Clinical Characterization of the Lacrimal Functional Unit in Patients with Chronic Ocular Pain Associated with Dry Eye Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Sample
2.2. Study Design
2.3. Symptoms
2.4. Clinical Tests
2.5. Statistical Analysis
3. Results
3.1. Sample Description
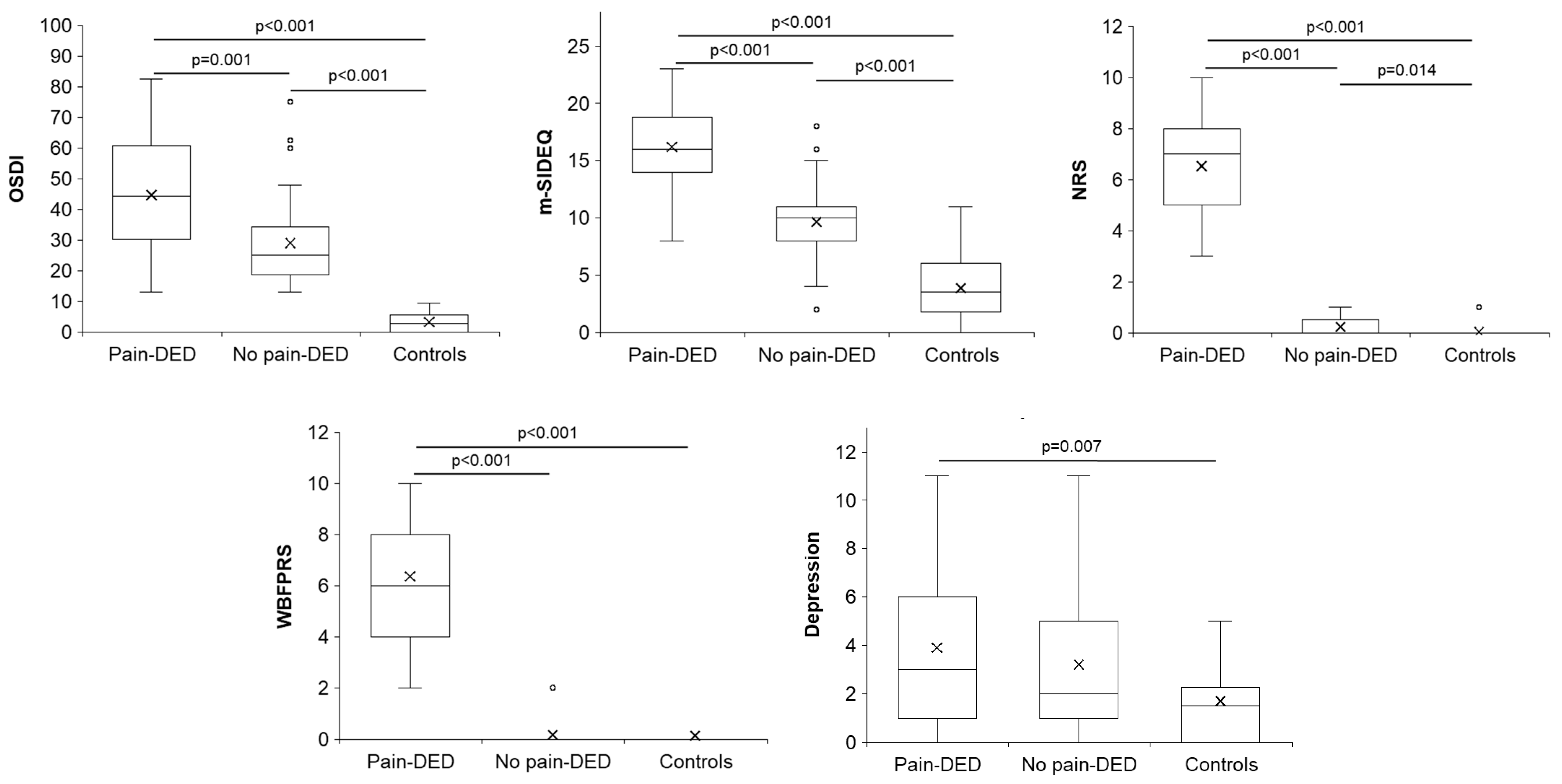
3.2. Symptoms
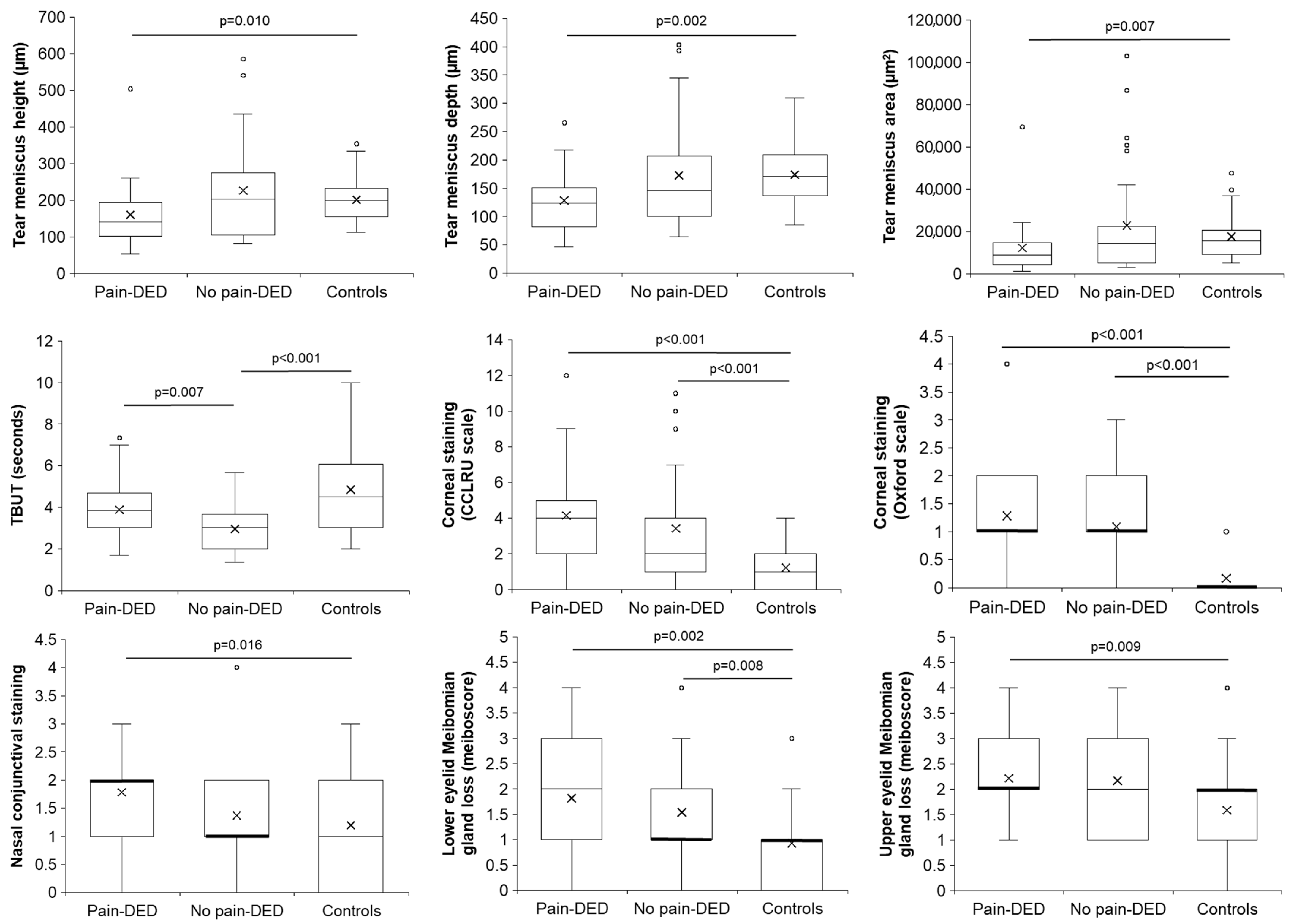
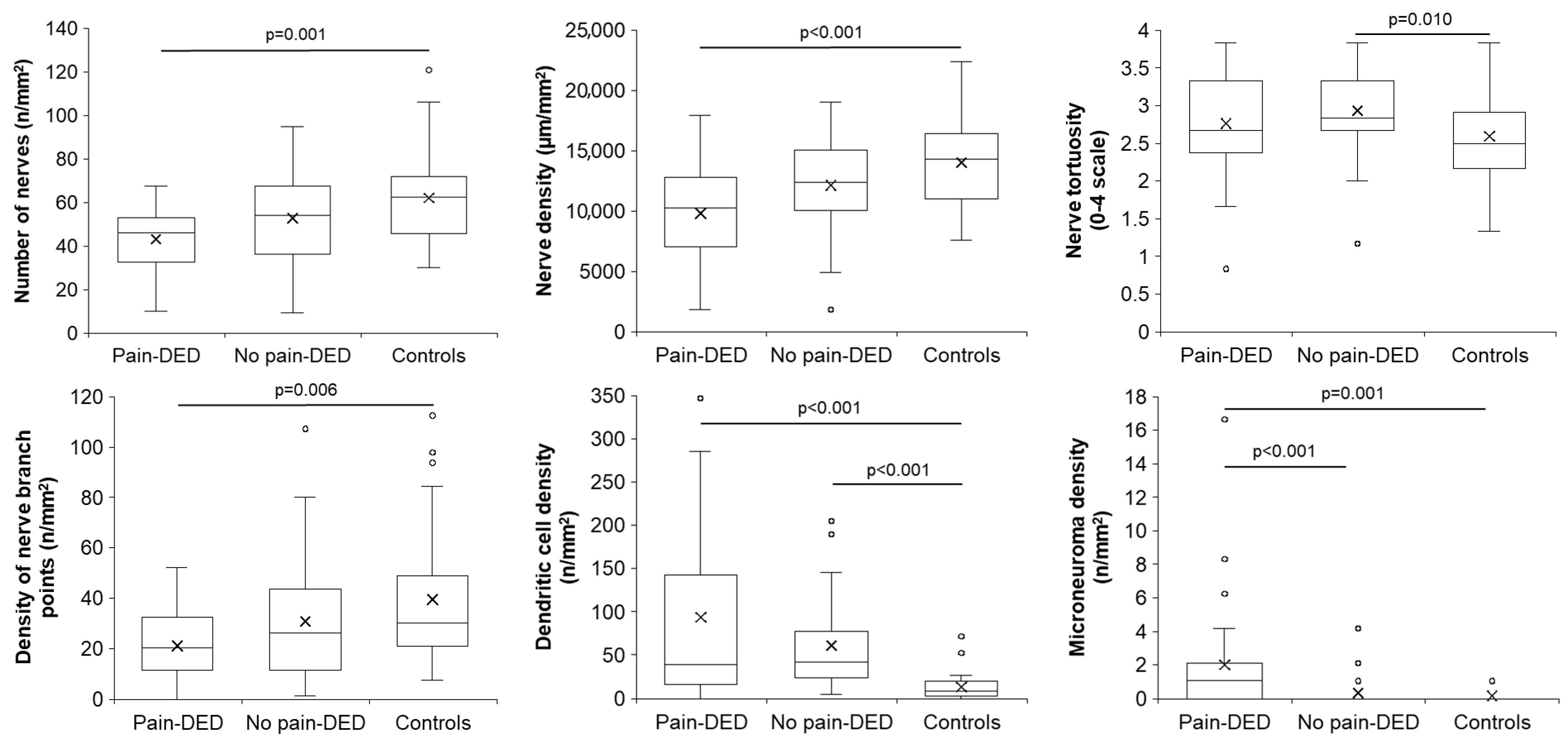
3.3. Clinical Tests
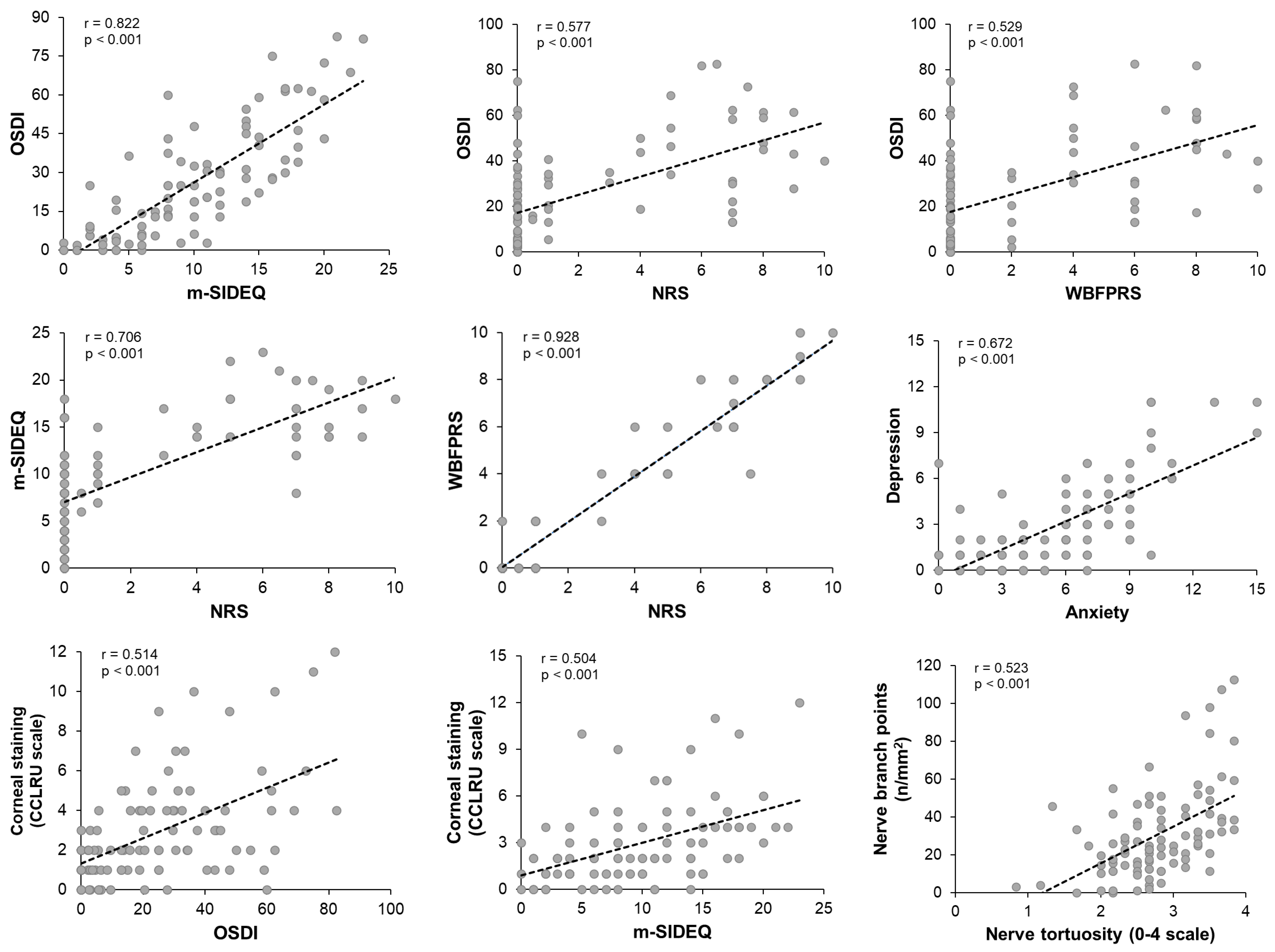
3.4. Correlations Among Clinical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CCLRU | Cornea and Contact Lens Research Unit |
| CELab | Controlled Environment Laboratory |
| Controls | Subjects without dry eye disease or ocular pain |
| DED | Dry eye disease |
| ETDRS | Early Treatment Diabetic Retinopathy Study |
| GRC | Global Rating of Change |
| HADS | Hospital Anxiety and Depression Scale |
| ICC | Intraclass correlation coefficient |
| LFU | Lacrimal functional unit |
| m-SIDEQ | Modified Single-Item score Dry Eye Questionnaire |
| No pain-DED | Patients with dry eye disease but no pain |
| NRS | Numerical Rating Scale |
| OSDI | Ocular Surface Disease Index |
| Pain-DED | Patients with chronic ocular pain associated with dry eye disease |
| TBUT | Tear break-up time |
| WBFPRS | Wong–Baker Faces® Pain-Rating Scale |
References
