Effectiveness of Ultrasound-Guided Lavage for Rotator Cuff Calcific Tendinopathy: A Case Series Study from a Clinical and Radiological Perspective
Abstract
1. Introduction
2. Materials and Methods
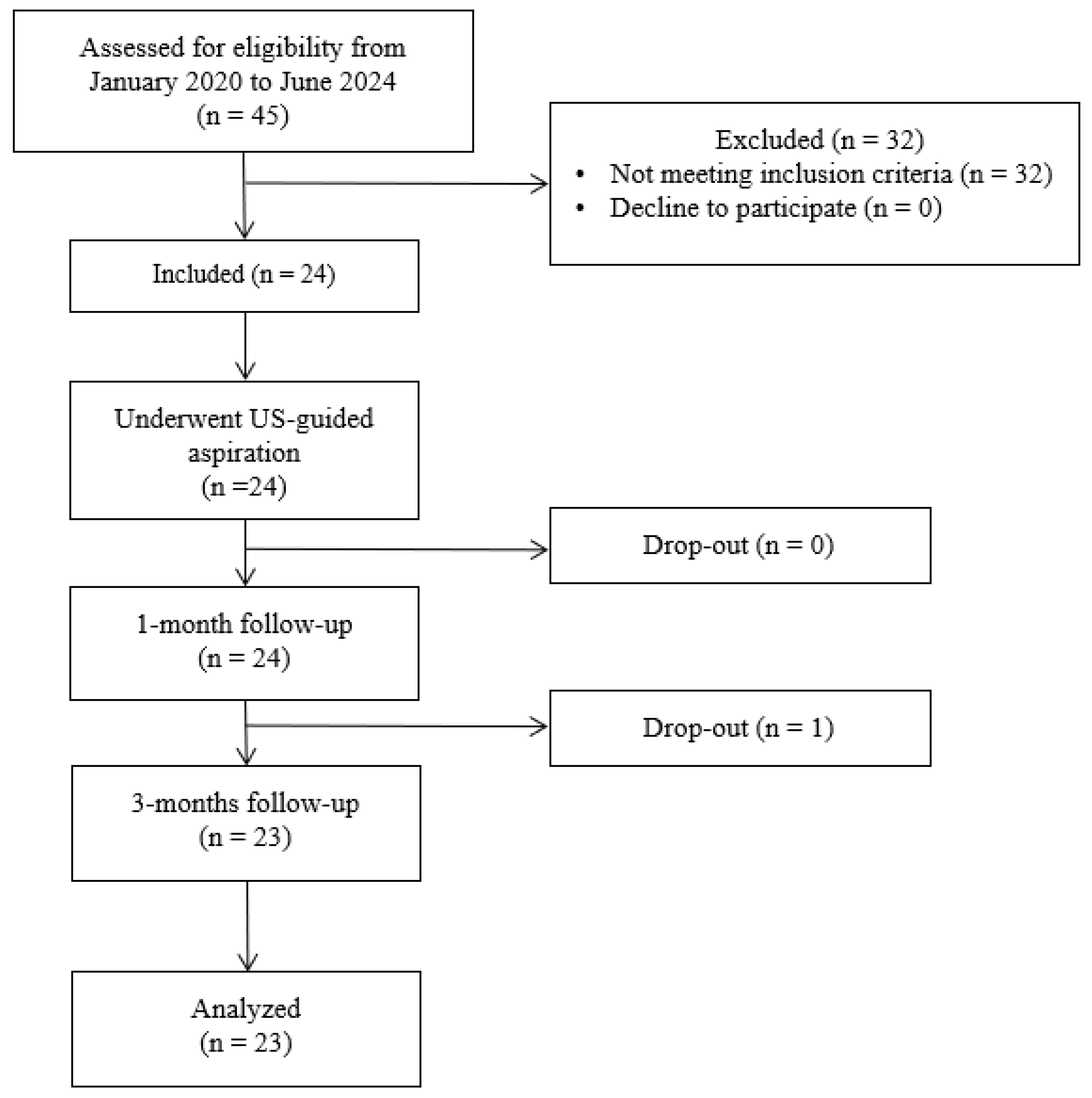
2.1. Participants
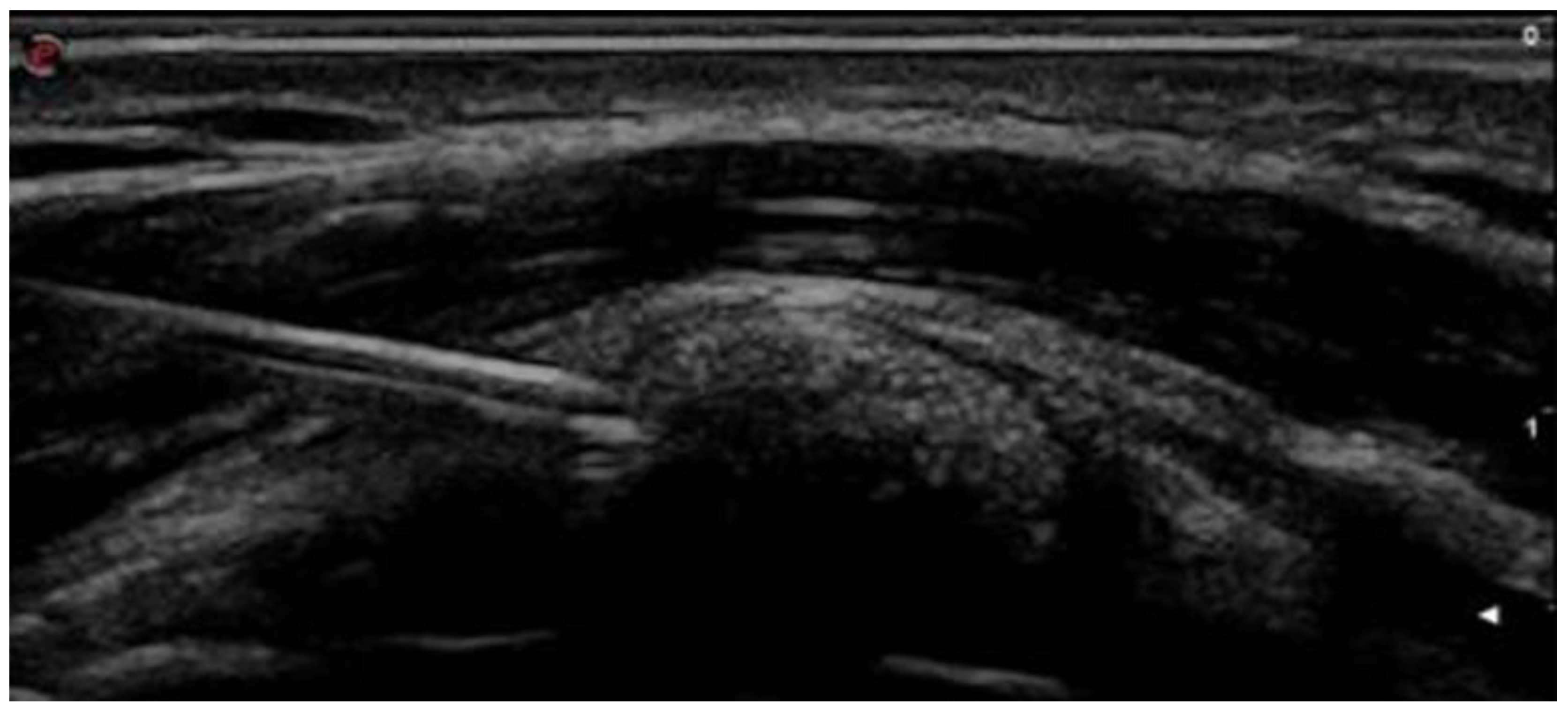
2.2. Intervention
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
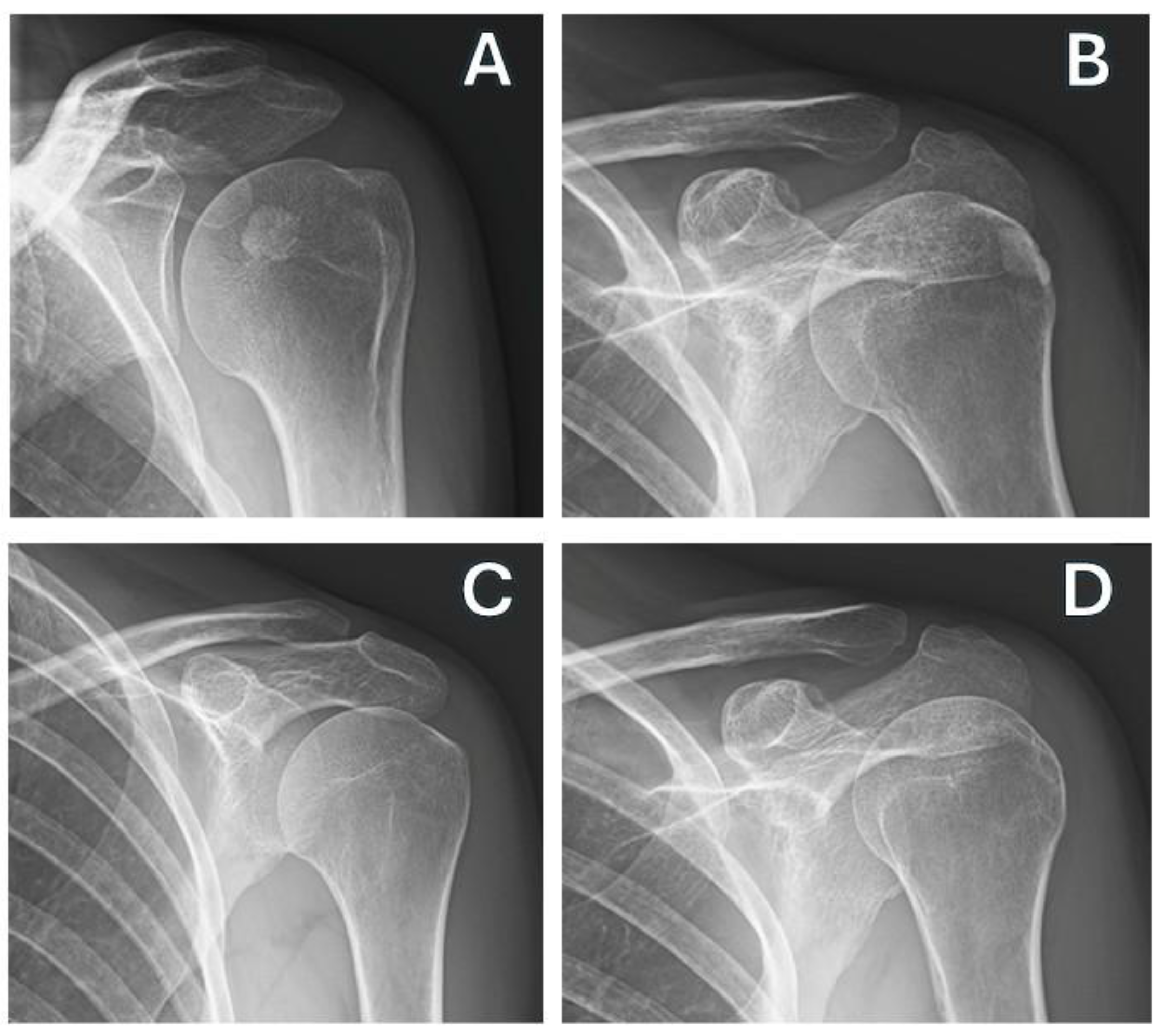
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RCCT | rotator cuff calcific tendinopathy |
| ESWT | focal extracorporeal shockwave therapy |
| STROBE | strengthening the reporting of observational studies in epidemiology |
| NRS | Numeric Rating Scale |
| DASH | Disability of Arm, Shoulder and Hand |
| CMS | Constant–Murley score |
| PROM | passive range of motion |
References
- Louwerens, J.K.G.; Sierevelt, I.N.; van Hove, R.P.; van den Bekerom, M.P.J.; van Noort, A. Prevalence of Calcific Deposits within the Rotator Cuff Tendons in Adults with and without Subacromial Pain Syndrome: Clinical and Radiologic Analysis of 1219 Patients. J. Shoulder Elb. Surg. 2015, 24, 1588–1593. [Google Scholar] [CrossRef]
- Čota, S.; Delimar, V.; Žagar, I.; Kovač Durmiš, K.; Kristić Cvitanović, N.; Žura, N.; Perić, P.; Laktašić Žerjavić, N. Efficacy of Therapeutic Ultrasound in the Treatment of Chronic Calcific Shoulder Tendinitis: A Randomized Trial. Eur. J. Phys. Rehabil. Med. 2023, 59, 75–84. [Google Scholar] [CrossRef]
- Darrieutort-Laffite, C.; Arnolfo, P.; Garraud, T.; Adrait, A.; Couté, Y.; Louarn, G.; Trichet, V.; Layrolle, P.; Le Goff, B.; Blanchard, F. Rotator Cuff Tenocytes Differentiate into Hypertrophic Chondrocyte-Like Cells to Produce Calcium Deposits in an Alkaline Phosphatase-Dependent Manner. J. Clin. Med. 2019, 8, 1544. [Google Scholar] [CrossRef]
- De Carli, A.; Pulcinelli, F.; Rose, G.D.; Pitino, D.; Ferretti, A. Calcific Tendinitis of the Shoulder. Joints 2014, 2, 130–136. [Google Scholar] [CrossRef]
