Quadruple Fenestrated Stentgrafts for Complex Aortic Aneurysms: Outcomes of Non-Stented Celiac Artery Fenestrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Outcomes
2.2. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wanhainen, A.; Van Herzeele, I.; Goncalves, F.B.; Montoya, S.B.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef]
- Oderich, G.S.; Greenberg, R.K.; Farber, M.; Lyden, S.; Sanchez, L.; Fairman, R.; Jia, F.; Bharadwaj, P.; Zenith Fenestrated Study Investigators. Results of the United States multicenter prospective study evaluating the Zenith fenestrated endo- vascular graft for treatment of juxtarenal abdominal aortic aneurysms. J. Vasc. Surg. 2014, 60, 1420–1428.e5. [Google Scholar] [CrossRef]
- Marcondes, G.B.; Tenorio, E.R.; Lima, G.B.; Mendes, B.C.; Oderich, G.S. Branched Endovascular Aortic Repair of Thoracoabdominal Aortic Aneurysm Using Total Percutaneous Transfemoral Approach. Oper. Tech. Thorac. Cardiovasc. Surg. 2021, 26, 3–19. [Google Scholar] [CrossRef]
- Maurel, B.; Resch, T.; Spear, R.; Roeder, B.; Bracale, U.M.; Haulon, S.; Mastracci, T.M. Early experience with a modified preloaded system for fenestrated endovascular aortic repair. J. Vasc. Surg. 2017, 65, 972–980. [Google Scholar] [CrossRef]
- Mirza, A.K.; Tenorio, E.R.; Kärkkäinen, J.M.; Pather, K.; Kratzberg, J.; Mendes, B.C.; DeMartino, R.R.; Oderich, G.S. Outcomes of a novel upper extremity preloaded delivery system for fenestrated-branched endovascular repair of thoracoabdominal aneurysms. J. Vasc. Surg. 2020, 72, 470–479. [Google Scholar] [CrossRef]
- Gallitto, E.; Faggioli, G.; Spath, P.; Feroldi, F.M.; Pini, R.; Logiacco, A.; Sufali, G.; Caputo, S.; Gargiulo, M. New Preloaded System for Renal and Visceral Arteries in Fenestrated Endografting. J. Endovasc. Ther. 2023, 32, 669–678. [Google Scholar] [CrossRef]
