The Role of 6-Hour ECG in Patients with Left Bundle Branch Block After TAVI in Determining Same-Day Discharge
Abstract
1. Introduction
2. Methods
- ΔPR6H (difference in PR intervals between ECGs at 6 h and immediately post-TAVI)
- ΔQRS6H (difference in QRS duration between ECGs at 6 h and immediately post-TAVI)
- ΔPR24H (difference in PR intervals between ECGs at 24 h and immediately post-TAVI)
- ΔQRS24H (difference in QRS duration between ECGs at 24 h and immediately post-TAVI)
Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Coisne, A.; Lancellotti, P.; Habib, G.; Garbi, M.; Dahl, J.S.; Barbanti, M.; Vannan, M.A.; Vassiliou, V.S.; Dudek, D.; Chioncel, O.; et al. ACC/AHA and ESC/EACTS Guidelines for the Management of Valvular Heart Diseases: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2023, 82, 721–734. [Google Scholar] [CrossRef]
- Forrest, J.K.; Yakubov, S.J.; Deeb, G.M.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Crouch, J.; Merhi, W.; Wai Sang, S.L.; et al. 5-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2025, 85, 1523–1532. [Google Scholar] [CrossRef]
- Arora, S.; Strassle, P.D.; Kolte, D.; Ramm, C.J.; Falk, K.; Jack, G.; Caranasos, T.G.; Cavender, M.A.; Rossi, J.S.; Vavalle, J.P. Length of Stay and Discharge Disposition After Transcatheter Versus Surgical Aortic Valve Replacement in the United States. Circ. Cardiovasc. Interv. 2018, 11, e006929. [Google Scholar] [CrossRef] [PubMed]
- Kermanshahchi, J.; Thind, B.; Davoodpour, G.; Hirsch, M.; Chen, J.; Reddy, A.J.; Yu, Z.; Falkenstein, B.E.; Javidi, D. Transcatheter Aortic Valve Replacement (TAVR) Versus Surgical Aortic Valve Replacement (SAVR): A Review on the Length of Stay, Cost, Comorbidities, and Procedural Complications. Cureus 2024, 16, e54435. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.A.; Lauck, S.B.; Cairns, J.A.; Humphries, K.H.; Cook, R.; Welsh, R.; Leipsic, J.; Genereux, P.; Moss, R.; Jue, J.; et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) Clinical Pathway Facilitates Safe Next-Day Discharge Home at Low-, Medium-, and High-Volume Transfemoral Transcatheter Aortic Valve Replacement Centers: The 3M TAVR Study. JACC Cardiovasc. Interv. 2019, 12, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; van Mourik, M.S.; Spence, M.S.; Iacovelli, F.; Martinelli, G.L.; Muir, D.F.; Saia, F.; Bortone, A.S.; Densem, C.G.; van der Kley, F.; et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: The multicentre European FAST-TAVI trial. EuroIntervention 2019, 15, 147–154. [Google Scholar] [CrossRef]
- Barker, M.; Sathananthan, J.; Perdoncin, E.; Devireddy, C.; Keegan, P.; Grubb, K.; Pop, A.M.; Depta, J.P.; Rai, D.; Abtahian, F.; et al. Same-Day Discharge Post-Transcatheter Aortic Valve Replacement During the COVID-19 Pandemic: The Multicenter PROTECT TAVR Study. JACC Cardiovasc. Interv. 2022, 15, 590–598. [Google Scholar] [CrossRef]
- Perdoncin, E.; Greenbaum, A.B.; Grubb, K.J.; Babaliaros, V.C.; Keegan, P.; Ceretto-Clark, B.; Wei, J.; Guyton, R.A.; Paone, G.; Byku, I.; et al. Safety of same-day discharge after uncomplicated, minimalist transcatheter aortic valve replacement in the COVID-19 era. Catheter. Cardiovasc. Interv. 2021, 97, 940–947. [Google Scholar] [CrossRef]
- Lilly, S.M.; Deshmukh, A.J.; Epstein, A.E.; Ricciardi, M.J.; Shreenivas, S.; Velagapudi, P.; Wyman, J.F. 2020 ACC Expert Consensus Decision Pathway on Management of Conduction Disturbances in Patients Undergoing Transcatheter Aortic Valve Replacement: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 2391–2411. [Google Scholar] [CrossRef]
- Siontis, G.C.; Juni, P.; Pilgrim, T.; Stortecky, S.; Bullesfeld, L.; Meier, B.; Wenaweser, P.; Windecker, S. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: A meta-analysis. J. Am. Coll. Cardiol. 