- Merolla, G.; Singh, S.; Paladini, P.; Porcellini, G. Calcific Tendinitis of the Rotator Cuff: State of the Art in Diagnosis and Treatment. J. Orthop. Traumatol. 2016, 17, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Mavrikakis, M.E.; Drimis, S.; Kontoyannis, D.A.; Rasidakis, A.; Moulopoulou, E.S.; Kontoyannis, S. Calcific Shoulder Periarthritis (Tendinitis) in Adult Onset Diabetes Mellitus: A Controlled Study. Ann. Rheum. Dis. 1989, 48, 211–214. [Google Scholar] [CrossRef]
- Uhthoff, H.K.; Sarkar, K.; Maynard, J.A. Calcifying Tendinitis: A New Concept of Its Pathogenesis. Clin. Orthop. Relat. Res. 1976, 118, 164–168. [Google Scholar] [CrossRef]
- Ricci, V.; Mezian, K.; Chang, K.-V.; Özçakar, L. Clinical/Sonographic Assessment and Management of Calcific Tendinopathy of the Shoulder: A Narrative Review. Diagnostics 2022, 12, 3097. [Google Scholar] [CrossRef]
- Louwerens, J.K.G.; Sierevelt, I.N.; van Noort, A.; van den Bekerom, M.P.J. Evidence for Minimally Invasive Therapies in the Management of Chronic Calcific Tendinopathy of the Rotator Cuff: A Systematic Review and Meta-Analysis. J. Shoulder Elb. Surg. 2014, 23, 1240–1249. [Google Scholar] [CrossRef]
- Gartner, J.; Heyer, A. Calcifying Tendinitis of the Shoulder. Orthopade 1995, 24, 284–302. [Google Scholar]
- Sconfienza, L.M.; Albano, D.; Allen, G.; Bazzocchi, A.; Bignotti, B.; Chianca, V.; Facal de Castro, F.; Drakonaki, E.E.; Gallardo, E.; Gielen, J.; et al. Clinical Indications for Musculoskeletal Ultrasound Updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) Consensus. Eur. Radiol. 2018, 28, 5338–5351. [Google Scholar] [CrossRef]
- Familiari, F.; Ammendolia, A.; Rupp, M.-C.; Russo, R.; Pujia, A.; Montalcini, T.; Marotta, N.; Mercurio, M.; Galasso, O.; Millett, P.J.; et al. Efficacy of Intra-Articular Injections of Hyaluronic Acid in Patients with Glenohumeral Joint Osteoarthritis: A Systematic Review and Meta-Analysis. J. Orthop. Res. 2023, 41, 2345–2358. [Google Scholar] [CrossRef]
- Catapano, M.; Robinson, D.M.; Schowalter, S.; McInnis, K.C. Clinical Evaluation and Management of Calcific Tendinopathy: An Evidence-Based Review. J. Osteopath. Med. 2022, 122, 141–151. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Midiri, F.; Mauri, G.; Aliprandi, A.; Catapano, M.; Pescatori, L.C.; Monaco, C.G.; Gitto, S.; et al. Rotator Cuff Calcific Tendinopathy: From Diagnosis to Treatment. Acta Biomed. 2018, 89, 186–196. [Google Scholar] [CrossRef]
