Transforming Patient Experience: Real-World Impact of Mepolizumab on Symptom Burden in Chronic Rhinosinusitis with Nasal Polyps—A Multicenter Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical Endpoints
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
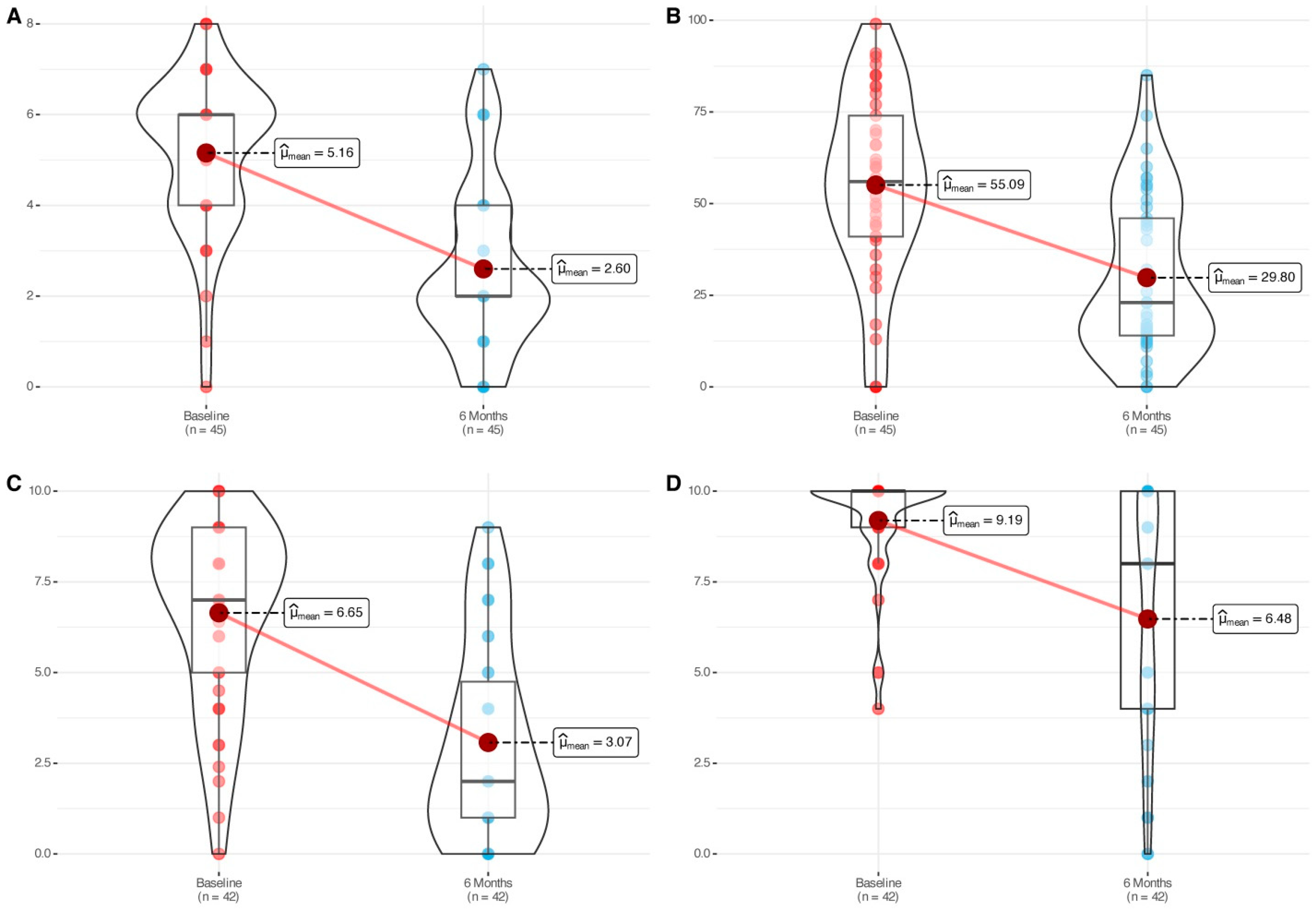
3.2. Nasal Polyp Score
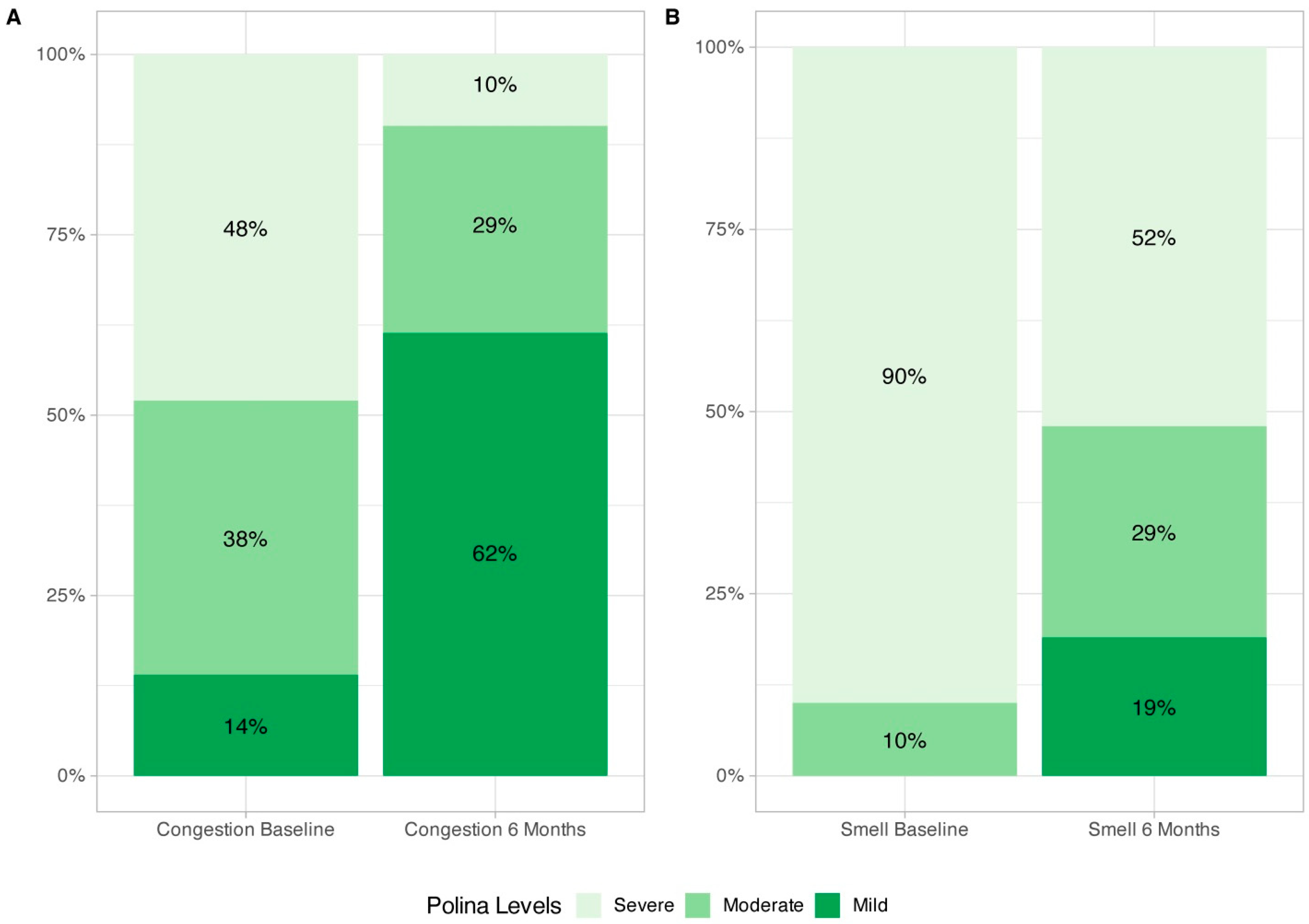
3.3. SNOT-22, Nasal Congestion VAS, and Smell Impairment VAS
3.4. ESS Events, SCS Intake, and Inflammatory Biomarkers
3.5. Asthma Control
3.6. Safety and Treatment Discontinuation Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRSwNP | chronic rhinosinusitis with nasal polyps |
| QoL | quality of life |
| SCS | systemic corticosteroids |
| ESS | endoscopic sinus surgery |
| mAb | monoclonal antibodies |
| NPS | nasal polyp score |
| SNOT-22 | Sinonasal Outcome Test-22 items |
| VAS | visual analog scale |
| ACT | Asthma Control Test |
| MCID | minimal clinically important difference |
| N-ERD | non-steroidal anti-inflammatory drug-exacerbated respiratory disease |
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Bhattacharyya, N.; Desrosiers, M.; Khan, A.H. Burden of Disease in Chronic Rhinosinusitis with Nasal Polyps. J. Asthma Allergy 2021, 14, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hellings, P.W.; Alobid, I.; Anselmo-Lima, W.T.; Bernal-Sprekelsen, M.; Bjermer, L.; Caulley, L.; Chaker, A.; Constantinidis, J.; Conti, D.M.; De Corso, E.; et al. EUFOREA/EPOS2020 statement on the clinical considerations for chronic rhinosinusitis with nasal polyps care. Allergy 2024, 79, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Han, J.K.; Bachert, C.; Fokkens, W.; Desrosiers, M.; Wagenmann, M.; Lee, S.E.; Smith, S.G.; Martin, N.; Mayer, B.; Yancey, S.W.; et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Galletti, C.; Ciodaro, F.; Barbieri, M.A.; Gambino, F.; Ferrisi, M.G.; Portelli, D.; Catalano, N.; Spina, E.; Freni, F.; Galletti, B. Effectiveness and safety profile of mepolizumab in chronic rhinosinusitis with nasal polyps: Real life data in a tertiary care. Am. J. Otolaryngol. 2024, 45, 104329. [Google Scholar] [CrossRef] [PubMed]