2014, 64, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Omari, M.; Durrani, T.; Diaz Nuila, M.E.; Thompson, A.; Irvine, T.; Edwards, R.; Das, R.; Zaman, A.; Farag, M.; Alkhalil, M. Cardiac output in patients with small annuli undergoing transcatheter aortic valve implantation with self-expanding versus balloon expandable valve (COPS-TAVI). Cardiovasc. Revasc Med. 2024, 73, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Al-Atta, A.; Farag, M.; Jeyalan, V.; Gazzal Asswad, A.; Thompson, A.; Irvine, T.; Edwards, R.; Das, R.; Zaman, A.; Alkhalil, M. Low Transvalvular Flow Rate in Patients Undergoing Transcatheter Aortic Valve Implantation (TAVI) Is a Predictor of Mortality: The TFR-TAVI Study. Heart Lung Circ. 2023, 32, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Piayda, K.; Hellhammer, K.; Veulemans, V.; Sievert, H.; Gafoor, S.; Afzal, S.; Hennig, I.; Makosch, M.; Polzin, A.; Jung, C.; et al. Navigating the “Optimal Implantation Depth” With a Self-Expandable TAVR Device in Daily Clinical Practice. JACC Cardiovasc. Interv. 2020, 13, 679–688. [Google Scholar] [CrossRef]
- Qureshi, W.T.; Kundu, A.; Mir, T.; Khan, A.; Anwaruddin, S.; Sattar, Y.; Ogunsua, A.; Dutta, A.; Majeed, C.N.; Walker, J.; et al. Meta-analysis of minimalist versus standard care approach for transcatheter aortic valve replacement. Expert Rev. Cardiovasc. Ther. 2021, 19, 565–574. [Google Scholar] [CrossRef]
- Varc-3 Writing, C.; Genereux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Chen, S.; Chau, K.H.; Nazif, T.M. The incidence and impact of cardiac conduction disturbances after transcatheter aortic valve replacement. Ann. Cardiothorac. Surg. 2020, 9, 452–467. [Google Scholar] [CrossRef]
- Prihadi, E.A.; Leung, M.; Vollema, E.M.; Ng, A.C.T.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Prevalence and Prognostic Relevance of Ventricular Conduction Disturbances in Patients With Aortic Stenosis. Am. J. Cardiol. 2017, 120, 2226–2232. [Google Scholar] [CrossRef]