- Minici, R.; Mercurio, M.; Iannò, B.; Galasso, O.; Gasparini, G.; Laganà, D. Advantages of the Use of Axial Traction Magnetic Resonance Imaging (MRI) of the Shoulder in Patients with Suspected Rota-Tor Cuff Tears: An Exploratory Pilot Study. Healthcare 2023, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Michener, L.A.; Snyder, A.R.; Leggin, B.G. Responsiveness of the Numeric Pain Rating Scale in Patients with Shoulder Pain and the Effect of Surgical Status. J. Sport. Rehabil. 2011, 20, 115–128. [Google Scholar] [CrossRef]
- Umamahesvaran, B.; Sambandam, S.N.; Mounasamy, V.; Gokulakrishnan, P.P.; Ashraf, M. Calcifying Tendinitis of Shoulder: A Concise Review. J. Orthop. 2018, 15, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Germann, G.; Wind, G.; Harth, A. The DASH(Disability of Arm-Shoulder-Hand) Questionnaire—A new instrument for evaluating upper extremity treatment outcome. Handchir. Mikrochir. Plast. Chir. 1999, 31, 149–152. [Google Scholar] [CrossRef]
- Constant, C.R.; Murley, A.H. A Clinical Method of Functional Assessment of the Shoulder. Clin. Orthop. Relat. Res. 1987, 214, 160–164. [Google Scholar] [CrossRef]
- Constant, C.R.; Gerber, C.; Emery, R.J.H.; Søjbjerg, J.O.; Gohlke, F.; Boileau, P. A Review of the Constant Score: Modifications and Guidelines for Its Use. J. Shoulder Elb. Surg. 2008, 17, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G. Effect Size Guidelines for Individual and Group Differences in Physiotherapy. Arch. Phys. Med. Rehabil. 2025. [Google Scholar] [CrossRef] [PubMed]
- Correll, J.; Mellinger, C.; Pedersen, E.J. Flexible Approaches for Estimating Partial Eta Squared in Mixed-Effects Models with Crossed Random Factors. Behav. Res. Methods 2022, 54, 1626–1642. [Google Scholar] [CrossRef] [PubMed]
- Lanza, E.; Banfi, G.; Serafini, G.; Lacelli, F.; Orlandi, D.; Bandirali, M.; Sardanelli, F.; Sconfienza, L.M. Ultrasound-Guided Percutaneous Irrigation in Rotator Cuff Calcific Tendinopathy: What Is the Evidence? A Systematic Review with Proposals for Future Reporting. Eur. Radiol. 2015, 25, 2176–2183. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Viganò, S.; Martini, C.; Aliprandi, A.; Randelli, P.; Serafini, G.; Sardanelli, F. Double-Needle Ultrasound-Guided Percutaneous Treatment of Rotator Cuff Calcific Tendinitis: Tips & Tricks. Skelet. Radiol. 2013, 42, 19–24. [Google Scholar] [CrossRef]