- Verhoeven, E.L.; Katsargyris, A.; e Fernandes, R.F.; Bracale, U.M.; Houthoofd, S.; Maleux, G. Practical points of attention beyond instructions for use with the Zenith fenestrated stent graft. J. Vasc. Surg. 2014, 60, 246–252. [Google Scholar] [CrossRef]
- Higashiura, W. Endovascular Treatment for Thoracoabdominal Aortic Aneurysm and Complex Abdominal Aortic Aneurysm Using Fenestrated and Branched Grafts. Interv. Radiol. 2020, 5, 103–113. [Google Scholar] [CrossRef]
- Monahan, T.S.; Schneider, D.B. Fenestrated and Branched Stent Grafts for Repair of Complex Aortic Aneurysms. Semin. Vasc. Surg. 2009, 22, 132–139. [Google Scholar] [CrossRef]
- de Marino, P.M.; Katsargyris, A.; Ibraheem, A.; Gafur, N.; Botos, B.; Verhoeven, E.L. Editor’s Choice—Four fenestration endovascular aortic aneurysm repair without stenting of the coeliac artery in selected cases. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 652–653. [Google Scholar] [CrossRef]
- Suh, G.-Y.; Choi, G.; Herfkens, R.J.; Dalman, R.L.; Cheng, C.P. Three-dimensional modeling analysis of visceral arteries and kidneys during respiration. Ann. Vasc. Surg. 2016, 34, 250–260. [Google Scholar] [CrossRef]
- Lee, V.S.; Morgan, J.N.; Tan, A.G.S.; Pandharipande, P.V.; Krinsky, G.A.; Barker, J.A.; Lo, C.; Weinreb, J.C. Celiac artery compression by the median arcuate ligament: A pitfall of end-expiratory MR imaging. Radiology 2003, 228, 437–442. [Google Scholar] [CrossRef]
- Witheford, M.; Au, D.; Mastracci, T.M. Mastracci: Coeliac Incorporation Strategy Impacts Visceral Branch Vessel Stability in Fenestrated Endovascular Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2022, 64, 321–330. [Google Scholar] [CrossRef]