- Stern, M.E.; Beuerman, R.W.; Fox, R.I.; Gao, J.; Mircheff, A.K.; Pflugfelder, S.C. The Pathology of Dry Eye: The Interaction between the Ocular Surface and Lacrimal Glands. Cornea 1998, 17, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II Pain and Sensation Report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Guidelines on the Pharmacological Treatment of Persisting Pain in Children with Medical Illnesses; World Health Organization (WHO): Geneva, Switzerland, 2012; ISBN 9789241548120. [Google Scholar]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic Pain as a Symptom or a Disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.S.; McGee, S.J. Pain as a Global Public Health Priority. BMC Public Health 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.; Von Korff, M.; Lee, S.; Alonso, J.; Karam, E.; Angermeyer, M.C.; Borges, G.L.G.; Bromet, E.J.; de Girolamo, G.; de Graaf, R.; et al. Common Chronic Pain Conditions in Developed and Developing Countries: Gender and Age Differences and Comorbidity with Depression-Anxiety Disorders. J. Pain 2008, 9, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Henschke, N.; Kamper, S.J.; Maher, C.G. The Epidemiology and Economic Consequences of Pain. Mayo Clin. Proc. 2015, 90, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M.T. Corneal Nerves: Structure, Contents and Function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Levitt, R.C.; Felix, E.R.; Martin, E.R.; Sarantopoulos, C.D. Neuropathic Ocular Pain: An Important yet Underevaluated Feature of Dry Eye. Eye 2015, 29, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Rusciano, D.; Bagnoli, P.; Gallar, J.; Galor, A. Editorial: Eye Pain: Etiology and Therapeutic Approacheses. Front. Pharmacol. 2022, 13, 914809. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, P.; Liang, H.; Reboussin, E.; Rabut, G.; Warcoin, E.; Brignole-Baudouin, F.; Melik-Parsadaniantz, S.; Baudouin, C.; Labbe, A.; Goazigo, A.R. Le Proinflammatory Markers, Chemokines, and Enkephalin in Patients Suffering from Dry Eye Disease. Int. J. Mol. Sci. 2018, 19, 1221. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.S. Diagnosis and Treatment of Ocular Pain: The Ophthalmologist’s Perspective. Curr. Ophthalmol. Rep. 2017, 5, 275. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Vázquez, M.; Vázquez, A.; Fernández, I.; Novo-Diez, A.; Martínez-Plaza, E.; García-Vázquez, C.; González-García, M.J.; Sobas, E.M.; Calonge, M.; Enríquez-de-Salamanca, A. Inflammation-Related Molecules in Tears of Patients with Chronic Ocular Pain and Dry Eye Disease. Exp. Eye Res. 2022, 219, 109057. [Google Scholar] [CrossRef] [PubMed]
- Wong-Baker FACES Foundation. Wong-Baker FACES® Pain Rating Scale. Available online: https://wongbakerfaces.org/ (accessed on 22 April 2022).
- Galor, A.; Hamrah, P.; Haque, S.; Attal, N.; Labetoulle, M. Understanding Chronic Ocular Surface Pain: An Unmet Need for Targeted Drug Therapy. Ocul. Surf. 2022, 26, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Calonge, M.; Pinto-Fraga, J.; González-García, M.J.; Enríquez-de-Salamanca, A.; López-de la Rosa, A.; Fernández, I.; López-Miguel, A. Effects of the External Environment on Dry Eye Disease. Int. Ophthalmol. Clin. 2017, 57, 23–40. [Google Scholar] [CrossRef] [PubMed]