- Auffret, V.; Puri, R.; Urena, M.; Chamandi, C.; Rodriguez-Gabella, T.; Philippon, F.; Rodes-Cabau, J. Conduction Disturbances After Transcatheter Aortic Valve Replacement: Current Status and Future Perspectives. Circulation 2017, 136, 1049–1069. [Google Scholar] [CrossRef]
- Piazza, N.; Onuma, Y.; Jesserun, E.; Kint, P.P.; Maugenest, A.M.; Anderson, R.H.; de Jaegere, P.P.; Serruys, P.W. Early and persistent intraventricular conduction abnormalities and requirements for pacemaking after percutaneous replacement of the aortic valve. JACC Cardiovasc. Interv. 2008, 1, 310–316. [Google Scholar] [CrossRef]
- Oestreich, B.A.; Mbai, M.; Gurevich, S.; Nijjar, P.S.; Adabag, S.; Bertog, S.; Kelly, R.; Garcia, S. Computed tomography (CT) assessment of the membranous septal anatomy prior to transcatheter aortic valve replacement (TAVR) with the balloon-expandable SAPIEN 3 valve. Cardiovasc. Revasc Med. 2018, 19, 626–631. [Google Scholar] [CrossRef]
- Mangieri, A.; Montalto, C.; Pagnesi, M.; Lanzillo, G.; Demir, O.; Testa, L.; Colombo, A.; Latib, A. TAVI and Post Procedural Cardiac Conduction Abnormalities. Front. Cardiovasc. Med. 2018, 5, 85. [Google Scholar] [CrossRef]
- Badertscher, P.; Knecht, S.; Zeljkovic, I.; Sticherling, C.; de Asmundis, C.; Conte, G.; Barra, S.; Jedrzej, K.; Kuhne, M.; Boveda, S. Management of conduction disorders after transcatheter aortic valve implantation: Results of the EHRA survey. Europace 2022, 24, 1179–1185. [Google Scholar] [CrossRef]
- Regueiro, A.; Abdul-Jawad Altisent, O.; Del Trigo, M.; Campelo-Parada, F.; Puri, R.; Urena, M.; Philippon, F.; Rodes-Cabau, J. Impact of New-Onset Left Bundle Branch Block and Periprocedural Permanent Pacemaker Implantation on Clinical Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2016, 9, e003635. [Google Scholar] [CrossRef]
- Ream, K.; Sandhu, A.; Valle, J.; Weber, R.; Kaizer, A.; Wiktor, D.M.; Borne, R.T.; Tumolo, A.Z.; Kunkel, M.; Zipse, M.M.; et al. Ambulatory Rhythm Monitoring to Detect Late High-Grade Atrioventricular Block Following Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 73, 2538–2547. [Google Scholar] [CrossRef]