- Tenorio, E.R.; Schanzer, A.; Timaran, C.H.; Schneider, D.B.; Mendes, B.C.; Eagleton, M.J.; Farber, M.A.; Parodi, F.E.; Gasper, W.J.; Beck, A.W.; et al. Mid-term Renal and Mesenteric Artery Outcomes During Fenestrated and Branched Endovascular Aortic Repair for Complex Abdominal and Thoracoabdominal Aortic Aneurysms in the United States Aortic Research Consortium. Ann. Surg. 2023, 278, e893–e902. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; e Melo, R.G.; Schanzer, A.; Sobocinski, J.; Austermann, M.; Chiesa, R.; Resch, T.; Gargiulo, M.; Timaran, C.; Maurel, B.; et al. Transatlantic multicenter study on the use of a modified preloaded delivery system for fenestrated endovascular aortic repair. J. Vasc. Surg. 2023, 78, 863–873.e3. [Google Scholar] [CrossRef] [PubMed]
- Oderich, G.S.; Forbes, T.L.; Chaer, R.; Davies, M.G.; Lindsay, T.F.; Mastracci, T.; Singh, M.J.; Timaran, C.; Woo, E.Y. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J. Vasc. Surg. 2021, 73, 4S–52S. [Google Scholar] [CrossRef] [PubMed]
- Mastracci, T.M.; Greenberg, R.K.; Eagleton, M.J.; Hernandez, A.V. Durability of branches in branched and fenestrated endografts. J. Vasc. Surg. 2013, 57, 926–933. [Google Scholar] [CrossRef]
- Lopes, J.A.; Jorge, S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin. Kidney J. 2013, 6, 8–14. [Google Scholar] [CrossRef]
- Oderich, G.S.; Ribeiro, M.; Hofer, J.; Wigham, J.; Cha, S.; Chini, J.; Macedo, T.A.; Gloviczki, P. Prospective, nonrandomized study to evaluate endovascular repair of pararenal and thoracoabdominal aortic aneurysms using fenestrated-branched endografts based on supraceliac sealing zones. J. Vasc. Surg. 2017, 65, 1249–1259.e10. [Google Scholar] [CrossRef]
- Sveinsson, M.; Sobocinski, J.; Resch, T.; Sonesson, B.; Dias, N.; Haulon, S.; Kristmundsson, T. Early versus late experience in fenestrated endovascular repair for abdominal aortic aneurysm. J. Vasc. Surg. 2015, 61, 895–901. [Google Scholar] [CrossRef]
