Application of Hybrid Platelet Technology for Platelet Count Improves Accuracy of PLT Measurement in Samples from Patients with Different Types of Anemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Included Samples
2.2. Impedance Platelets Measurement
2.3. Hybrid Platelet Measurement
2.4. Fluorescent Platelet Measurement
2.5. Statistical Analysis
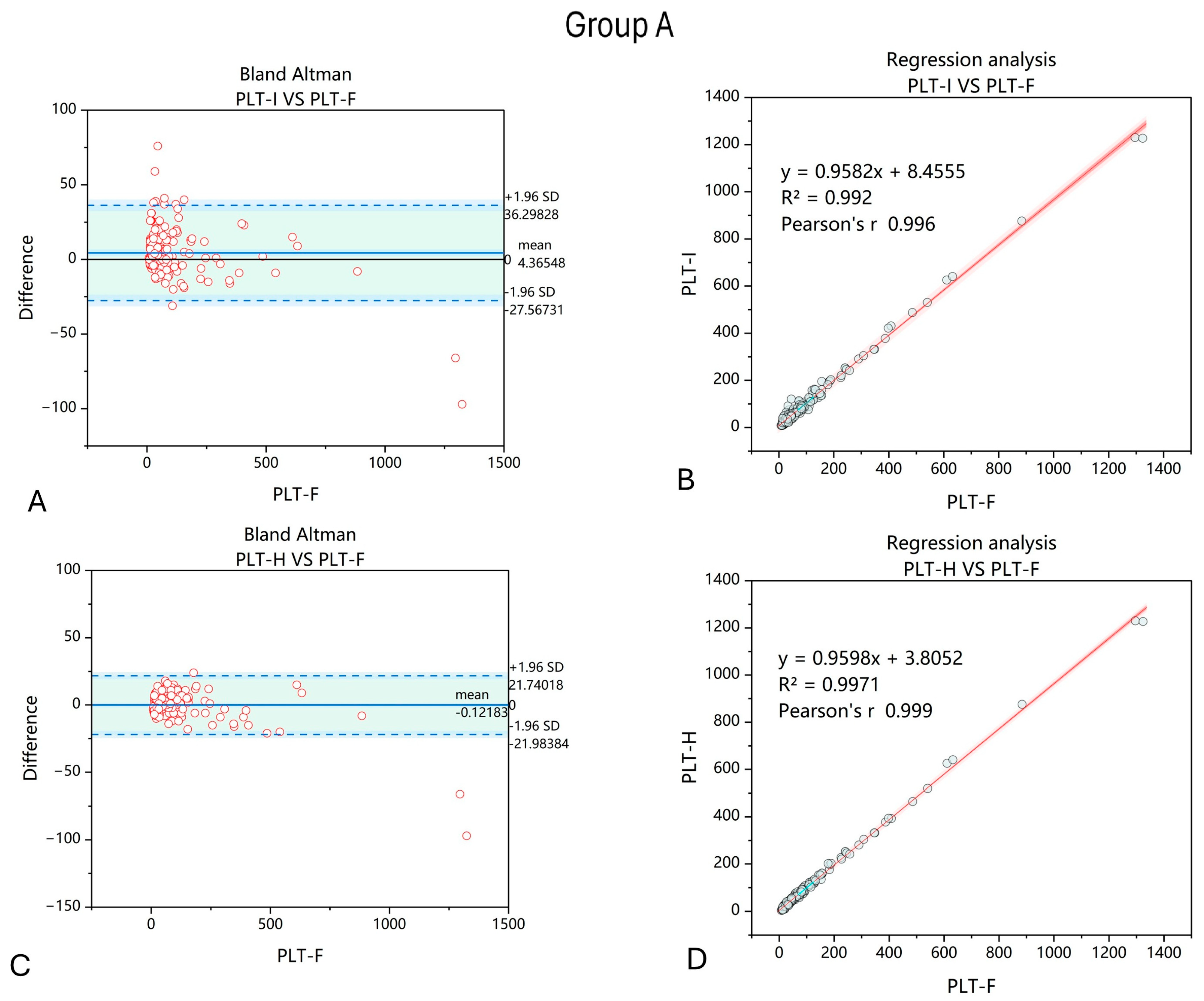
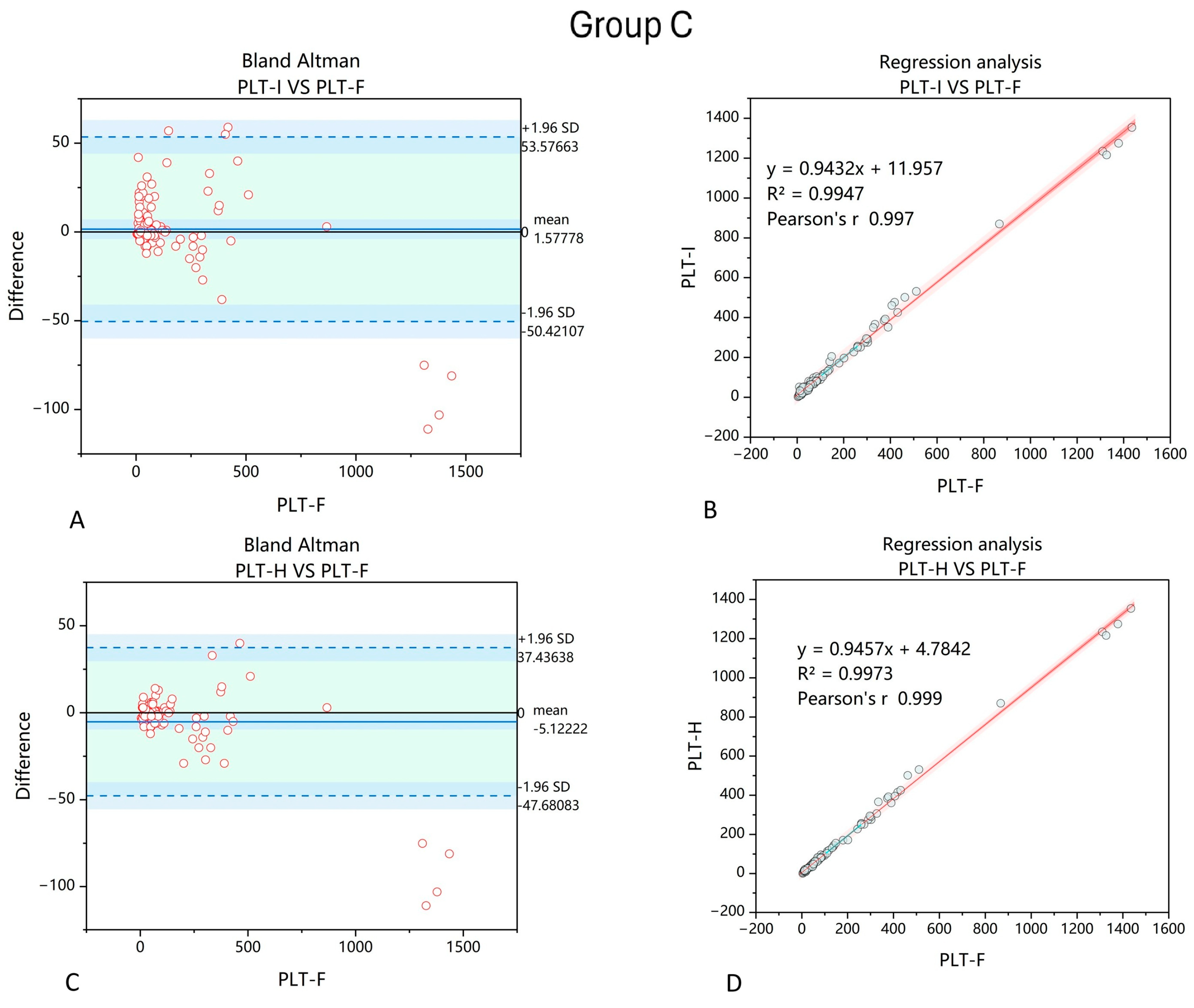
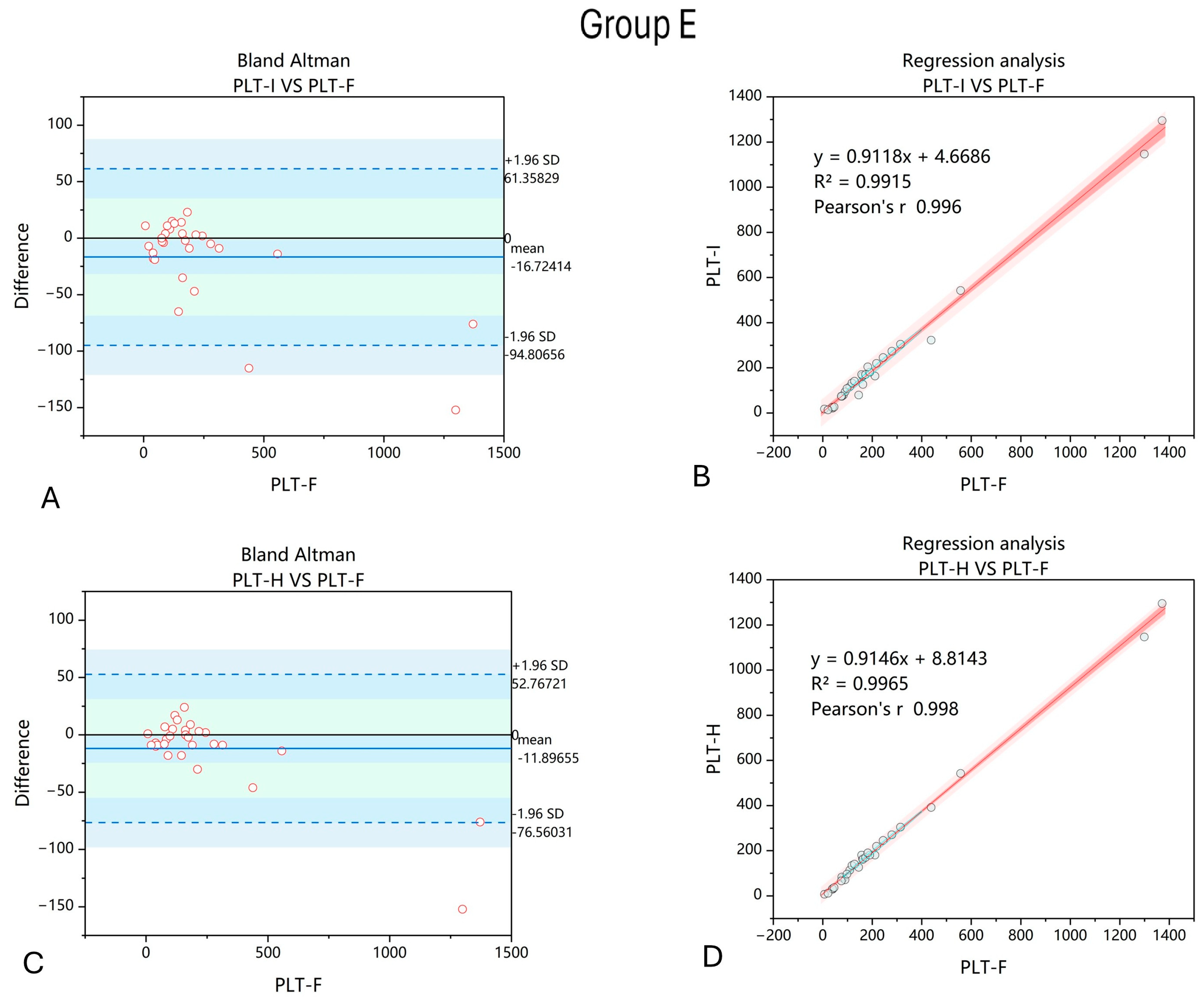
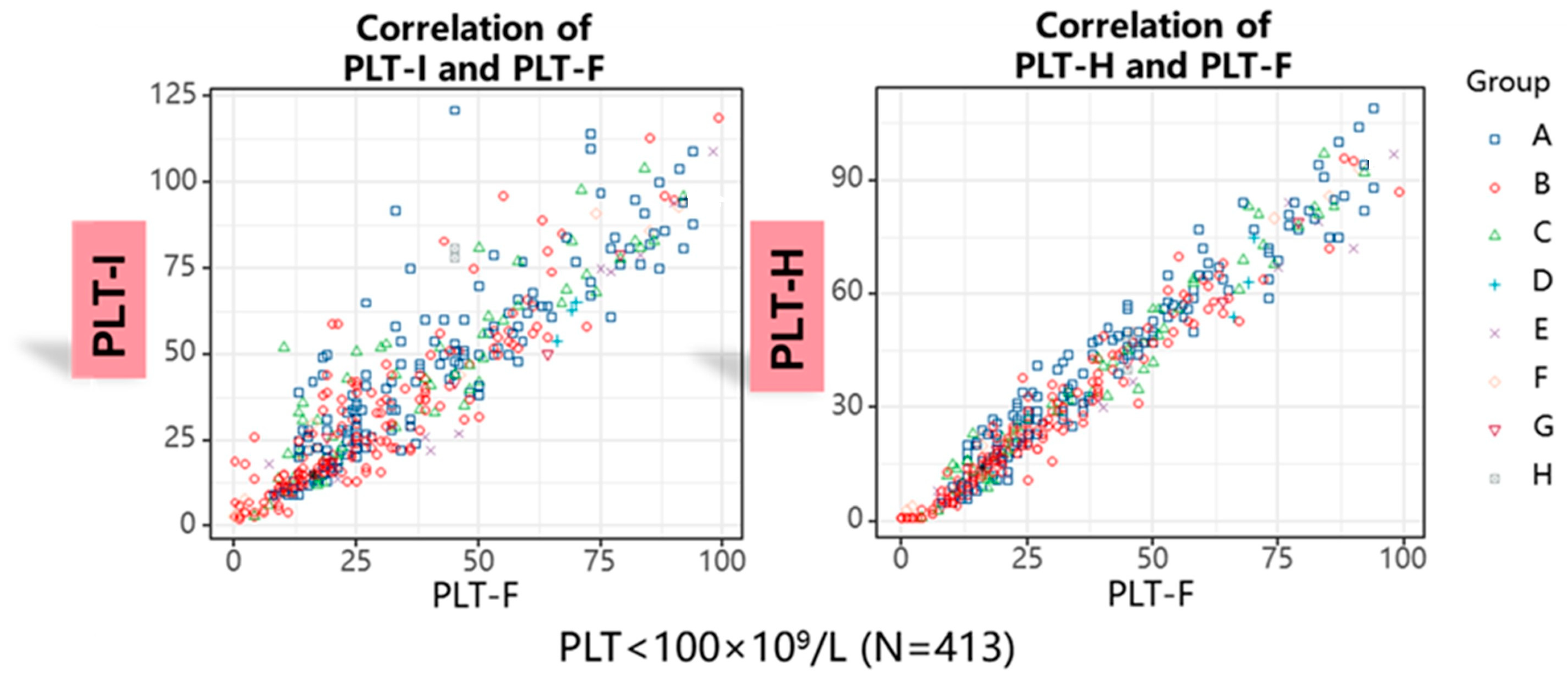
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassebaum, N.J.; GBD 2013 Anemia Collaborators. The Global Burden of Anemia. Hematol. Oncol. Clin. N. Am. 2016, 30, 247–308. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ramirez, S.; Jerico, C.; Munoz, M. Perioperative anemia: Prevalence, consequences and pathophysiology. Transfus. Apher. Sci. 2019, 58, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Yang, J.J.; Suh, J.T.; Lee, W.I.; Lee, H.J.; Park, T.S. Mean platelet volume/platelet count ratio in anemia. Platelets 2013, 24, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Talamo, G.; Oyeleye, O.; Paudel, A.; Tayyab, H.; Khan, M.; Meseeha, M.G.; Bhatta, G. Platelet levels before and after iron replacement therapy in patients with iron deficiency anemia. Hematology 2025, 30, 2458358. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Qin, X.; Liu, W. Platelet-Acute Leukemia Interactions. Clin. Chim. Acta 2022, 536, 29–38. [Google Scholar] [CrossRef]
- Cox, D. Sepsis—It is all about the platelets. Front. Immunol. 2023, 14, 1210219. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, L.; Ye, F.; Shen, G.; Wang, Y. Spurious Platelet Counts due to Cell Fragmentation in a Patient with Influenza B Virus. Clin. Lab. 2023, 69, 1041–1044. [Google Scholar] [CrossRef]
- Mylemans, M.; Boeckx, N.; Dierickx, D.; Tajdar, M.; van Laer, C. Blood smear and fluorescence based platelet count are key in a case of cryoglobulin masked thrombocytopenia. Int. J. Lab. Hematol. 2023, 45, 825–827. [Google Scholar] [CrossRef]
- Noris, P.; Balduini, C.L. Small red blood cells mimicking platelets. Blood 2014, 123, 4014. [Google Scholar] [CrossRef][Green Version]
- Bassi, C.; Richard, C.; Bene, M.C.; Eveillard, M. Interference in platelet count due to microspherocytosis related to extensive burn injury. Int. J. Lab. Hematol. 2022, 44, 57–58. [Google Scholar] [CrossRef]
- Briggs, C.; Harrison, P.; Machin, S.J. Continuing developments with the automated platelet count. Int. J. Lab. Hematol. 2007, 29, 77–91. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Hu, H. Advances and challenges in platelet counting: Evolving from traditional microscopy to modern flow cytometry. J. Lab. Med. 2024, 49, 2–13. [Google Scholar] [CrossRef]