- Fischer, Q.; Himbert, D.; Webb, J.G.; Eltchaninoff, H.; Munoz-Garcia, A.J.; Tamburino, C.; Nombela-Franco, L.; Nietlispach, F.; Moris, C.; Ruel, M.; et al. Impact of Preexisting Left Bundle Branch Block in Transcatheter Aortic Valve Replacement Recipients. Circ. Cardiovasc. Interv. 2018, 11, e006927. [Google Scholar] [CrossRef]
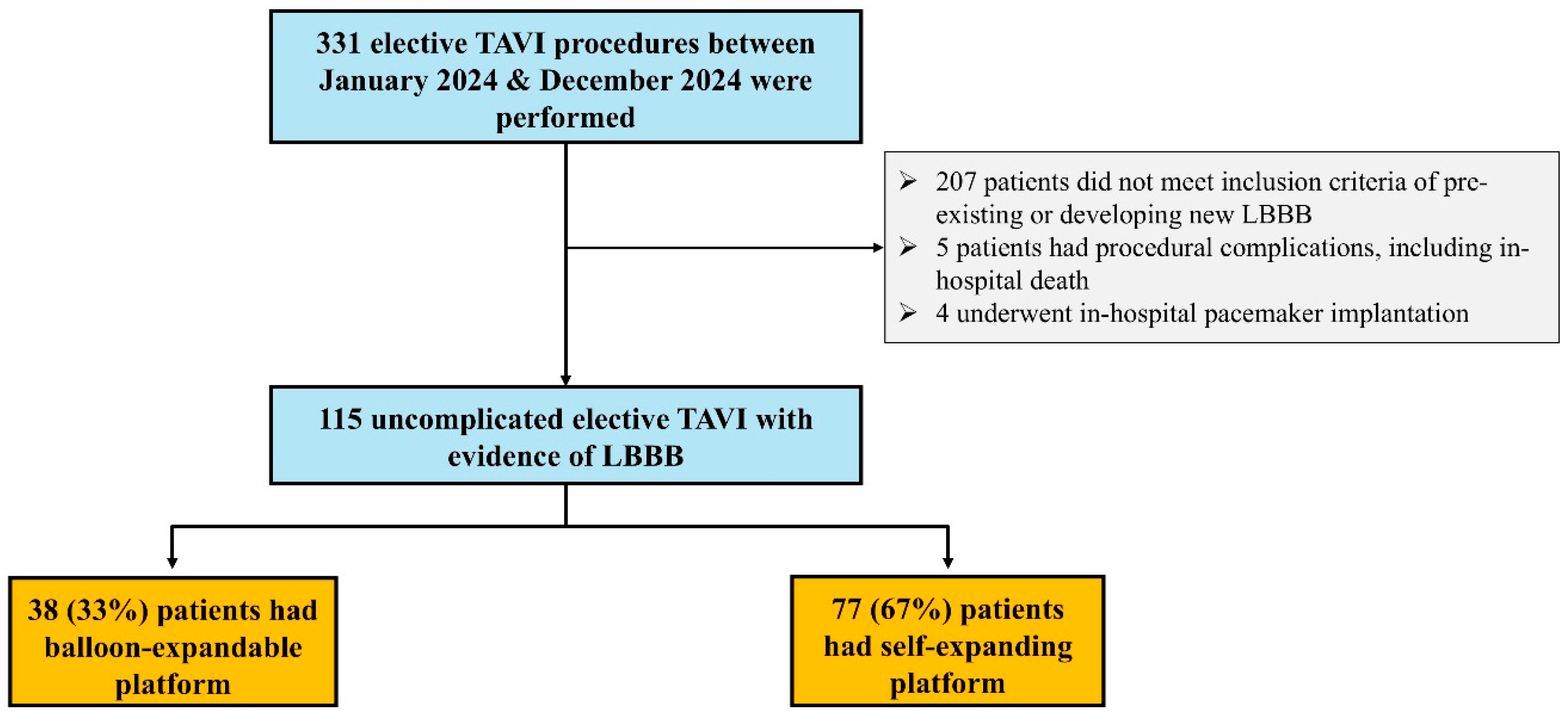
| Whole Cohort (n = 115) | Self-Expanding Platform (n = 77) | Balloon-Expandable Platform (n = 38) | p Value | |
|---|---|---|---|---|
| Age, yrs (mean ± SD) | 81 ± 7 | 81 ± 6 | 80 ± 8 | 0.21 |
| Male gender (n, %) | 62 (54%) | 38 (49%) | 24 (63%) | 0.16 |
| Body mass index, kg/m2 (mean ± SD) | 30 ± 6 | 30 ± 6 | 30 ± 7 | 0.95 |
| Diabetes (n, %) | 30 (26%) | 25 (33%) | 5 (13%) | 0.027 |
| Previous smoker (n, %) | 41 (36%) | 27 (35%) | 14 (37%) | 0.85 |
| Obstructive lung disease (n, %) | 4 (4%) | 4 (5%) | 0 (0%) | 0.15 |
| Creatinine, mmol/L (mean ± SD) | 97 ± 37 | 92 ± 32 | 105 ± 43 | 0.11 |
| Previous CVA/TIA (n, %) | 4 (4%) | 3 (4%) | 1 (3%) | 0.73 |
| Atrial fibrillation (n, %) | 26 (23%) | 17 (22%) | 9 (24%) | 0.85 |
| NYHA III/IV (n, %) | 72 (63%) | 46 (60%) | 26 (68%) | 0.37 |
| Mean gradient, mmHg (mean ± SD) | 45 ± 12 | 45 ± 13 | 44 ± 11 | 0.77 |
| Peak gradient, mmHg (mean ± SD) | 76 ± 19 | 76 ± 20 | 71 ± 17 | 0.97 |
| Aortic valve area, cm2 (mean ± SD) | 0.75 ± 0.47 | 0.80 ± 0.55 | 0.66 ± 0.21 | 0.27 |
| Moderate MR (n, %) | 2 (2%) | 2 (3%) | 0 (0%) | 0.32 |
| Preserved LV function (n, %) | 106 (92%) | 70 (91%) | 36 (95%) | 0.47 |
| Same-day discharge (n, %) | 14 (12%) | 11 (14%) | 3 (8%) | 0.32 |
| Whole Cohort (n = 115) | Self-Expanding Platform (n = 77) | Balloon-Expandable Platform (n = 38) | p Value | |
|---|---|---|---|---|
| Pre TAVI | ||||
| Sinus rhythm (n, %) | 88 (77%) | 59 (77%) | 29 (76%) | 0.77 |