- Galletti, C.; Sireci, F.; Stilo, G.; Barbieri, M.A.; Messina, G.; Manzella, R.; Portelli, D.; Zappalà, A.G.; Diana, M.; Frangipane, S.; et al. Mepolizumab in chronic rhinosinusitis with nasal polyps: Real life data in a multicentric Sicilian experience. Am. J. Otolaryngol. 2025, 46, 104597. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Sosa, M.S.; Cabrera-Ramírez, M.S.; Marrero-Ramos, M.D.C.; Dávila-Quintana, D.; Cabrera-López, C.; Carrillo-Díaz, T.; del Rosalrio, J.J.B. Real-Life Effectiveness of Mepolizumab in Refractory Chronic Rhinosinusitis with Nasal Polyps. Biomedicines 2023, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Orlando, P.; Vivarelli, E.; Minzoni, A.; Licci, G.; Accinno, M.; Brugnoli, B.; Matucci, A.; Vultaggio, A.; Maggiore, G. Effects of Mepolizumab in the treatment of type 2 CRSwNP: A real-life clinical study. Eur. Arch. Otorhinolaryngol. 2025, 282, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; Loperfido, A.; Ciofalo, A.; Di Michele, L.; Begvarfaj, E.; Bellocchi, G.; Bugani, M.; de Vincentiis, M.; Greco, A.; Millarelli, S.; et al. Real-Life Evidence of Mepolizumab Treatment in Chronic Rhinosinusitis with Nasal Polyps: A Multicentric Study. J. Clin. Med. 2024, 13, 3575. [Google Scholar] [CrossRef] [PubMed]
- Armengot-Carceller, M.; Gómez-Gómez, M.J.; García-Navalón, C.; Doménech-Campos, E.; Muñoz-Fernández, N.; de Miguel, A.G.-L.; Marco-Algarra, J.; Palop-Cervera, M.; Piñero, A.G. Effects of Omalizumab Treatment in Patients with Recalcitrant Nasal Polyposis and Mild Asthma: A Multicenter Retrospective Study. Am. J. Rhinol. Allergy 2021, 35, 516–524. [Google Scholar] [CrossRef] [PubMed]