- López-Miguel, A.; Tesón, M.; Martín-Montañez, V.; Enríquez-de-Salamanca, A.; Stern, M.E.; Calonge, M.; González-García, M.J. Dry Eye Exacerbation in Patients Exposed to Desiccating Stress under Controlled Environmental Conditions. Am. J. Ophthalmol. 2014, 157, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.L.; Walt, J.G.; Mink, D.R.; Satram-Hoang, S.; Wilson, S.E.; Perry, H.D.; Asbell, P.A.; Pflugfelder, S.C. Minimal Clinically Important Difference for the Ocular Surface Disease Index. Arch. Ophthalmol. 2010, 128, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Simmons, P.A.; Vehige, J.G.; Carlisle, C.; Felix, C. Comparison of Dry Eye Signs in Self-Described Mild and Moderate Patients. In Proceedings of the ARVO Annual Meeting, Florida, FL, USA, 4–9 May 2003. [Google Scholar]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of Four Pain Intensity Rating Scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, Y.; Lu, A.T.H.; Liu, P.; Tang, M.; Yiu, S.C.; Huang, D. Reproducibility of Tear Meniscus Measurement by Fourier-Domain Optical Coherence Tomography: A Pilot Study. Ophthalmic Surg. Lasers Imaging 2009, 40, 447. [Google Scholar] [CrossRef] [PubMed]
- Tukenmez-Dikmen, N.; Yildiz, E.H.; Imamoglu, S.; Turan-Vural, E.; Sevim, M.S. Correlation of Dry Eye Workshop Dry Eye Severity Grading System with Tear Meniscus Measurement by Optical Coherence Tomography and Tear Osmolarity. Eye Contact Lens 2016, 42, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Takehisa, Y.; Kinoshita, S. Correlation of Tear Lipid Layer Interference Patterns with the Diagnosis and Severity of Dry Eye. Am. J. Ophthalmol. 