- Verhoeven, E.; Katsargyris, A. SS17: Comparison of standard double fenestrated EVAR vs triple or quadruple fenestrated EVAR in the treatment of complex aortic aneurysms. VAM 2016 Abstracts. J. Vasc. Surg. 2016, 63, 136S–137S. [Google Scholar] [CrossRef]
- Mezzetto, L.; Scorsone, L.; Silingardi, R.; Gennai, S.; Piffaretti, G.; Mantovani, A.; Bush, R.L.; Haulon, S.; Veraldi, G.F. Bridging stents in fenestrated and branched endovascular aneurysm repair: A systematic review. Ann. Vasc. Surg. 2021, 73, 454–462. [Google Scholar] [CrossRef]
- Wattez, H.; Martin-Gonzalez, T.; Lopez, B.; Spear, R.; Clough, R.E.; Hertault, A.; Sobocinski, J.; Haulon, S. Results of celiac trunk stenting during fenestrated or branched aortic endografting. J. Vasc. Surg. 2016, 64, 1595–1601. [Google Scholar] [CrossRef]
- Scott, C.K.; Timaran, D.E.; Soto-Gonzalez, M.; Malekpour, F.; Kirkwood, M.L.; Timaran, C.H. Effects of preoperative visceral artery stenosis on target artery outcomes after fenestrated/branched endovascular aortic aneurysm repair. J. Vasc. Surg. 2021, 73, 1504–1512. [Google Scholar] [CrossRef]

| Variables | Unstented (n = 29) | Stented (n = 72) | p Value |
|---|---|---|---|
| Age | 74.1 ± 5.3 | 73.8 ± 5.6 | 0.83 |
| Male sex | 26 (89.7) | 63 (87.5) | 0.75 |
| Hypertension | 19 (65.5) | 53 (73.6) | 0.41 |
| Diabetes | 2 (6.9) | 10 (13.9) | 0.32 |
| Chronic kidney disease | 7 (24.1) | 23 (31.94) | 0.43 |
| Coronary artery disease | 9 (31.0) | 26 (36.1) | 0.62 |
| Peripheral arterial occlusive disease | 5 (17.2) | 10 (13.9) | 0.67 |
| Smoking status | 0.0001 | ||
| Never | 2 (6.9) | 6 (8.3) | |
| Former | 13 (44.8) | 45 (62.5) | |
| Current | 14 (48.3) | 21 (29.2) |
| Preoperative Anatomical Features of CA Univariate Analysis | Unstented (n = 29) | Stented (n = 72) | p Value |
|---|---|---|---|
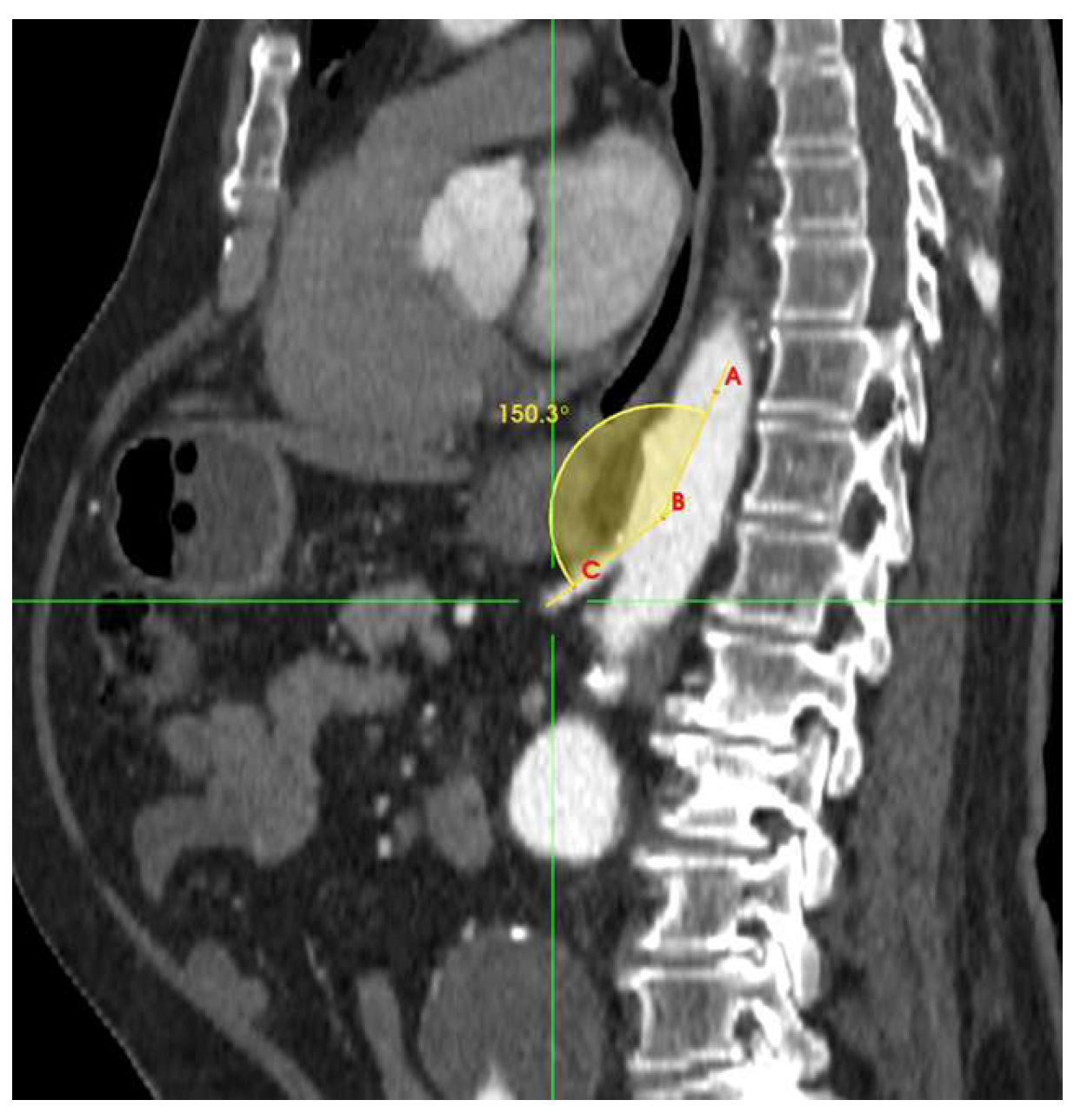
| Takeoff angulation | 0.0184 | ||
