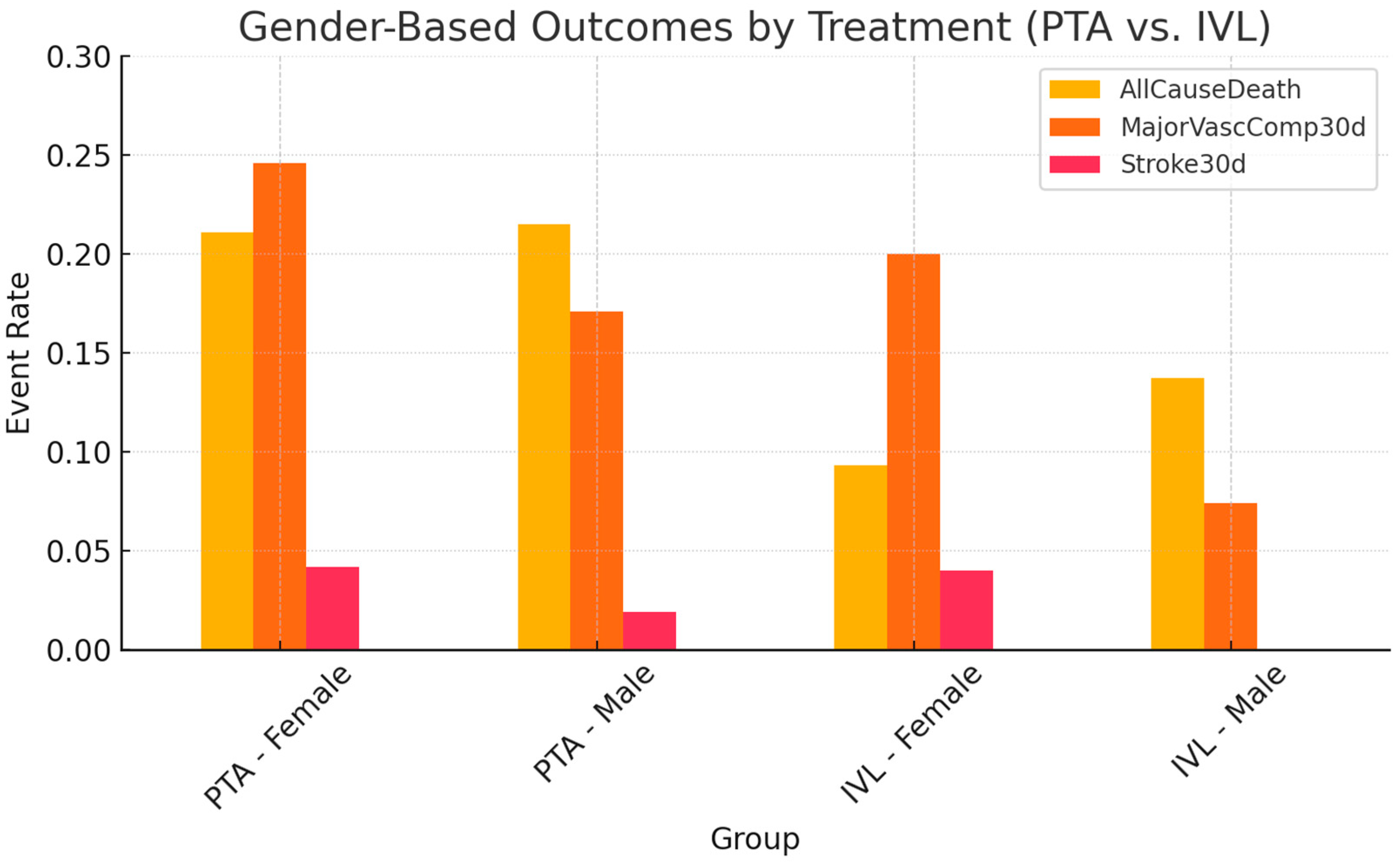
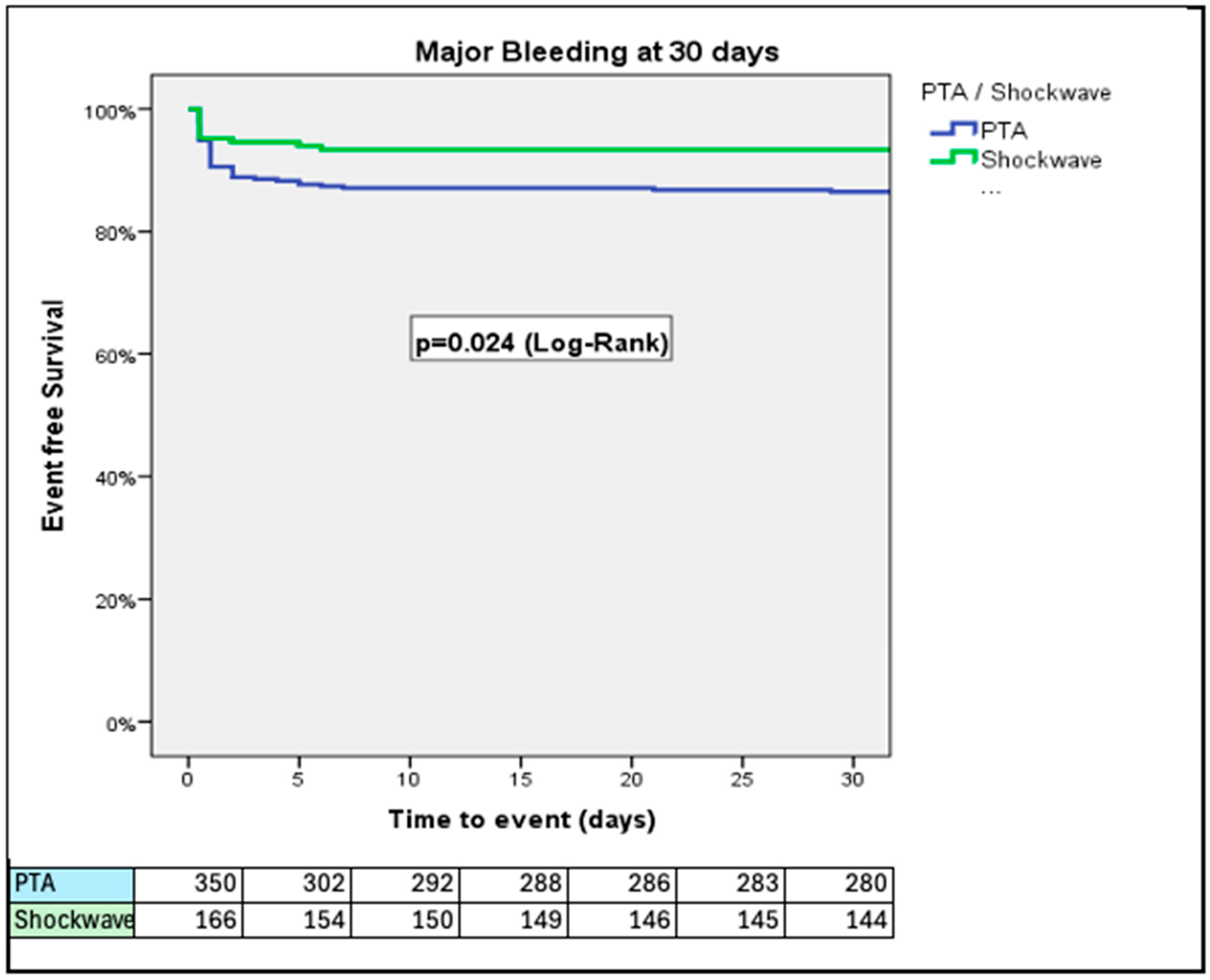
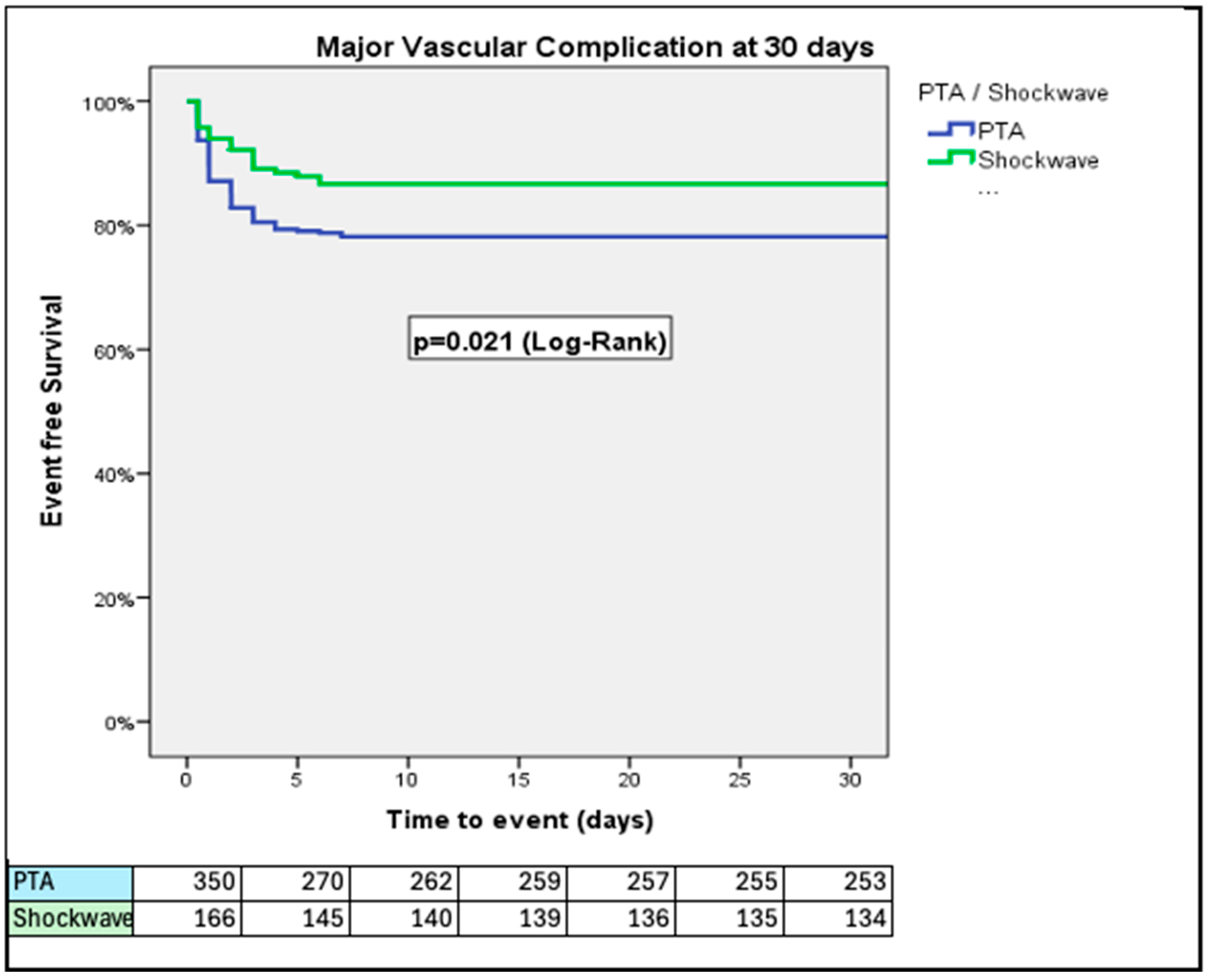
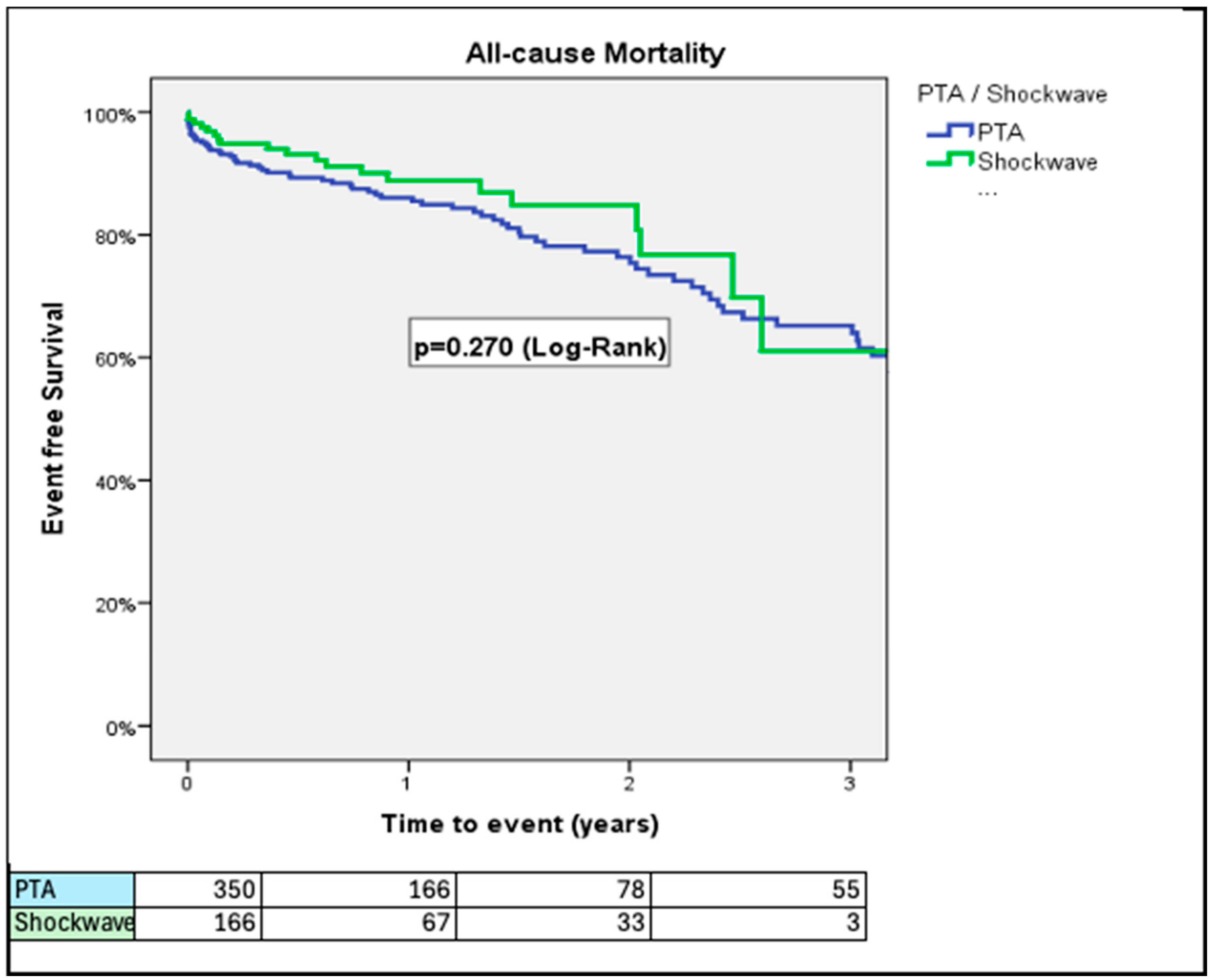
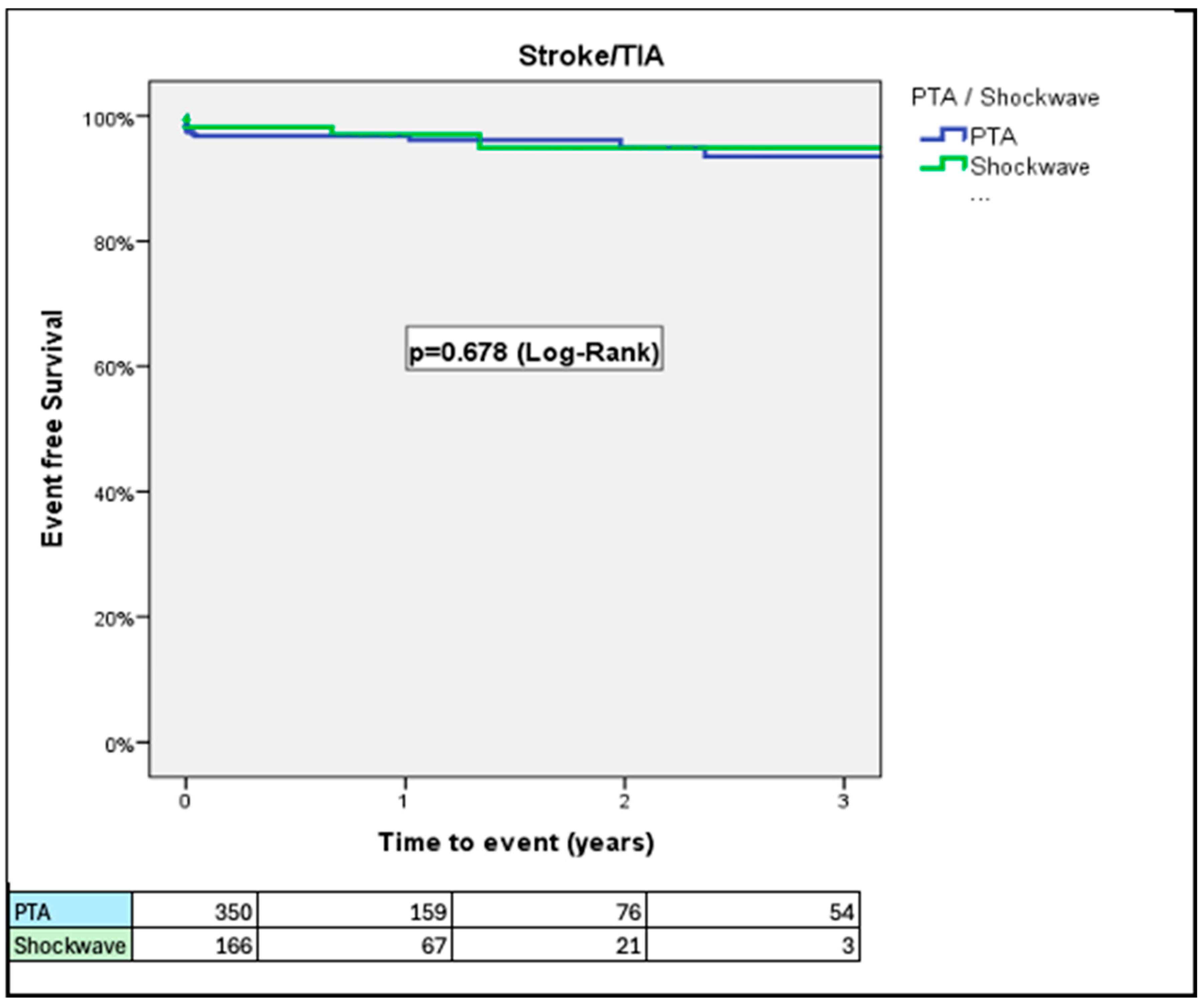
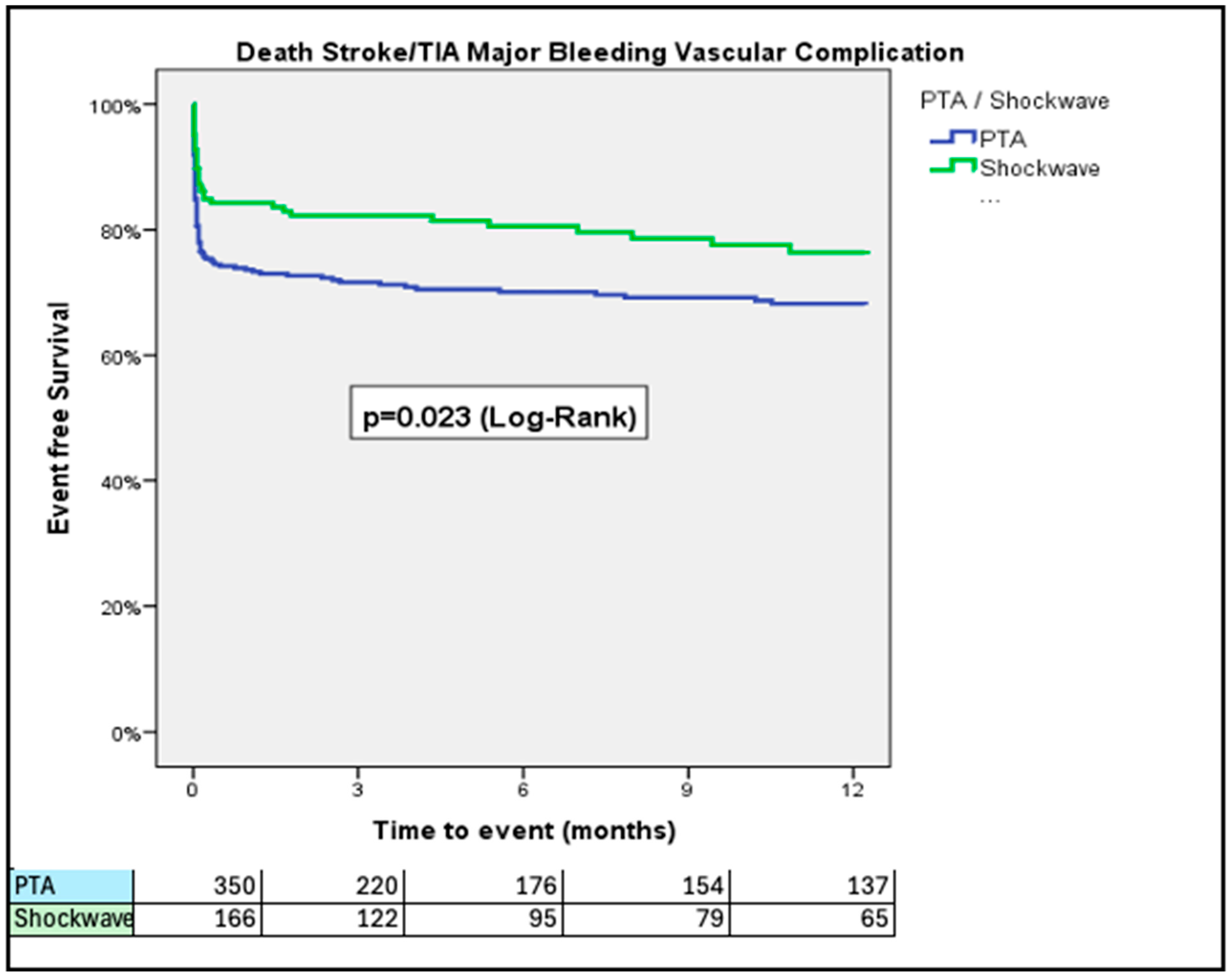
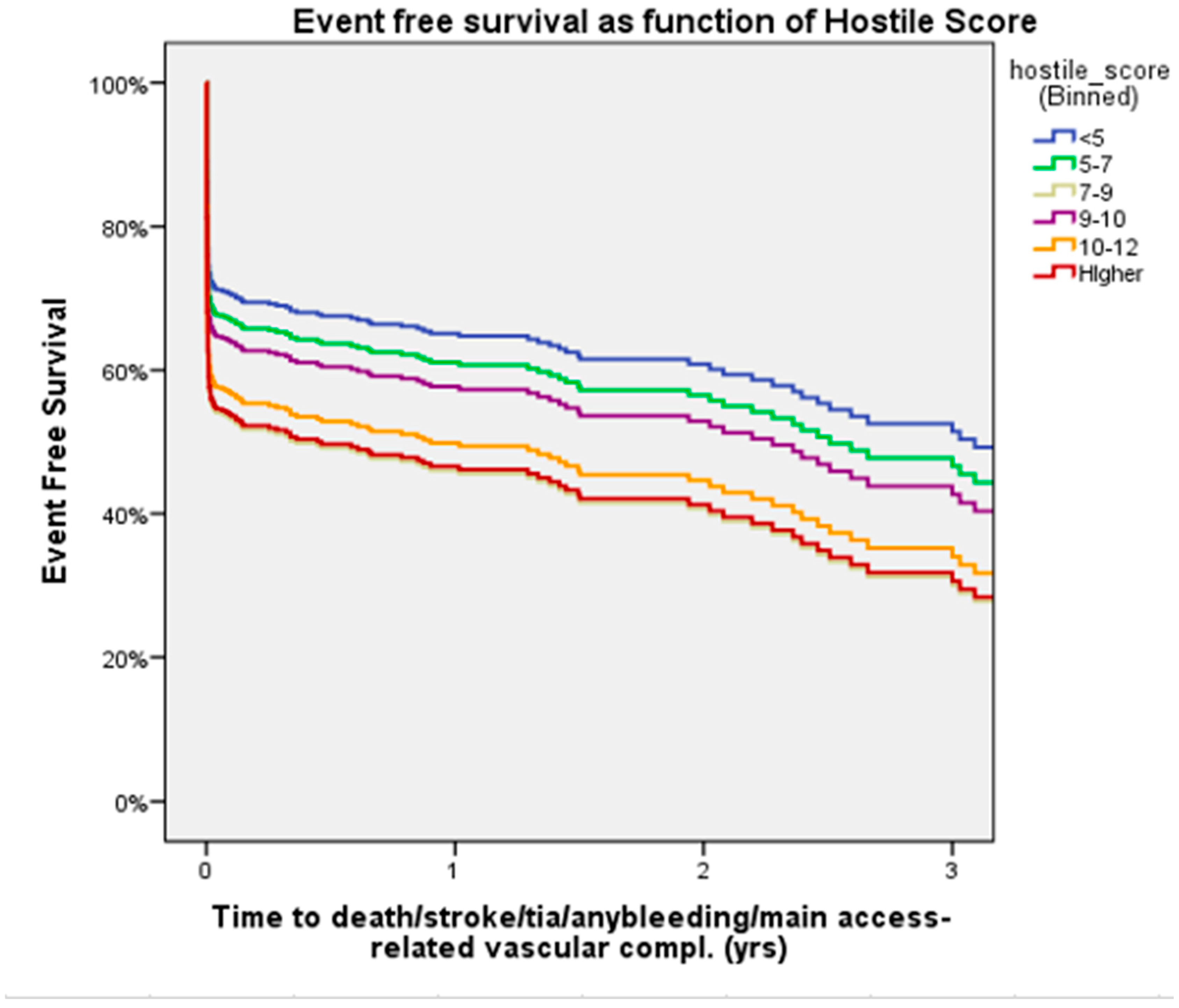
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | Log(OR) | Std. Error | z | p-Value | OR | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|---|
| IVL | −0.488 | 0.288 | −1.697 | 0.09 | 0.614 | 0.349 | 1.079 |
| PTA | 11.78 | 565.135 | 0.021 | 0.983 | 130,674.63 | 0.0 | ∞ |
| Closure Device: Combination of devices | 0.446 | 1.302 | 0.342 | 0.732 | 1.561 | 0.122 | 20.022 |
| Closure Device: MANTA | −2.03 | 1.611 | −1.26 | 0.208 | 0.131 | 0.006 | 3.089 |
| Closure Device: ProGlide | −0.626 | 1.245 | −0.503 | 0.615 | 0.535 | 0.047 | 6.132 |
| Closure Device: Prostar | −0.608 | 1.329 | −0.458 | 0.647 | 0.544 | 0.04 | 7.358 |
| Closure Device: Surgical | 10.765 | 565.137 | 0.019 | 0.985 | 47,357.814 | 0.0 | ∞ |
References
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.J.; Davidson, C.J. Transcatheter Treatment of Valvular Heart Disease: A Review. JAMA 2021, 325, 2480–2494. [Google Scholar] [CrossRef] [PubMed]