- International Council for Standardization in Haematology Expert Panel on Cytometry; International Society of Laboratory Hematology Task Force on Platelet Counting. Platelet counting by the RBC/platelet ratio method: A Reference Method. Am. J. Clin. Pathol. 2001, 115, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Cai, Q.; Chen, G.; Liu, Z.; Liu, Y.; Bian, X.; Yao, D.; Xu, K. A New Anti-Interfering Platelet Counting Technology Utilizing Conventional Impedance and White Blood Cell Differential Channel. Int. J. Lab. Hematol. 2025, 47, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Lin, H.; Guo, P. Performance evaluation of PLT-H (hybrid-channel platelet) under various interferences and application studies for platelet transfusion decisions. Platelets 2023, 34, 2287064. [Google Scholar] [CrossRef]
- Park, S.H.; Park, C.J.; Kim, M.J.; Han, M.Y.; Lee, B.R.; Cho, Y.U.; Jang, S. The Sysmex XN-2000 hematology autoanalyzer provides a highly accurate platelet count than the former Sysmex XE-2100 system based on comparison with the CD41/CD61 immunoplatelet reference method of flow cytometry. Ann. Lab. Med. 2014, 34, 471–474. [Google Scholar] [CrossRef]
- Deng, J.; Xie, S.; Chen, Y.; Ma, Q.; He, Y.; Liu, M.; Wang, D.; Yu, X. Application of the Fluorescence Method. on Sysmex XN9000 Hematology Analyzer for Correcting Platelet Count in Individuals with Microcytosis. Lab. Med. 2023, 54, e10–e13. [Google Scholar] [CrossRef]
- Westgard Quality Control. 2025 CLIA Proposed Acceptance Limits for Proficiency Testing. Available online: https://westgard.com/clia-a-quality/quality-requirements/2024-clia-requirements.html (accessed on 30 May 2025).