| <140° | 9 (31.0) | 41 (56.9) | 0.018 |
| ≥140° | 20 (68.9) | 31 (43.0) | 0.019 |
| Proximal diameter | 0.0005 | ||
| ≤6.5 mm | 10 (34.4) | 5 (6.9) | 0.0001 |
| >6.5 mm | 19 (65.5) | 67 (93.0) | 0.025 |
| Distal diameter | 0.388 | ||
| ≤6.5 mm | 9 (31.0) | 29 (40.2) | 0.285 |
| >6.5 mm | 20 (68.9) | 43 (59.7) | 0.424 |
| Patency overall | 0.0029 | ||
| No Stenosis | 3 (10.3) | 26 (36.1) | 0.009 |
| Stenosis < 50% | 8 (27.5) | 26 (36.1) | 0.414 |
| Stenosis ≥ 50% | 18 (62.0) | 20 (27.7) | 0.001 |
| Length of stenosis | 0.00001 | ||
| ≤6.5 mm | 9 (31.0) | 38 (52.7) | 0.02 |
| >6.5 mm | 20 (68.9) | 34 (47.2) | 0.05 |
| Calcification | 19 (65.5) | 46 (63.8) | 0.87 |
| Early division (main trunk < 6 mm) | 1 (3.4) | 0 | 0.11 |
| Artery Tortuosity | 16 (55.1) | 14 (19.4) | 0.00037 |
| Aneurysm | 0 | 1 (1.3) | 0.52 |
| Anatomical Abnormalities | 4 (13.7) | 6 (8.3) | 0.40 |
| Preoperative and Perioperative Characteristics | Unstented (n = 29) | Stented (n = 72) | p Value |
|---|---|---|---|
| cAAA type | |||
| Type IV | 1 (3.4) | 1 (1.4) | 0.51 |
| Paravisceral | 2 (6.9) | 0 | 0.025 |
| Pararenal | 10 (34.5) | 11 (15.3) | 0.032 |
| Juxtarenal | 16 (55.1) | 60 (83.3) | 0.003 |
| Preoperative aortic diameter -mm | 68.9 ± 12.7 | 67.3 ± 9.8 | 0.49 |
| Cook zenith fenestrated CMD | |||
| standard | 15 (51.7) | 48 (66.7) | 0.16 |
| preloaded | 14 (48.3) | 24 (33.3) | 0.16 |
| General anesthesia | 29 (100.0) | 72 (100.0) | ns |
| Access site | |||
| percutaneous femoral access | 29 (100) | 72 (100) | ns |
| percutaneous brachial access | 4 (13.7) | 3 (4.2) | 0.91 |
| percutaneous axillary access | 1 (3.5) | 1 (1.4) | 0.50 |
| Intraoperative CA complication | |||
| failed catheterization | 8 (27.6) | 0 | 0.0001 |
| unsuccessful attempted stenting | 20 (69.0) | 0 | <0.0001 |
| Stenting in CA was deemed unnecessary | 1 (3.4) | 0 | 0.11 |