1996, 122, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Efron, N. Grading Scales for Contact Lens Complications. Ophthalmic Physiol. Opt. 1998, 18, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.L.; Schnider, C.M.; Holden, B.A.; Cornish, R.; Grant, T.; Sweeney, D.; La Hood, D.; Back, A. CCLRU Standards for Success of Daily and Extended Wear Contact Lenses. Optom. Vis. Sci. 1993, 70, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of Corneal and Conjunctival Staining in the Context of Other Dry Eye Tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Korb, D.R.; Herman, J.P.; Greiner, J.V.; Scaffidi, R.C.; Finnemore, V.M.; Exford, J.M.; Blackie, C.A.; Douglass, T. Lid Wiper Epitheliopathy and Dry Eye Symptoms. Eye Contact Lens 2005, 31, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; Benjamin, L.; Snibson, G.R. Meibomian Gland Disease. Classification and Grading of Lid Changes. Eye 1991, 5, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Goto, E.; Ono, M.; Shimmura, S.; Tsubota, K. Meibomian Gland Dysfunction in Patients with Sjögren Syndrome. Ophthalmology 1998, 105, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Pult, H.; Riede-Pult, B. Comparison of Subjective Grading and Objective Assessment in Meibography. Cont. Lens Anterior Eye 2013, 36, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Acosta, M.C.; Schmelz, M.; Gallar, J. Measurement of Corneal Sensitivity to Mechanical and Chemical Stimulation with a CO2 Esthesiometer. Investig. Ophthalmol. Vis. Sci. 1999, 40, 513–519. [Google Scholar]