- Hummel, K.; van Dun, L.; Sachse, M.; Beeger, C.; Hoffmann, J. Leukocyte fragments may interfere in the fluorescent platelet count of Sysmex XN hematology analyzers. Int. J. Lab. Hematol. 2020, 42, e167–e169. [Google Scholar] [CrossRef]
- Krishnamurthy, V.; Shivamurthy, A.; Kumar, P.V. Platelet count in impedance-based hematology analyzer: Beware of trap! Asian J. Transfus. Sci. 2023, 17, 131–132. [Google Scholar] [CrossRef]
- Boulassel, M.R.; Al-Farsi, R.; Al-Hashmi, S.; Al-Riyami, H.; Khan, H.; Al-Kindi, S. Accuracy of Platelet Counting by Optical and Impedance Methods in Patients with Thrombocytopaenia and Microcytosis. Sultan Qaboos Univ. Med. J. 2015, 15, e463–e468. [Google Scholar] [CrossRef][Green Version]
- Chen, S.; Mao, Z.; Wang, S.; Deng, J.; Liao, H.; Zheng, Q. Micro-Red Blood Cell, Fragmented Red Blood Cell, Platelet Distribution Width, Mean Platelet Volume, and Platelet-Large Cell Ratio on Sysmex XN Series Hematology Analyzers Can Be Used for the Reflex Test of Impedance Platelet Count in Clinical Practice. Arch. Pathol. Lab. Med. 2024, 148, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Tantanate, C. Spuriously high platelet counts by various automated hematology analyzers in a patient with disseminated intravascular coagulation. Clin. Chem. Lab. Med. 2015, 53, e257–e259. [Google Scholar] [CrossRef] [PubMed]
- Barros Pinto, M.P. The importance of schistocytes in a patient in VA-ECMO. Morphologie 2024, 108, 100777. [Google Scholar] [CrossRef] [PubMed]
- Barros Pinto, M.P. Spurious thrombocytosis, red cell count and mean cell volume in a patient with thermal burns. Br. J. Haematol. 2023, 200, 121. [Google Scholar] [CrossRef]
- Lesesve, J.F.; Salignac, S.; Alla, F.; Defente, M.; Benbih, M.; Bordigoni, P.; Lecompte, T. Comparative evaluation of schistocyte counting by an automated method and by microscopic determination. Am. J. Clin. Pathol. 2004, 121, 739–745. [Google Scholar] [CrossRef]
- Lam, W.K.; Law, Y.F.W.; Yip, S.F. Resolution of platelet count interference due to cytoplasmic fragments of leukaemic cells by flow cytometry in acute myeloid leukaemia. Int. J. Lab. Hematol. 2022, 44, 983–985. [Google Scholar] [CrossRef]
- Frotscher, B.; Salignac, S.; Muller, M.; Latger-Cannard, V.; Feugier, P.; Lesesve, J.F. Interference of blast cell fragments with automated platelet counting. Int. J. Lab. Hematol. 2015, 37, 613–619. [Google Scholar] [CrossRef]
- Briggs, C.J.; Machin, S.J. Discrepancy between impedance and immunofluorescence platelet counting has implications for clinical decision making in patients with idiopathic thrombocytopenia purpura. Br. J. Haematol. 2006, 135, 416–417. [Google Scholar] [CrossRef]
- Gonzalez-Casas, R.; Jones, E.A.; Moreno-Otero, R. Spectrum of anemia associated with chronic liver disease. World J. Gastroenterol. 2009, 15, 4653–4658. [Google Scholar] [CrossRef]
- Gaspar, B.L.; Sharma, P.; Das, R. Anemia in malignancies: Pathogenetic and diagnostic considerations. Hematology 2015, 20, 18–25. [Google Scholar] [CrossRef]
- Evstatiev, R.; Bukaty, A.; Jimenez, K.; Kulnigg-Dabsch, S.; Surman, L.; Schmid, W.; Eferl, R.; Lippert, K.; Scheiber-Mojdehkar, B.; Kvasnicka, H.M.; et al. Iron deficiency alters megakaryopoiesis and platelet phenotype independent of thrombopoietin. Am. J. Hematol. 2014, 89, 524–529. [Google Scholar] [CrossRef]