| intraoperative endoleak from CA | 1 (3.4) | 0 | 0.11 |
| CA bridging stent | |||
| BeGraft | 43 (59.7) | na | |
| BeGraft plus | 1 (1.39) | na | |
| VBX | 13 (18.0) | na | |
| V12 Advanta | 15 (20.8) | na | |
| Technical success for CA | 28 (96.5) | 72 (100.0) | 0.11 |
| Primary Technical success | 28 (96.5) | 65 (90.2) | 0.29 |
| Total operating time min | 256 ± 93.8 | 237.1 ± 79.7 | 0.30 |
| Fluoroscopy time-min | 104.4 ± 40.5 | 103.4 ± 39.5 | 0.90 |
| Postoperative Characteristics | Unstented (n = 29) | Stented (n = 72) | p Value |
|---|---|---|---|
| In-hospital (days) | 5.1 ± 5.5 | 4.7 ± 4.7 | 0.71 |
| Early reintervention for endoleak or migration—before discharge | 0 | 0 | na |
| Early reintervention for rupture or bleeding—before discharge | 1 (3.4) | 4 (5.6) | 0.64 |
| Reintervention (central arterial perforation) | 0 | 3 (4.2) | 0.26 |
| Hematoma | 0 | 1 (1.4) | 0.52 |
| False aneurysm | 1 (3.4) | 0 | 0.11 |
| Early reintervention for lower limb ischemia Access-site, device, and procedure-related. | 1 (3.4) | 3 (4.2) | 0.85 |
| Postoperative adverse events | |||
| spinal cord ischemia | 1 (3.4) | 2 (2.8) | 0.87 |
| mesenteric ischemia | 0 | 3 (4.2) | 0.26 |
| renal ischemia/failure | 3 (10.3) | 4 (5.6) | 0.40 |
| aortic dissection | 0 | 1 (1.4) | 0.52 |
| cerebral event | 0 | 1 (1.4) | 0.52 |
| cardiac event | 0 | 2 (2.8) | 0.36 |
| pulmonary event | 0 | 1 (1.4) | 0.52 |
| multiorgan failure | 0 | 1 (1.4) | 0.52 |
| intensive care > 1 day | 0 | 1 (1.4) | 0.52 |
| other | 2 (6.9) | 1 (1.4) | 0.14 |
| Early reintervention for access-site lymphocele and/or infection | 0 | 0 | na |
| Unstented (n = 29) | Stented (n = 72) | p Value | |
|---|---|---|---|
| <50% Stenosis | 1 (3.4) | 0 | 0.11 |
| ≥50% Stenosis | 0 | 0 | na |
| CA Occlusion | 4 (13.7) | 2 (2.7) | 0.034 |
| CA stent stenosis | NA | 2 (2.7) | na |