- Albano, D.; Gambino, A.; Messina, C.; Chianca, V.; Gitto, S.; Faenza, S.; Galia, M.; Sconfienza, L.M. Ultrasound-Guided Percutaneous Irrigation of Rotator Cuff Calcific Tendinopathy (US-PICT): Patient Experience. Biomed. Res. Int. 2020, 2020, 3086395. [Google Scholar] [CrossRef]
- Farin, P.U.; Räsänen, H.; Jaroma, H.; Harju, A. Rotator Cuff Calcifications: Treatment with Ultrasound-Guided Percutaneous Needle Aspiration and Lavage. Skelet. Radiol. 1996, 25, 551–554. [Google Scholar] [CrossRef]
- Comfort, T.H.; Arafiles, R.P. Barbotage of the Shoulder with Image-Intensified Fluoroscopic Control of Needle Placement for Calcific Tendinitis. Clin. Orthop. Relat. Res. 1978, 135, 171–178. [Google Scholar] [CrossRef]
- Compagnoni, R.; Menon, A.; Radaelli, S.; Lanzani, F.; Gallazzi, M.B.; Tassi, A.; Randelli, P.S. Long-Term Evolution of Calcific Tendinitis of the Rotator Cuff: Clinical and Radiological Evaluation 10 Years after Diagnosis. J. Orthop. Traumatol. 2021, 22, 42. [Google Scholar] [CrossRef]
- Verstraelen, F.; Verhagen, S.; Giesberts, A.; Bonneux, I.; Koot, H.; Boer, W.; van der Steen, M. Needle Aspiration of Calcific Deposits versus Shock Wave Therapy for Conservative Therapy Resistant Calcifying Tendinitis of the Shoulder: Protocol of a Randomized, Controlled Trial. BMC Musculoskelet. Disord. 2022, 23, 308. [Google Scholar] [CrossRef]
- Lin, J.T.; Adler, R.S.; Bracilovic, A.; Cooper, G.; Sofka, C.; Lutz, G.E. Clinical Outcomes of Ultrasound-Guided Aspiration and Lavage in Calcific Tendinosis of the Shoulder. HSS J. 2007, 3, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Oudelaar, B.W.; Veld, R.H.I.; Schepers-Bok, R.; Ooms, E.M.; Nelissen, R.G.H.H.; Vochteloo, A.J.H. Prognostic Factors for the Outcome of Needle Aspiration of Calcific Deposits for Rotator Cuff Calcific Tendinitis. Eur. Radiol. 2020, 30, 4082–4090. [Google Scholar] [CrossRef]