| Heart rate (mean ± SD) | 73 ± 13 | 73 ± 14 | 72 ± 12 | 0.65 |
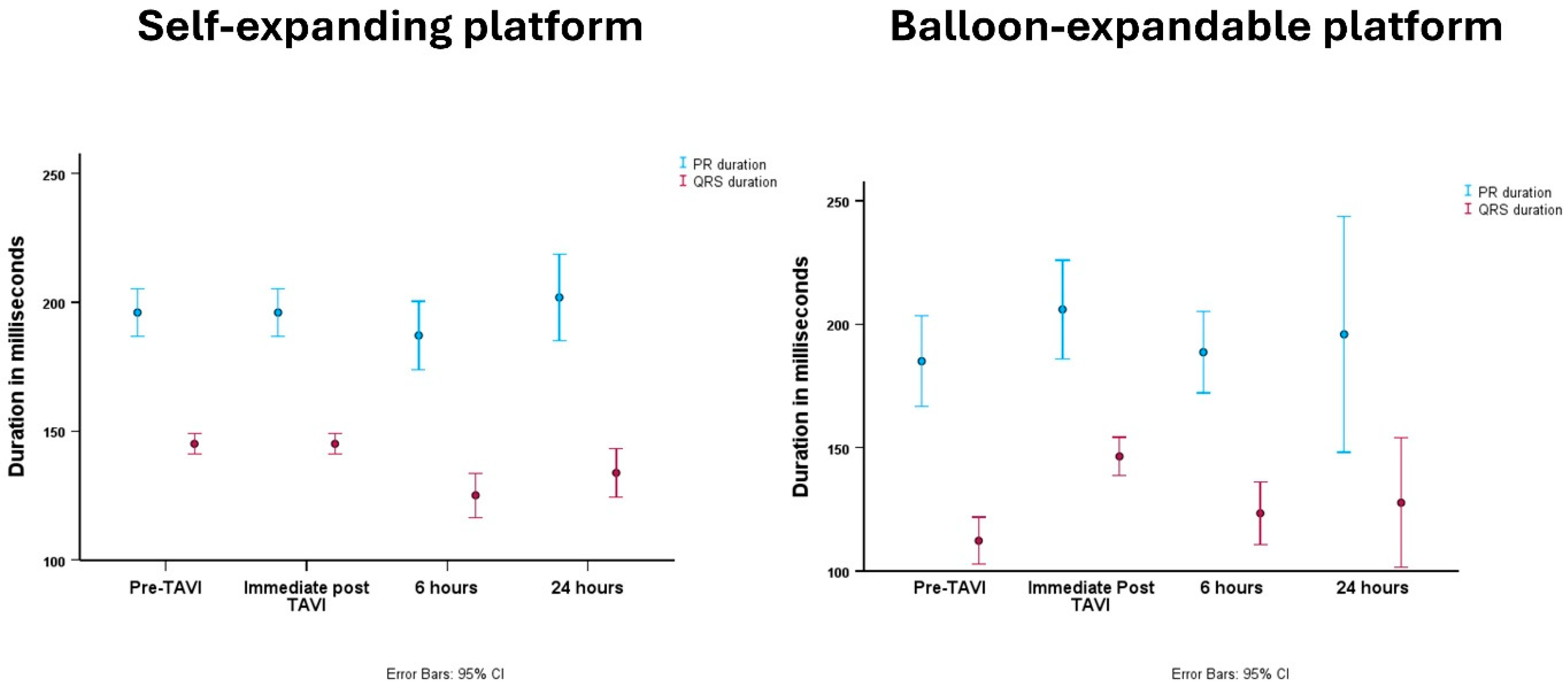
| PR interval (mean ± SD) | 180 ± 37 | 177 ± 32 | 185 ± 46 | 0.37 |
| QRS duration (mean ± SD) | 106 ± 26 | 102 ± 27 | 113 ± 24 | 0.051 |
| Immediately Post TAVI | ||||
| Sinus rhythm (n, %) | 88 (77%) | 58 (75%) | 30 (79%) | 0.67 |
| Heart rate (mean ± SD) | 68 ± 13 | 69 ± 13 | 68 ± 13 | 0.94 |
| PR interval (mean ± SD) | 199 ± 42 | 196 ± 36 | 206 ± 53 | 0.30 |
| QRS duration (mean ± SD) | 146 ± 17 | 145 ± 16 | 147 ± 19 | 0.74 |
| At 6 h post TAVI | ||||
| Sinus rhythm (n, %) | 88 (77%) | 59 (77%) | 29 (76%) | 0.77 |
| Heart rate (mean ± SD) | 70 ± 14 | 71 ± 14 | 70 ± 14 | 0.77 |
| PR interval (mean ± SD) | 188 ± 42 | 187 ± 45 | 189 ± 37 | 0.89 |
| QRS duration (mean ± SD) | 126 ± 28 | 127 ± 28 | 124 ± 29 | 0.69 |
| At 24 h post TAVI | ||||
| Sinus rhythm (n, %) | 91 (79%) | 61 (79%) | 30 (79%) | 0.77 |
| Heart rate (mean ± SD) | 73 ± 13 | 73 ± 13 | 73 ± 13 | 0.87 |
| PR interval (mean ± SD) | 200 ± 51 | 201 ± 40 | 196 ± 71 | 0.75 |
| QRS duration (mean ± SD) | 130 ± 26 | 131 ± 24 | 129 ± 31 | 0.77 |
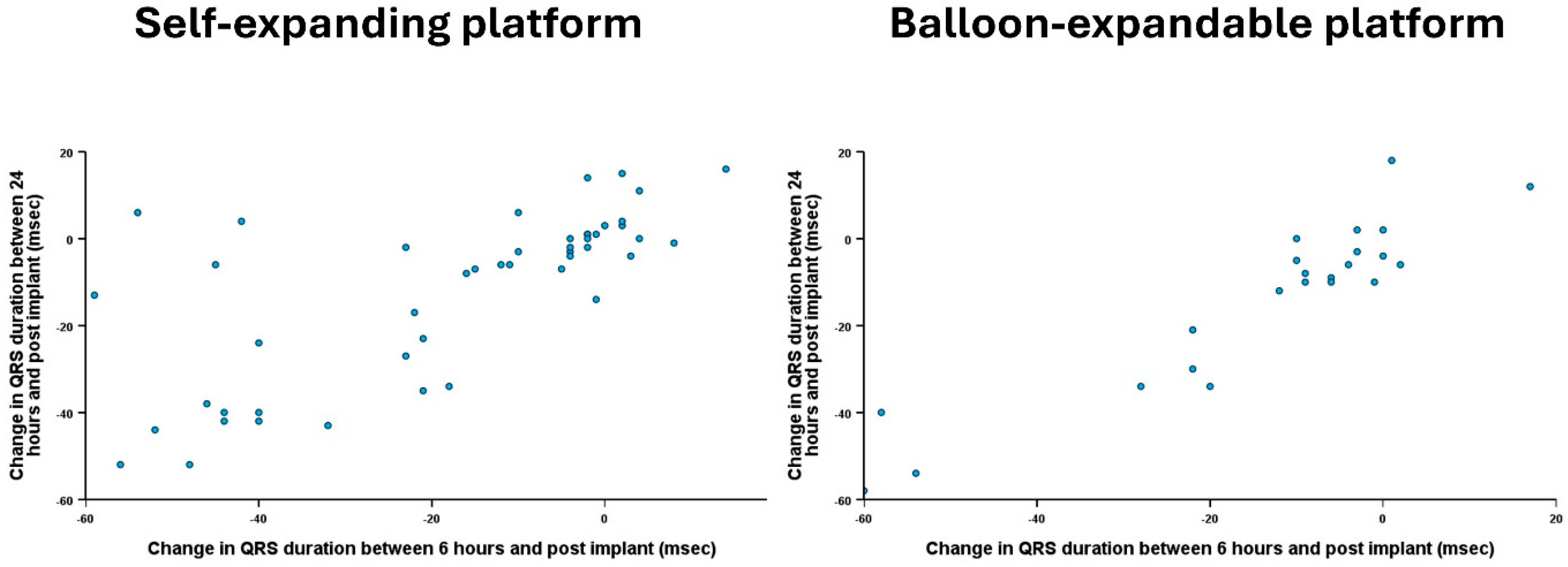
| Change in PR interval at 6 h * (median, IQR) | −11 (−20, 3) | −11 (−19, 4) | −13 (−21, 0) | 0.62 |
| Change in QRS duration at 6 h * (median, IQR) | −10 (−40, −2) | −10 (−40, −2) | −10 (−36, −3) | 0.48 |
| Change in PR interval at 24 h ⁋ (median, IQR) | −2 (−24, 16) | 3 (−27, 20) | −14 (−24, 3) | 0.29 |
| Change in QRS duration at 24 h ⁋ (median, IQR) | −7 (−34, 0) | −6 (−26, 1) | −10 (−40, −4) | 0.17 |
| LBBB Cohort (n = 115) | No LBBB Cohort (n = 174) | p Value | |
|---|---|---|---|
| Age, yrs (mean ± SD) | 81 ± 7 | 81 ± 6 | 0.57 |
| Male gender (n, %) | 62 (54%) | 110 (63%) | 0.12 |
| Body mass index, kg/m2 (mean ± SD) | 30 ± 6 | 28 ± 6 | 0.009 |
| Diabetes (n, %) | 30 (26%) | 33 (19%) | 0.15 |
| Previous smoker (n, %) | 41 (36%) | 70 (40%) | 0.43 |