- Avvedimento, M.; Angellotti, D.; Ilardi, F.; Leone, A.; Scalamogna, M.; Castiello, D.S.; Manzo, R.; Mariani, A.; Immobile Molaro, M.; Simonetti, F.; et al. Acute advanced aortic stenosis. Heart Fail Rev. 2023, 28, 1101–1111, Erratum in: Heart Fail Rev. 2023, 28, 1235. https://doi.org/10.1007/s10741-023-10320-7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beurtheret, S.; Karam, N.; Resseguier, N.; Houel, R.; Modine, T.; Folliguet, T.; Chamandi, C.; Com, O.; Gelisse, R.; Bille, J.; et al. Femoral Versus Nonfemoral Peripheral Access for Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 2728–2739. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.S.; Krishnaswamy, A.; Svensson, L.G.; Tuzcu, E.M.; Mick, S.; Kapadia, S.R. Access Options for Transcatheter Aortic Valve Replacement in Patients with Unfavorable Aortoiliofemoral Anatomy. Curr. Cardiol. Rep. 2016, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Dato, I.; Burzotta, F.; Trani, C.; Crea, F.; Ussia, G.P. Percutaneous management of vascular access in transfemoral transcatheter aortic valve implantation. World J. Cardiol. 2014, 6, 836–846. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nardi, G.; De Backer, O.; Saia, F.; Søndergaard, L.; Ristalli, F.; Meucci, F.; Stolcova, M.; Mattesini, A.; Demola, P.; Wang, X.; et al. Peripheral intravascular lithotripsy for transcatheter aortic valve implantation: A multicentre observational study. EuroIntervention 2022, 17, e1397–e1406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palmerini, T.; Saia, F.; Kim, W.K.; Renker, M.; Iadanza, A.; Fineschi, M.; Bruno, A.G.; Ghetti, G.; Vanhaverbeke, M.; Søndergaard, L.; et al. Vascular Access in Patients With Peripheral Arterial Disease Undergoing TAVR: The Hostile Registry. JACC Cardiovasc. Interv. 2023, 16, 396–411. [Google Scholar] [CrossRef] [PubMed]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R., Jr.; Nishimura, R.A.; Grover, F.L.; Brindis, R.G.; Carroll, J.D.; Edwards, F.H.; Peterson, E.D.; Rumsfeld, J.S.; Shahian, D.M.; Thourani, V.H.; et al. STS/ACC TVT Registry. Annual Outcomes With Transcatheter Valve Therapy: From the STS/ACC TVT Registry. Ann. Thorac. Surg. 2016, 101, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Webb, J.G.; Svensson, L.G.; Kodali, S.K.; Satler, L.F.; Fearon, W.F.; Davidson, C.J.; Eisenhauer, A.C.; Makkar, R.R.; Bergman, G.W.; et al. PARTNER Trial Investigators. Vascular complications after transcatheter aortic valve replacement: Insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J. Am. Coll. Cardiol. 2012, 60, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Head, S.J.; Van Mieghem, N.M.; Kodali, S.; Kirtane, A.J.; Xu, K.; Smith, C.; Serruys, P.W.; Kappetein, A.P.; Leon, M.B. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: A weighted meta-analysis of 3,519 patients from 16 studies. J. Am. Coll. Cardiol. 2012, 59, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Brodmann, M.; Werner, M.; Holden, A.; Tepe, G.; Scheinert, D.; Schwindt, A.; Wolf, F.; Jaff, M.; Lansky, A.; Zeller, T. Primary outcomes and mechanism of action of intravascular lithotripsy in calcified, femoropopliteal lesions: Results of Disrupt PAD II. Catheter. Cardiovasc. Interv. 2019, 93, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; Deeb, G.M.; Søndergaard, L.; Grube, E.; Windecker, S.; Gada, H.; Mumtaz, M.; Olsen, P.S.; Heiser, J.C.; Merhi, W.; et al. SURTAVI Trial Investigators. Self-expanding Transcatheter vs Surgical Aortic Valve Replacement in Intermediate-Risk Patients: 5-Year Outcomes of the SURTAVI Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 1000–1008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ullah, W.; Satti, D.I.; Sana, M.K.; Osler, B.; Khattak, F.; Ahmed, M.; Vishnevsky, A. Trends and Outcomes of Transcatheter Aortic Valve Implantation in Patients With Peripheral Arterial Disease: Insights From the National Readmissions Database. Curr. Probl. Cardiol. 2023, 48, 101605. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, H.; Schmermund, A.; Voigtländer, T.; Kahlert, P.; Erbel, R.; Mehta, R.H. Risk of stroke after transcatheter aortic valve implantation (TAVI): A meta-analysis of 10,037 published patients. EuroIntervention 2012, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Wayangankar, S.A.; Elgendy, I.Y.; Xiang, Q.; Jneid, H.; Vemulapalli, S.; Khachatryan, T.; Pham, D.; Hilliard, A.A.; Kapadia, S.R. Length of Stay After Transfemoral Transcatheter Aortic Valve Replacement: An Analysis of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. JACC Cardiovasc. Interv. 