- De Conti, G.; Marchioro, U.; Dorigo, A.; Boscolo, N.; Vio, S.; Trevisan, M.; Meneghini, A.; Baldo, V.; Angelini, F. Percutaneous Ultrasound-Guided Treatment of Shoulder Tendon Calcifications: Clinical and Radiological Follow-up at 6 Months. J. Ultrasound 2010, 13, 188–198. [Google Scholar] [CrossRef]
- AL-Bashaireh, A.M.; Haddad, L.G.; Weaver, M.; Kelly, D.L.; Chengguo, X.; Yoon, S. The Effect of Tobacco Smoking on Musculoskeletal Health: A Systematic Review. J. Environ. Public Health 2018, 2018, 4184190. [Google Scholar] [CrossRef]
- Oudelaar, B.W.; Ooms, E.M.; Huis In ’t Veld, R.M.H.A.; Schepers-Bok, R.; Vochteloo, A.J. Smoking and Morphology of Calcific Deposits Affect the Outcome of Needle Aspiration of Calcific Deposits (NACD) for Calcific Tendinitis of the Rotator Cuff. Eur. J. Radiol. 2015, 84, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Banfi, G.; Orlandi, D.; Lacelli, F.; Serafini, G.; Mauri, G.; Secchi, F.; Silvestri, E.; Sconfienza, L.M. Ultrasound-Guided Interventional Procedures around the Shoulder. Br. J. Radiol. 2016, 89, 20150372. [Google Scholar] [CrossRef]
- Orlandi, D.; Mauri, G.; Lacelli, F.; Corazza, A.; Messina, C.; Silvestri, E.; Serafini, G.; Sconfienza, L.M. Rotator Cuff Calcific Tendinopathy: Randomized Comparison of US-Guided Percutaneous Treatments by Using One or Two Needles. Radiology 2017, 285, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- del Cura, J.L.; Torre, I.; Zabala, R.; Legórburu, A. Sonographically Guided Percutaneous Needle Lavage in Calcific Tendinitis of the Shoulder: Short- and Long-Term Results. Am. J. Roentgenol. 2007, 189, W128–W134. [Google Scholar] [CrossRef]
- Gerdesmeyer, L.; Wagenpfeil, S.; Haake, M.; Maier, M.; Loew, M.; Wörtler, K.; Lampe, R.; Seil, R.; Handle, G.; Gassel, S.; et al. Extracorporeal Shock Wave Therapy for the Treatment of Chronic Calcifying Tendonitis of the Rotator Cuff: A Randomized Controlled Trial. JAMA 2003, 290, 2573–2580. [Google Scholar] [CrossRef]
- de Filippis, R.; Mercurio, M.; Segura-Garcia, C.; De Fazio, P.; Gasparini, G.; Galasso, O. Defining the Minimum Clinically Important Difference (MCID) in the Hospital Anxiety and Depression Scale (HADS) in Patients Undergoing Total Hip and Knee Arthroplasty. Orthop. Traumatol. Surg. Res. 2024, 110, 103689. [Google Scholar] [CrossRef]