- Yarnitsky, D.; Ochoa, J.L. Studies of Heat Pain Sensation in Man: Perception Thresholds, Rate of Stimulus Rise and Reaction Time. Pain 1990, 40, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.C.; Belmonte, C.; Gallar, J. Sensory Experiences in Humans and Single-Unit Activity in Cats Evoked by Polymodal Stimulation of the Cornea. J. Physiol. 2001, 534, 525. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.C.; Berenguer-Ruiz, L.; García-Gálvez, A.; Perea-Tortosa, D.; Gallar, J.; Belmonte, C. Changes in Mechanical, Chemical, and Thermal Sensitivity of the Cornea after Topical Application of Nonsteroidal Anti-Inflammatory Drugs. Investig. Ophthalmol. Vis. Sci. 2005, 46, 282–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chao, C.; Stapleton, F.; Badarudin, E.; Golebiowski, B. Ocular Surface Sensitivity Repeatability with Cochet-Bonnet Esthesiometer. Optom. Vis. Sci. 2015, 92, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Kamper, S.J.; Maher, C.G.; Mackay, G. Global Rating of Change Scales: A Review of Strengths and Weaknesses and Considerations for Design. J. Man. Manip. Ther. 2009, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Soto, L.; Efron, N. Morphology of Corneal Nerves Using Confocal Microscopy. Cornea 2001, 20, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1988; ISBN 9780203771587. [Google Scholar]
- IBM Corp. IBM SPSS Statistics. Available online: https://www.ibm.com/es-es/products/spss-statistics (accessed on 30 April 2022).