| Symptoms | 0 | 0 | na |
| Lost to Fu | 4 (13.7) | 19 (26.3) | 0.17 |
| No info | 1 (3.4) | 0 | 0.11 |
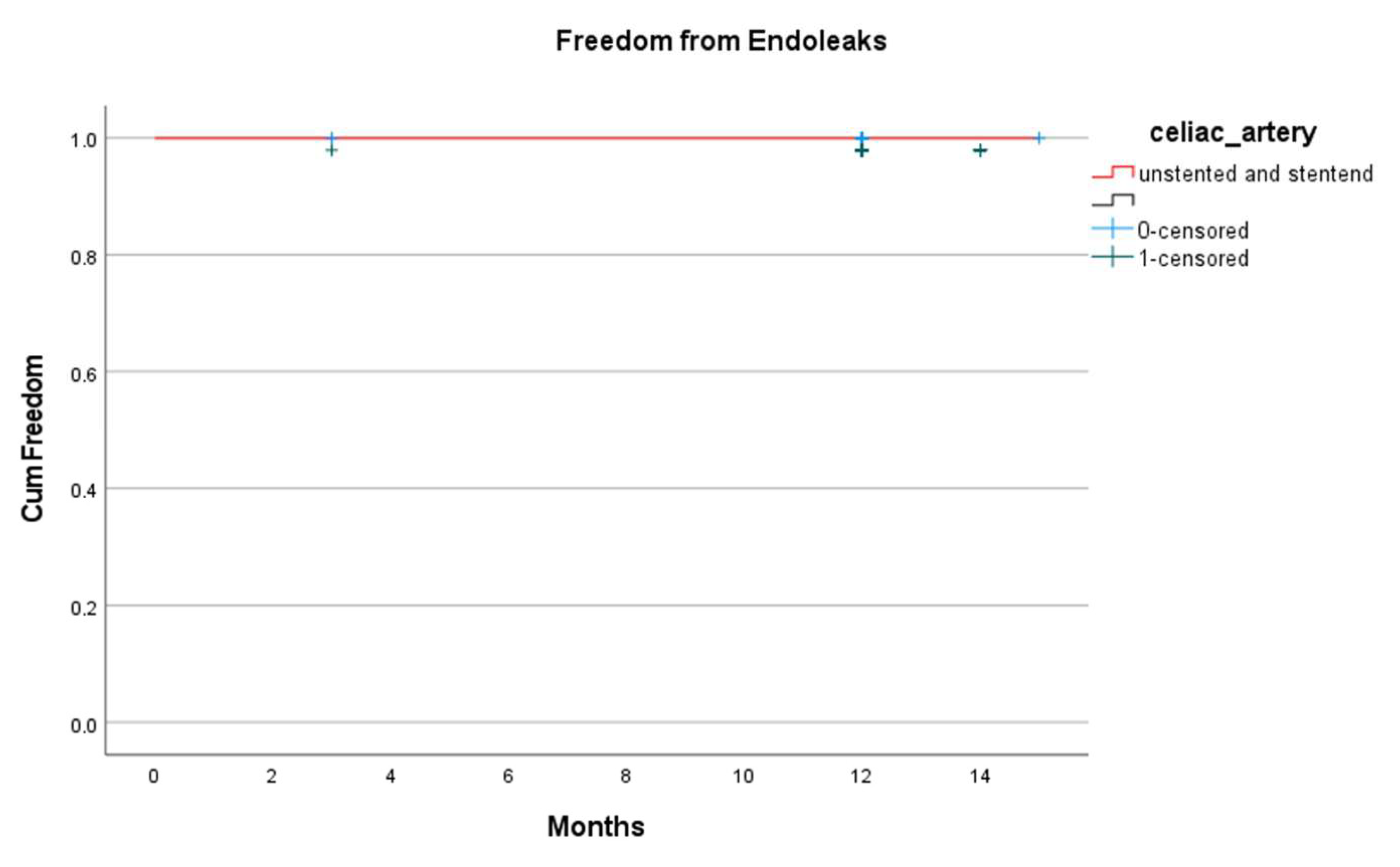
| Endoleaks | 0 | 0 | na |
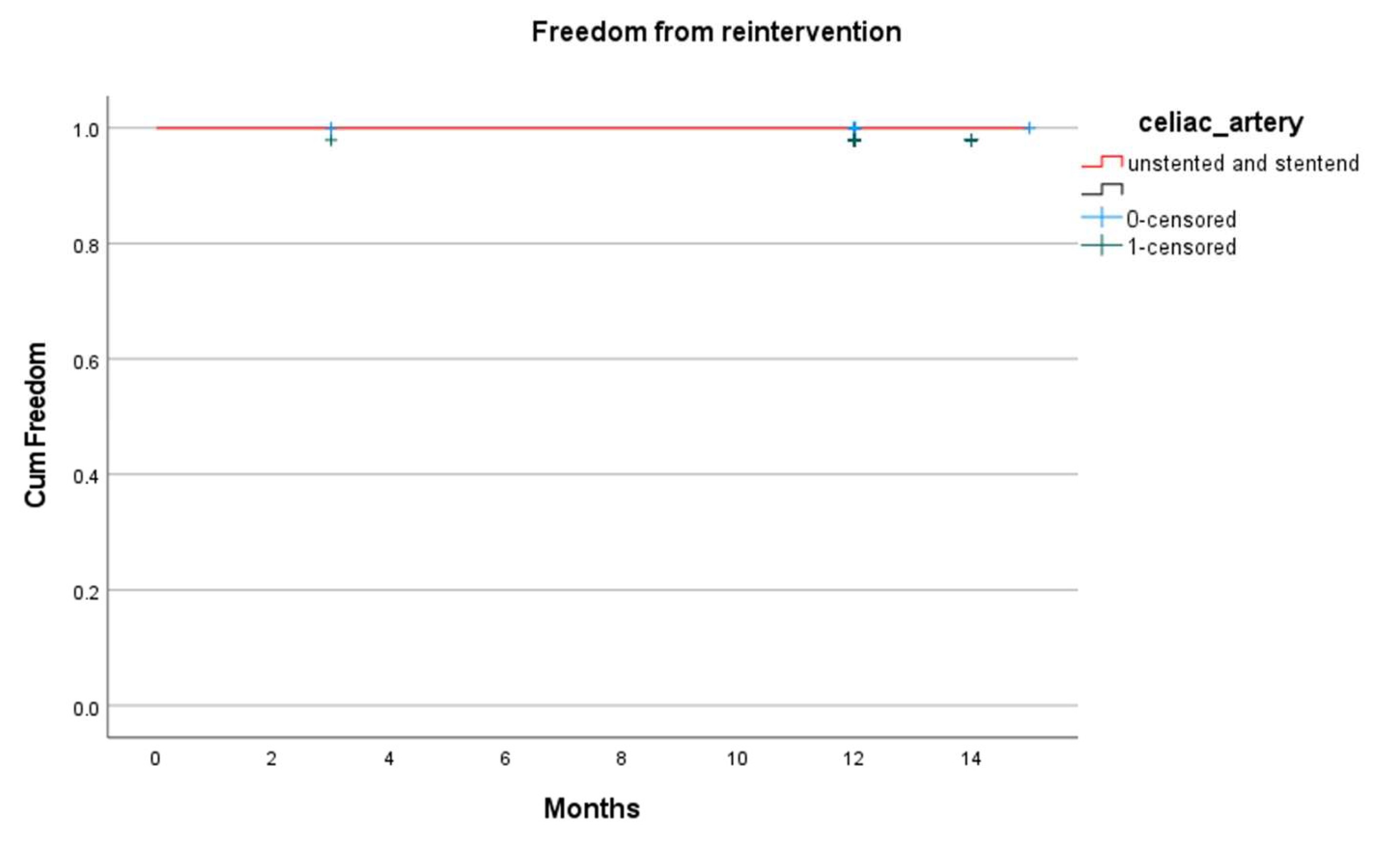
| Reinterventions | 0 | 0 | na |
| Death | 1 (3.4) | 5 (6.9) | 0.50 |
| CA instability | 5 (17.2) | 4 (5.5) | 0.06 |
| Unstented (n = 29) | Stented (n =72) | p Value | |
|---|---|---|---|
| Endoleaks | |||
| Type Ic | 1 (3.4) | 0 | 0.11 |
| Type III | 0 | 0 | na |
| Branch stenosis | 0 | 0 | na |
| Branch occlusion | 1 (3.4) | 2 (2.7) | 0.85 |
| Branch reintervention | 1 (3.4) | 0 | 0.11 |
| Non CA TV instability | 3 (10.3) | 2 (2.8) | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro, D.; Bredahl, K.; Björses, K.; Ohrlander, T.; Vogt, K.; Resch, T. Quadruple Fenestrated Stentgrafts for Complex Aortic Aneurysms: Outcomes of Non-Stented Celiac Artery Fenestrations. J. Clin. Med. 2025, 14, 5189. https://doi.org/10.3390/jcm14155189
Toro D, Bredahl K, Björses K, Ohrlander T, Vogt K, Resch T. Quadruple Fenestrated Stentgrafts for Complex Aortic Aneurysms: Outcomes of Non-Stented Celiac Artery Fenestrations. Journal of Clinical Medicine. 2025; 14(15):5189. https://doi.org/10.3390/jcm14155189
Chicago/Turabian StyleToro, Daniela, Kim Bredahl, Katarina Björses, Tomas Ohrlander, Katja Vogt, and Timothy Resch. 2025. "Quadruple Fenestrated Stentgrafts for Complex Aortic Aneurysms: Outcomes of Non-Stented Celiac Artery Fenestrations" Journal of Clinical Medicine 14, no. 15: 5189. https://doi.org/10.3390/jcm14155189
APA StyleToro, D., Bredahl, K., Björses, K., Ohrlander, T., Vogt, K., & Resch, T. (2025). Quadruple Fenestrated Stentgrafts for Complex Aortic Aneurysms: Outcomes of Non-Stented Celiac Artery Fenestrations. Journal of Clinical Medicine, 14(15), 5189. https://doi.org/10.3390/jcm14155189