- de Filippis, R.; Mercurio, M.; Spina, G.; De Fazio, P.; Segura-Garcia, C.; Familiari, F.; Gasparini, G.; Galasso, O. Antidepressants and Vertebral and Hip Risk Fracture: An Updated Systematic Review and Meta-Analysis. Healthcare 2022, 10, 803. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.; Castioni, D.; de Filippis, R.; De Fazio, P.; Paone, A.; Familiari, F.; Gasparini, G.; Galasso, O. Postoperative Psychological Factors and Quality of Life but Not Shoulder Brace Adherence Affect Clinical Outcomes after Arthroscopic Rotator Cuff Repair. J. Shoulder Elb. Surg. 2023, 32, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Galasso, O.; Mercurio, M.; Luciano, F.; Mancuso, C.; Gasparini, G.; De Benedetto, M.; Orlando, N.; Castricini, R. Arthroscopic Capsular Release for Frozen Shoulder: When Etiology Matters. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5248–5254. [Google Scholar] [CrossRef] [PubMed]

| Age | Sex | Height | Weight | BMI | Smoke | Dysthyroidism | Diabetes Mellitus | Affected Side | Dominant Side | Previous Treatment | Type of Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 59 | F | 1.75 | 80 | 26.1 | No | No | Yes | Right | Right | Yes | Intra-articular injection |
| 65 | M | 1.65 | 58 | 21.3 | Yes | No | No | Right | Right | No | |
| 58 | M | 1.75 | 71 | 23.1 | No | No | No | Left | Right | No | |
| 56 | F | 1.65 | 58 | 21.3 | No | No | No | Left | Right | No | |
| 61 | F | 1.58 | 57 | 22.8 | No | No | No | Right | Right | Yes | ESWT |
| 69 | M | 1.78 | 90 | 28.4 | No | No | Yes | Right | Left | No | |
| 51 | M | 1.73 | 68 | 22.7 | No | Yes | No | Left | Left | No | |
| 50 | F | 1.63 | 65 | 24.4 | No | Yes | No | Right | Left | No | |
| 52 | M | 1.76 | 64 | 20.6 | No | Yes | No | Right | Right | No | |
| 48 | F | 1.88 | 80 | 22.6 | No | No | No | Left | Left | Yes | ESWT |
| 54 | M | 1.62 | 61 | 23.2 | No | Yes | No | Right | Right | No | |
| 55 | F | 1.66 | 82 | 29.7 | No | No | Yes | Right | Right | No | |
| 41 | F | 1.58 | 50 | 20.0 | Yes | Yes | No | Right | Right | No | |