- De Corso, E.; Pasquini, E.; Trimarchi, M.; La Mantia, I.; Pagella, F.; Ottaviano, G.; Garzaro, M.; Pipolo, C.; Torretta, S.; Seccia, V.; et al. Dupireal Italian Study Group. Dupilumab in the treatment of severe uncontrolled chronic rhinosinusitis with nasal polyps (CRSwNP): A multicentric observational Phase IV real-life study (DUPIREAL). Allergy 2023, 78, 2669–2683. [Google Scholar] [CrossRef] [PubMed]
- de Loos, D.D.; Cornet, M.; Hopkins, C.; Fokkens, W.; Reitsma, S. Measuring control of disease in Chronic Rhinosinusitis; assessing the correlation between SinoNasal Outcome Test-22 and Visual Analogue Scale item scores. Rhinol. J. 2023, 61, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Alobid, I.; Colás, C.; Castillo, J.; Arismendi, E.; del Cuvillo, A.; Gómez-Outes, A.; Sastre, J.; Mullol, J. POLINA group. Spanish Consensus on the Management of Chronic Rhinosinusitis with Nasal Polyps (POLIposis NAsal/POLINA 2.0). J. Investig. Allergol. Clin. Immunol. 2023, 33, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Plaza, V.; Alobid, I.; Castillo Vizuete, J.A.; Colás Sanz, C. Guía POLINA; Documento de consenso sobre rinosinusitis crónica con poliposis nasal; Madrid © Sociedad Española de Otorrinolaringología y Cirugía de Cabeza y Cuello: Madrid, Spain, 2023; ISBN 978-84-19069-76-4. [Google Scholar]
- de los Santos, G.; Reyes, P.; del Castillo, R.; Fragola, C.; Royuela, A. Cross-cultural adaptation and validation of the sino-nasal outcome test (SNOT-22) for Spanish-speaking patients. Eur. Arch. Otorhinolaryngol. 2015, 272, 3335–3340. [Google Scholar] [CrossRef] [PubMed]
- RStudio: Integrated Development Environment for R Posit Software; PBC: Boston, MA, USA, 2025; Available online: http://www.posit.co/ (accessed on 5 May 2025).
- Farhood, Z.; Schlosser, R.J.; Pearse, M.E.; Storck, K.A.; Nguyen, S.A.; Soler, Z.M. Twenty-two-item Sino-Nasal Outcome Test in a control population: A cross-sectional study and systematic review. Int. Forum. Allergy Rhinol. 2016, 6, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Krouse, J.H.; Brown, R.W.; Fineman, S.M.; Han, J.K.; Heller, A.J.; Joe, S.; Krouse, H.J.; Pillsbury, H.C.; Ryan, M.W.; Veling, M.C. Asthma and the unified airway. Otolaryngol. Head Neck Surg. 2007, 136 (Suppl. S5), S75–S106. [Google Scholar] [CrossRef] [PubMed]