| Obstructive lung disease (n, %) | 4 (4%) | 7 (4%) | 0.81 |
| Creatinine, mmol/L (mean ± SD) | 97 ± 37 | 95 ± 41 | 0.76 |
| Previous CVA/TIA (n, %) | 4 (4%) | 11 (6%) | 0.29 |
| Atrial fibrillation (n, %) | 26 (23%) | 39 (22%) | 0.97 |
| NYHA III/IV (n, %) | 72 (63%) | 105 (60%) | 0.70 |
| Mean gradient, mmHg (mean ± SD) | 45 ± 12 | 46 ± 12 | 0.45 |
| Peak gradient, mmHg (mean ± SD) | 76 ± 19 | 73 ± 22 | 0.21 |
| Aortic valve area, cm2 (mean ± SD) | 0.75 ± 0.47 | 0.75 ± 0.17 | 0.91 |
| Moderate MR (n, %) | 2 (2%) | 2 (1%) | 0.67 |
| Preserved LV function (n, %) | 106 (92%) | 160 (92%) | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omari, M.; Memon, S.; Stewart, D.; Ali, M.; Edwards, R.; Das, R.; Cartlidge, T.; Zaman, A.; Farag, M.; Alkhalil, M. The Role of 6-Hour ECG in Patients with Left Bundle Branch Block After TAVI in Determining Same-Day Discharge. J. Clin. Med. 2025, 14, 5408. https://doi.org/10.3390/jcm14155408
Omari M, Memon S, Stewart D, Ali M, Edwards R, Das R, Cartlidge T, Zaman A, Farag M, Alkhalil M. The Role of 6-Hour ECG in Patients with Left Bundle Branch Block After TAVI in Determining Same-Day Discharge. Journal of Clinical Medicine. 2025; 14(15):5408. https://doi.org/10.3390/jcm14155408
Chicago/Turabian StyleOmari, Muntaser, Saif Memon, Debbie Stewart, Mohamed Ali, Richard Edwards, Rajiv Das, Timothy Cartlidge, Azfar Zaman, Mohamed Farag, and Mohammad Alkhalil. 2025. "The Role of 6-Hour ECG in Patients with Left Bundle Branch Block After TAVI in Determining Same-Day Discharge" Journal of Clinical Medicine 14, no. 15: 5408. https://doi.org/10.3390/jcm14155408
APA StyleOmari, M., Memon, S., Stewart, D., Ali, M., Edwards, R., Das, R., Cartlidge, T., Zaman, A., Farag, M., & Alkhalil, M. (2025). The Role of 6-Hour ECG in Patients with Left Bundle Branch Block After TAVI in Determining Same-Day Discharge. Journal of Clinical Medicine, 14(15), 5408. https://doi.org/10.3390/jcm14155408