| 58 | F | 1.6 | 49 | 19.1 | No | No | No | Right | Right | No | |
| 80 | M | 1.83 | 75 | 22.4 | No | Yes | Yes | Left | Right | No | |
| 59 | M | 1.78 | 70 | 22.0 | No | Yes | Yes | Right | Left | No | |
| 69 | M | 1.86 | 85 | 24.5 | Yes | No | No | Right | Right | No | |
| 40 | F | 1.5 | 45 | 20.0 | Yes | Yes | Yes | Right | Right | Yes | ESWT |
| 59 | F | 1.65 | 55 | 20.2 | No | Yes | No | Left | Right | No | |
| 55 | M | 1.8 | 78 | 24.0 | Yes | Yes | No | Left | Left | No | |
| 53 | M | 1.85 | 83 | 24.2 | No | No | Yes | Right | Left | Yes | Intra-articular injection |
| 62 | F | 1.63 | 56 | 21.0 | Yes | Yes | No | Left | Right | No | |
| 69 | F | 1.7 | 60 | 20.7 | No | No | Yes | Right | Left | No | |
| 55.5 (7.75) | 1.69 (0.10) | 66.5 (19.75) | 23.0 (2.57) |
| NRS | Gartner | DASH | CMS | Calcification Measure (cm) | PROM Flexion (°) | PROM Abduction (°) |
|---|---|---|---|---|---|---|
| 8 | 2 | 74 | 23 | 1.2 | 90 | 60 |
| 7 | 1 | 65 | 40 | 1.4 | 90 | 90 |
| 5 | 2 | 55 | 60 | 1.6 | 100 | 90 |
| 6 | 3 | 53 | 52 | 1.6 | 120 | 110 |
| 7 | 1 | 69 | 48 | 1.7 | 80 | 80 |
| 5 | 2 | 47 | 76 | 1.8 | 120 | 120 |
| 6 | 2 | 61 | 67 | 1.9 | 110 | 90 |
| 5 | 3 | 41 | 55 | 2.5 | 110 | 100 |
| 7 | 2 | 67 | 43 | 0.8 | 70 | 70 |
| 7 | 2 | 71 | 38 | 1.5 | 100 | 90 |
| 5 | 2 | 44 | 90 | 1.3 | 150 | 130 |
| 8 | 1 | 63 | 40 | 0.8 | 70 | 70 |
| 6 | 2 | 73 | 43 | 1.3 | 80 | 70 |
| 8 | 2 | 66 | 60 | 1.3 | 100 | 80 |
| 5 | 1 | 50 | 55 | 1.7 | 110 | 100 |
| 9 | 2 | 55 | 46 | 1.5 | 110 | 100 |
| 8 | 1 | 67 | 80 | 1.75 | 90 | 90 |
| 7 | 1 | 49 | 60 | 1.75 | 110 | 110 |
| 8 | 2 | 59 | 55 | 1.9 | 120 | 100 |
| 6 | 3 | 43 | 40 | 2.8 | 100 | 90 |
| 9 | 1 | 65 | 40 | 0.7 | 80 | 80 |
| 10 | 1 | 75 | 90 | 1.55 | 90 | 70 |
| 7 | 2 | 40 | 60 | 1.3 | 150 | 130 |
| 7 (2) | 2 (1) | 0.61 (0.17) | 0.55 (0.18) | 1.55 (0.45) | 100 (20) | 90 (20) |
| Outcome Measures | T0 | T1 | T2 | p Value ΔT0–T1 | ES | 95%LCI | 95%UCI | p Value ΔT1–T2 | ES | 95%LCI | 95%UCI | p Value RM-ANOVA | η2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRS | 2 (2) | 3 (4) | 1 (1.5) | 0.001 * | 0.76 | 0.44 | 0.99 | 0.54 | 0.72 | 0.25 | 1.17 | <0.00001 * | 0.57 |
| Gartner | 2 (1) | 3 (1) | 3 (0.5) | 0.004 * | −0.89 | −1.56 | −0.80 | 0.157 | −0.15 | −0.56 | 0.27 | <0.00001 * | 0.37 |