- Mullol, J.; Backer, V.; Constantinidis, J.; Eguíluz-Gracia, I.; Moure, A.L.; Cuervo-Pinto, R.; Zhang, L.; Shah, P.; Kerr, W.; Hellings, P. Global airway disease: Mepolizumab simultaneously improves outcomes in severe CRSwNP and asthma. Rhinol. J. 2025, 63, 113–115. [Google Scholar] [CrossRef] [PubMed]
- AbuJabal, R.; Ramakrishnan, R.K.; Bajbouj, K.; Hamid, Q. Role of IL-5 in asthma and airway remodelling. Clin. Exp. Allergy 2024, 54, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, P.; Han, J.K.; Smith, S.G.; Sousa, A.R.; Howarth, P.H.; Yancey, S.W.; Chan, R.; Bachert, C. The roles of eosinophils and interleukin-5 in the pathophysiology of chronic rhinosinusitis with nasal polyps. Int. Forum. Allergy Rhinol. 2022, 12, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
| Variables | N = 47 | |
|---|---|---|
| Age (years) | Med [IQR] | 56.0 [45.5, 63.0] |
| Mean (std) | 54.3 (11.7) | |
| Sex (n, %) | Men | 24 (51.06%) |
| Women | 23 (48.94%) | |
| Atopy | No | 25 (53.19%) |
| Yes | 22 (46.81%) | |
| Total IgE (UI/mL) | Med [IQR] | 113.0 [52.0, 270.5] |
| Mean (std) | 248.1 (352.9) | |
| Age at CRSwNP diagnosis (years) | Med [IQR] | 40.0 [29.0, 45.0] |
| Mean (std) | 38.4 (11.6) | |
| Number of ESS | Med [IQR] | 2.0 [1.0, 3.0] |
| Mean (std) | 2.4 (2.4) | |
| Proportion of patients with 0, 1, >1 ESS: n (%) | 0 surgeries | 8 (17.02%) |
| 1 surgery | 7 (14.89%) | |
| >1 surgery | 32 (68.09%) | |
| Time from CRSwNP diagnosis to mepolizumab initiation (months) | Med [IQR] | 159.0 [72.0, 240.0] |
| Mean (std) | 176.3 (128.6) | |
| Time from last surgery to mepolizumab initiation (months) | Med [IQR] | 48.0 [12.0, 89.2] |
| Mean (std) | 59.8 (61.9) | |
| N-ERD, n (%) | No | 17 (36.17%) |
| Yes | 30 (63.83%) | |
| Asthma, n (%) | No | 4 (8.51%) |