- Ratner, B. The Correlation Coefficient: Its Values Range between 1/1, or Do They. J. Targeting Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef]
- Patel, S.; Felix, E.R.; Levitt, R.C.; Sarantopoulos, C.D.; Galor, A. Dysfunctional Coping Mechanisms Contribute to Dry Eye Symptoms. J. Clin. Med. 2019, 8, 901. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.G. Long-Term Consequences of Chronic Pain: Mounting Evidence for Pain as a Neurological Disease and Parallels with Other Chronic Disease States. Pain Med. 2011, 12, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Miljanović, B.; Dana, R.; Sullivan, D.A.; Schaumberg, D.A. Impact of Dry Eye Syndrome on Vision-Related Quality of Life. Am. J. Ophthalmol. 2007, 143, 415. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Breivik Hals, E.K.; Kvarstein, G.; Stubhaug, A. Assessment of Pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Moreno, A.; Liang, H.; Moreau, N.; Luzu, J.; Rabut, G.; Parsadaniantz, S.M.; Labbé, A.; Baudouin, C.; Goazigo, A.R. Le Corneal Nerve Abnormalities in Painful Dry Eye Disease Patients. Biomedicines 2021, 9, 1424. [Google Scholar] [CrossRef] [PubMed]
- Satitpitakul, V.; Kheirkhah, A.; Crnej, A.; Hamrah, P.; Dana, R. Determinants of Ocular Pain Severity in Patients with Dry Eye Disease. Am. J. Ophthalmol. 2017, 179, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, A.; Blanco-Vázquez, M.; Martínez-Plaza, E.; Sobas, E.M.; González-García, M.J.; López-Miguel, A.; Ortega, E.; Enríquez-de-Salamanca, A.; Calonge, M. Corneal Sensory Changes and Nerve Plexus Abnormalities in Chronic Neuropathic Ocular Pain and Dry Eye Post-Refractive Surgery. Am. J. Ophthalmol. 2025, 276, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, A.N.; Smith, S.D.; Siedlecki, A.R.; Hayek, S.M.; Sayegh, R.R. Ocular Pain Response to Treatment in Dry Eye Patients. Ocul. Surf. 2020, 18, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, A.; Martínez-Plaza, E.; Fernández, I.; Sobas, E.M.; González-García, M.J.; Enríquez-de-Salamanca, A.; Ortega, E.; López-Miguel, A.; Calonge, M. Phenotypic Characterization of Patients Developing Chronic Dry Eye and Pain after Refractive Surgery: A Cross-Sectional Study. Ocul. Surf. 2022, 26, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Felix, E.R.; Feuer, W.; Shalabi, N.; Martin, E.R.; Margolis, T.P.; Sarantopoulos, C.D.; Levitt, R.C. Dry Eye Symptoms Align More Closely to Non-Ocular Conditions than to Tear Film Parameters. Br. J. Ophthalmol. 2015, 99, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, X.; Han, J.; Shao, T.; Wang, Y. Association Between Sleep Quality, Mood Status, and Ocular Surface Characteristics in Patients with Dry Eye Disease. Cornea 2019, 38, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Vanathi, M.; Kishore, A.; Gupta, N.; Tandon, R. Evaluation of Strip Meniscometry, Tear Meniscus Height and Depth in the Diagnosis of Dry Eye Disease in Asian Indian Eyes. Ocul. Surf. 2019, 17, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Jiang, F.; Lu, Y.; Xue, C.; Zhu, X.; Wu, Y.; Huang, Z. Objective Optical Assessment of Tear-Film Quality Dynamics in Patients with Meibomian Gland Dysfunction and Aqueous-Deficient Dry Eye Optical Quality Changes in Different Dry Eye Subtypes. Indian J. Ophthalmol. 2019, 67, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Ayaki, M.; Kawashima, M.; Uchino, M.; Tsubota, K.; Negishi, K. Gender Differences in Adolescent Dry Eye Disease: A Health Problem in Girls. Int. J. Ophthalmol. 2018, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Labbé, A.; Alalwani, H.; Van Went, C.; Brasnu, E.; Georgescu, D.; Baudouin, C. The Relationship between Subbasal Nerve Morphology and Corneal Sensation in Ocular Surface Disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4926–4931. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xie, J.; Yang, T.; Su, P.; Liu, R.; Sun, T.; Zhou, Y.; Wang, H.; Feng, X.; Ma, S.; et al. Quantification of Increased Corneal Subbasal Nerve Tortuosity in Dry Eye Disease and Its Correlation with Clinical Parameters. Transl. Vis. Sci. Technol. 2021, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Villani, E.; Ceresara, G.; Beretta, S.; Magnani, F.; Viola, F.; Ratiglia, R. In Vivo Confocal Microscopy of Meibomian Glands in Contact Lens Wearers. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5215–5219. [Google Scholar] [CrossRef] [PubMed]
- Moein, H.R.; Akhlaq, A.; Dieckmann, G.; Abbouda, A.; Pondelis, N.; Salem, Z.; Müller, R.T.; Cruzat, A.; Cavalcanti, B.M.; Jamali, A.; et al. Visualization of Microneuromas by Using in Vivo Confocal Microscopy: An Objective Biomarker for the Diagnosis of Neuropathic Corneal Pain? Ocul. Surf. 2020, 18, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Y.; Li, W.; Huang, X. Semiautomated and Automated Quantitative Analysis of Corneal Sub-Basal Nerves in Patients with DED with Ocular Pain Using IVCM. Front. Med. 2022, 9, 831307. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Deshpande, K.; Deshmukh, R.; Jayadev, C.; Shroff, R. Bowman Break and Subbasal Nerve Plexus Changes in a Patient with Dry Eye Presenting with Chronic Ocular Pain and Vitamin D Deficiency. Cornea 2016, 35, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. Alterations in Corneal Stromal Dendritic Cell Phenotype and Distribution in Inflammation. Arch. Ophthalmol. 2003, 121, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Chen, Z.; Xie, C.; Liu, L.; Yang, H.; Wei, R. Relationship Between Dry Eye Disease and Emotional Disorder: The Mediating Effect of Health Anxiety. Front. Public Health 2022, 10, 771554. [Google Scholar] [CrossRef] [PubMed]
| Demographic Characteristics | Pain-DED | No Pain-DED | Controls | p-Value * |
|---|---|---|---|---|
| Age (years) | 60.89 ± 10.06 | 61.20 ± 13.01 | 58.87 ± 9.39 | 0.668 |
| n (%) females/males | 24/4 (85.7/14.3) | 25/10 (71.4/28.6) | 18/12 (60.0/40.0) | 0.092 |
| Years with DED-related symptoms | 9.34 ± 10.28 | 9.85 ± 8.94 | ---- | 0.637 |
| Years with ocular pain | 6.49 ± 6.14 | ---- | ---- | ---- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Vázquez, M.; Novo-Diez, A.; Vázquez, A.; Enríquez-de-Salamanca, A.; González-García, M.J.; Calonge, M. Clinical Characterization of the Lacrimal Functional Unit in Patients with Chronic Ocular Pain Associated with Dry Eye Disease. J. Clin. Med. 2025, 14, 5250. https://doi.org/10.3390/jcm14155250
Blanco-Vázquez M, Novo-Diez A, Vázquez A, Enríquez-de-Salamanca A, González-García MJ, Calonge M. Clinical Characterization of the Lacrimal Functional Unit in Patients with Chronic Ocular Pain Associated with Dry Eye Disease. Journal of Clinical Medicine. 2025; 14(15):5250. https://doi.org/10.3390/jcm14155250
Chicago/Turabian StyleBlanco-Vázquez, Marta, Andrea Novo-Diez, Amanda Vázquez, Amalia Enríquez-de-Salamanca, María J. González-García, and Margarita Calonge. 2025. "Clinical Characterization of the Lacrimal Functional Unit in Patients with Chronic Ocular Pain Associated with Dry Eye Disease" Journal of Clinical Medicine 14, no. 15: 5250. https://doi.org/10.3390/jcm14155250
APA StyleBlanco-Vázquez, M., Novo-Diez, A., Vázquez, A., Enríquez-de-Salamanca, A., González-García, M. J., & Calonge, M. (2025). Clinical Characterization of the Lacrimal Functional Unit in Patients with Chronic Ocular Pain Associated with Dry Eye Disease. Journal of Clinical Medicine, 14(15), 5250. https://doi.org/10.3390/jcm14155250