2019, 12, 422–430. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | PTA | IVL | p-Value |
|---|---|---|---|
| (n = 352) | (n = 166) | ||
| Age | 81.1 ± 6.4 | 79.6 ± 7.0 | 0.051 |
| Male | 184 (52.3) | 93 (56) | 0.451 |
| Hypertension | 316 (90.0) | 149 (89.8) | 1 |
| Diabetes mellitus | 116 (33.0) | 59 (35.5) | 0.619 |
| CAD | 246 (69.9) | 110 (66.3) | 0.418 |
| Prior stroke/TIA | 41 (11.6) | 8 (4.8) | 0.015 |
| Prior PCI | 115 (32.7) | 74 (44.6) | 0.925 |
| Prior MI | 99 (28.1) | 42 (25.3) | 0.527 |
| Prior CABG | 78 (22.2) | 39 (23.5) | 0.737 |
| Atrial fibrillation | 135 (38.4) | 60 (36.1) | 0.698 |
| Baseline serum creatinine | 1.48 ± 1.37 | 1.41 ± 1.1 | 0.586 |
| LVEF | 52.9 ± 11.8 | 52.6 ± 11.8 | 0.768 |
| BMI | 25.7 ± 4.6 | 25.6 ± 5.7 | 0.882 |
| NYHA class 1 to 4 | 2.7 ± 0.7 | 2.7 ± 0.6 | 0.7 |
| EuroSCORE II | 7.9 ± 6.8 | 8.1 ± 6.5 | 0.797 |
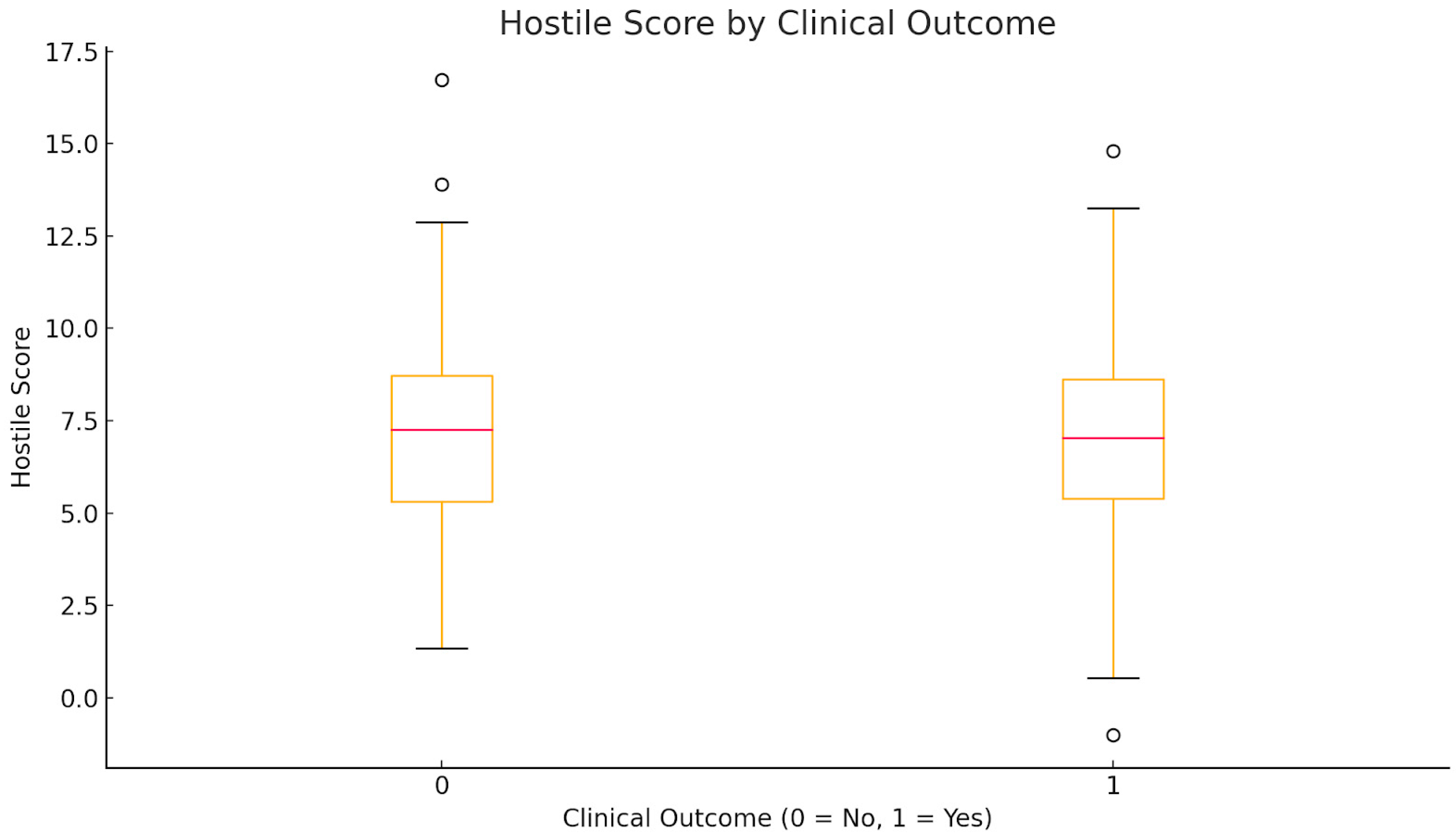
| Hostile Score | 7.1 ± 2.5 | 7.1 ± 2.6 | 0.877 |
| STS-PROM | 6.1 ± 4.4 | 5.6 ± 3.2 | 0.151 |