| Yes | 43 (91.49%) | |
| Asthma severity, n (%) | Mild | 9 (20.93%) |
| Moderate | 15 (34.88%) | |
| Severe | 19 (44.19%) | |
| Intranasal corticosteroid treatment, n (%) | Yes | 47 (100.00%) |
| No | 0 (0%) | |
| Saline rinses, n (%) | No | 14 (29.79%) |
| Yes | 33 (70.21%) | |
| Systemic corticosteroid courses in the previous 12 months | Med [IQR] | 2.0 [1.0, 3.0] |
| Mean (std) | 1.9 (1.7) | |
| Proportion of patients with 0, 1 >1 courses, n (%) | 0 courses | 9 (19.15%) |
| 1 course | 12 (25.53%) | |
| >1 course | 26 (55.32%) |
| Baseline | 6 Months | p Value | Median or Mean Difference (IC95%) 3 | Effect Size 4 | ||
|---|---|---|---|---|---|---|
| Total NPS | Med [IQR] | 6.0 [4.0, 6.0] | 2.0 [2.0, 4.0] | <0.0001 2 | −2.56 [−3.24, −1.88] | −1.42 (large) [−1.94, −0.89] |
| Mean (std) | 5.1 (1.7) | 2.7 (1.9) | ||||
| SNOT-22 | Med [IQR] | 56.0 [41.0, 74.0] | 23.0 [14.5, 47.5] | <0.0001 2 | −25.29 [−32.15, −18.42] | 1.06 (large) [−1.42, −0.71] |
| Mean (std) | 55.1 (25.1) | 30.0 (21.1) | ||||
| Nasal congestion VAS | Med [IQR] | 7.0 [5.0, 9.0] | 2.0 [1.0, 4.8] | <0.0001 2 | −3.57 [−4.50, −2.65] | −1.34 (large) [−1.81, −0.87] |
| Mean (std) | 6.6 (2.5) | 3.0 (2.7) | ||||
| Smell impairment VAS | Med [IQR] | 10.0 [9.0, 10.0] | 8 [4.0, 10.0] | <0.0001 1 | −4.0 [−5.50, −3.0] | −0.96 (large) [−1.39, −0.54] |
| Mean (std) | 9.2 (1.5) | 6.5 (3.4) | ||||
| Blood eosinophil count * | Med [IQR] | 670.0 [500.0, 937.5] | 95.0 [60.0, 180.0] | <0.0001 1 | −625.45 [−770.0, −495.0] | −1.41 (large) [−1.98, −0.84] |
| Mean (std) | 829.8 (619.4) | 155.5 (196.7) | ||||
| Tissue eosinophil count ** | Med [IQR] | 51.0 [36.2, 187.5] | 37.5 [6.2, 70.0] | <0.0001 1 | −52.5 [−119.9, −25.0] | −0.69 (medium) [−1.17, −0.20] |
| Mean (std) | 125.8 (131.3) | 51.1 (69.4) | ||||
| ACT | Med [IQR] | 15.0 [11.0, 21.0] | 24.0 [21.0, 25.0] | <0.001 1 | 8.0 [5.50, 11.99] | 1.34 (large) [0.71, 1.97] |