| DASH | 0.61 (0.17) | 0.33 (0.12) | 0.2 (0.11) | 0.002 * | 0.85 | 0.16 | 1.52 | 0.44 | 0.94 | 0.44 | 1.43 | <0.00001 * | 0.64 |
| CMS | 0.55 (0.18) | 0.85 (0.20) | 0.96 (0.08) | 0.003 * | −0.81 | −1.36 | −0.44 | 0.043 * | −0.76 | −1.22 | −0.29 | <0.00001 * | 0.56 |
| Calcification diameter (cm) | 1.55 (0.45) | 0.90 (0.35) | 0 (0.45) | 0.002 * | 0.84 | 0.16 | 1.51 | 0.102 | 0.74 | 0.34 | 1.21 | <0.00001 * | 0.70 |
| PROM flexion (°) | 100 (20) | 140 (27.5) | 180 (10) | 0.003 * | 0.90 | 0.45 | 1.66 | 0.108 | −0.75 | −1.22 | −0.51 | <0.00001 * | 0.68 |
| PROM abduction (°) | 90 (20) | 135 (27.5) | 180 (17.5) | 0.002 * | −0.96 | −0.78 | −1.32 | 0.043 * | −0.68 | −1.31 | −0.03 | <0.00001 * | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moggio, L.; Mercurio, M.; Marotta, N.; Longo, U.G.; Gasparini, G.; Ammendolia, A.; de Sire, A. Effectiveness of Ultrasound-Guided Lavage for Rotator Cuff Calcific Tendinopathy: A Case Series Study from a Clinical and Radiological Perspective. J. Clin. Med. 2025, 14, 5376. https://doi.org/10.3390/jcm14155376
Moggio L, Mercurio M, Marotta N, Longo UG, Gasparini G, Ammendolia A, de Sire A. Effectiveness of Ultrasound-Guided Lavage for Rotator Cuff Calcific Tendinopathy: A Case Series Study from a Clinical and Radiological Perspective. Journal of Clinical Medicine. 2025; 14(15):5376. https://doi.org/10.3390/jcm14155376
Chicago/Turabian StyleMoggio, Lucrezia, Michele Mercurio, Nicola Marotta, Umile Giuseppe Longo, Giorgio Gasparini, Antonio Ammendolia, and Alessandro de Sire. 2025. "Effectiveness of Ultrasound-Guided Lavage for Rotator Cuff Calcific Tendinopathy: A Case Series Study from a Clinical and Radiological Perspective" Journal of Clinical Medicine 14, no. 15: 5376. https://doi.org/10.3390/jcm14155376
APA StyleMoggio, L., Mercurio, M., Marotta, N., Longo, U. G., Gasparini, G., Ammendolia, A., & de Sire, A. (2025). Effectiveness of Ultrasound-Guided Lavage for Rotator Cuff Calcific Tendinopathy: A Case Series Study from a Clinical and Radiological Perspective. Journal of Clinical Medicine, 14(15), 5376. https://doi.org/10.3390/jcm14155376