| Characteristic | PTA | IVL | p-Value |
|---|---|---|---|
| (n = 352) | (n = 166) | ||
| Surgical cutdown | 48 (13.6) | 9 (5.4) | 0.003 |
| Percutaneous access | 304 (86.4) | 157 (94.6) | 0.451 |
| Ultrasound-guided femoral puncture | 128 (38.3) | 107 (81.1) | <0.001 |
| Type of valve | |||
| Evolut/CoreValve | 133 (37.8) | 64 (38.6) | |
| Sapien | 158 (44.9) | 64 (38.6) | |
| Other | 55 (15.6) | 37 (22.3) | |
| Not implanted | 2 (0.6) | 0 (0) | |
| Closure device | |||
| Angioseal | 4 (1.1) | 0 (0) | |
| MANTA | 14 (4.0) | 15 (9.0) | |
| ProGlide | 237 (67.3) | 127 (76.5) | |
| Prostar | 20 (5.7) | 7 (4.2) | |
| Surgical | 44 (12.5) | 0 (0) | |
| Combination of devices | 20 (5.7) | 8 (4.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkin, D.; Bental, T.; Palmerini, T.; Kornowski, R.; Codner, P. Standard Percutaneous Transluminal Angioplasty Versus Intravascular Lithotripsy to Facilitate Trans-Femoral Transcatheter Aortic Valve Implantation in Patients with Aortic Stenosis and Severe Peripheral Arterial Disease. J. Clin. Med. 2025, 14, 6335. https://doi.org/10.3390/jcm14176335