| Mean (std) | 15.2 (6.9) | 22.2 (3.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Piñero, A.; Pérez-Carbonell, T.; Gómez-Gómez, M.-J.; Domenech-Campos, E.; Martinez-Expósito, F.; Muñoz-Fernández, N.; Calvo-Gómez, J.; García-Navalón, C.; Fito-Martorell, L.; Ferrer-Baixauli, F.; et al. Transforming Patient Experience: Real-World Impact of Mepolizumab on Symptom Burden in Chronic Rhinosinusitis with Nasal Polyps—A Multicenter Perspective. J. Clin. Med. 2025, 14, 5248. https://doi.org/10.3390/jcm14155248
García-Piñero A, Pérez-Carbonell T, Gómez-Gómez M-J, Domenech-Campos E, Martinez-Expósito F, Muñoz-Fernández N, Calvo-Gómez J, García-Navalón C, Fito-Martorell L, Ferrer-Baixauli F, et al. Transforming Patient Experience: Real-World Impact of Mepolizumab on Symptom Burden in Chronic Rhinosinusitis with Nasal Polyps—A Multicenter Perspective. Journal of Clinical Medicine. 2025; 14(15):5248. https://doi.org/10.3390/jcm14155248
Chicago/Turabian StyleGarcía-Piñero, Alfonso, Tomás Pérez-Carbonell, María-José Gómez-Gómez, Encarna Domenech-Campos, Fernando Martinez-Expósito, Noelia Muñoz-Fernández, Jordi Calvo-Gómez, Carmen García-Navalón, Lucas Fito-Martorell, Felip Ferrer-Baixauli, and et al. 2025. "Transforming Patient Experience: Real-World Impact of Mepolizumab on Symptom Burden in Chronic Rhinosinusitis with Nasal Polyps—A Multicenter Perspective" Journal of Clinical Medicine 14, no. 15: 5248. https://doi.org/10.3390/jcm14155248
APA StyleGarcía-Piñero, A., Pérez-Carbonell, T., Gómez-Gómez, M.-J., Domenech-Campos, E., Martinez-Expósito, F., Muñoz-Fernández, N., Calvo-Gómez, J., García-Navalón, C., Fito-Martorell, L., Ferrer-Baixauli, F., García-Lliberós, A., Mosquera-Lloreda, N., Taleb, C., Zac-Romero, C., López-Valdivia, C., Pardo-Albiach, J., & Armengot-Carceller, M. (2025). Transforming Patient Experience: Real-World Impact of Mepolizumab on Symptom Burden in Chronic Rhinosinusitis with Nasal Polyps—A Multicenter Perspective. Journal of Clinical Medicine, 14(15), 5248. https://doi.org/10.3390/jcm14155248