- Li, J.; Duan, Q.J. Severe hemolytic anemia and acute renal failure after mitral valve repair associated with non-endothelialization of artificial chordae tendinae: Case report. J. Cardiothorac. Surg. 2021, 16, 303. [Google Scholar] [CrossRef]
- Aikins, J.; Koomson, A.; Ladele, M.; Al-Nusair, L.; Ahmed, A.; Ashry, A.; Harky, A. Anticoagulation and antiplatelet therapy in patients with prosthetic heart valves. J. Card. Surg. 2020, 35, 3521–3529. [Google Scholar] [CrossRef]
- Katoh, A.; Ikeda, H. Platelet count as a prognostic marker in patients with acute coronary syndromes. Circ. J. 2012, 76, 591–592. [Google Scholar] [CrossRef]
- Dahlen, B.; Schulz, A.; Gobel, S.; Trobs, S.O.; Schwuchow-Thonke, S.; Spronk, H.M.; Prochaska, J.H.; Arnold, N.; Lackner, K.J.; Gori, T.; et al. The impact of platelet indices on clinical outcome in heart failure: Results from the MyoVasc study. ESC Heart Fail. 2021, 8, 2991–3001. [Google Scholar] [CrossRef]




| Group | Cause of Anemia | Number of Samples |
|---|---|---|
| A (non-hematological metabolic disorders) | Alcoholic liver disease/ HCV infection/Cirrhosis/Sepsis/Qualification for liver transplantation/Primary biliary cholangitis/Diabetes | 197 |
| B (hematological cancers/disorders affecting WBC, RBC, and PLT production) | Aplastic anemia/Leukemia/Lymphoma/Multiple myeloma/Myeloproliferative neoplasm | 193 |
| C (non-hematopoietic-related solid tumors) | Hepatocellular carcinoma/Colon cancer/Gastric cancer/Lung adenocarcinoma | 90 |
| D (microcytic anemia) | Iron deficiency anemia/Thalassemia/Chronic blood loss | 39 |
| E (non-inherited hemolytic anemia) | Anemia associated with cardiovascular events | 29 |
| F (acute blood loss anemia) | Surgery/Acute gastrointestinal bleeding | 19 |
| G (ITP) | Idiopathic thrombocytopenia | 8 |
| H (TTP) | Thrombotic thrombocytopenic purpura | 2 |
| Obtained Value | Absolute Deviation | Percentage of Maximal Target Value |
|---|---|---|
| 0–10 × 109/L | ±5 × 109/L | 50% |
| 10–20 × 109/L | ±8 × 109/L | 40% |
| 20–50 × 109/L | ±10 × 109/L | 20% |
| 50–100 × 109/L | ±20 × 109/L | 20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wituska, M.; Ciepiela, O. Application of Hybrid Platelet Technology for Platelet Count Improves Accuracy of PLT Measurement in Samples from Patients with Different Types of Anemia. J. Clin. Med. 2025, 14, 5401. https://doi.org/10.3390/jcm14155401
Wituska M, Ciepiela O. Application of Hybrid Platelet Technology for Platelet Count Improves Accuracy of PLT Measurement in Samples from Patients with Different Types of Anemia. Journal of Clinical Medicine. 2025; 14(15):5401. https://doi.org/10.3390/jcm14155401
Chicago/Turabian StyleWituska, Małgorzata, and Olga Ciepiela. 2025. "Application of Hybrid Platelet Technology for Platelet Count Improves Accuracy of PLT Measurement in Samples from Patients with Different Types of Anemia" Journal of Clinical Medicine 14, no. 15: 5401. https://doi.org/10.3390/jcm14155401
APA StyleWituska, M., & Ciepiela, O. (2025). Application of Hybrid Platelet Technology for Platelet Count Improves Accuracy of PLT Measurement in Samples from Patients with Different Types of Anemia. Journal of Clinical Medicine, 14(15), 5401. https://doi.org/10.3390/jcm14155401