Belkin D, Bental T, Palmerini T, Kornowski R, Codner P. Standard Percutaneous Transluminal Angioplasty Versus Intravascular Lithotripsy to Facilitate Trans-Femoral Transcatheter Aortic Valve Implantation in Patients with Aortic Stenosis and Severe Peripheral Arterial Disease. Journal of Clinical Medicine. 2025; 14(17):6335. https://doi.org/10.3390/jcm14176335
Chicago/Turabian StyleBelkin, David, Tamir Bental, Tullio Palmerini, Ran Kornowski, and Pablo Codner. 2025. "Standard Percutaneous Transluminal Angioplasty Versus Intravascular Lithotripsy to Facilitate Trans-Femoral Transcatheter Aortic Valve Implantation in Patients with Aortic Stenosis and Severe Peripheral Arterial Disease" Journal of Clinical Medicine 14, no. 17: 6335. https://doi.org/10.3390/jcm14176335
APA StyleBelkin, D., Bental, T., Palmerini, T., Kornowski, R., & Codner, P. (2025). Standard Percutaneous Transluminal Angioplasty Versus Intravascular Lithotripsy to Facilitate Trans-Femoral Transcatheter Aortic Valve Implantation in Patients with Aortic Stenosis and Severe Peripheral Arterial Disease. Journal of Clinical Medicine, 14(17), 6335. https://doi.org/10.3390/jcm14176335







