Effect of Iron Deficiency on Right Ventricular Strain in Patients Diagnosed with Acute Heart Failure †
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. ID Parameters Assessment and Definitions
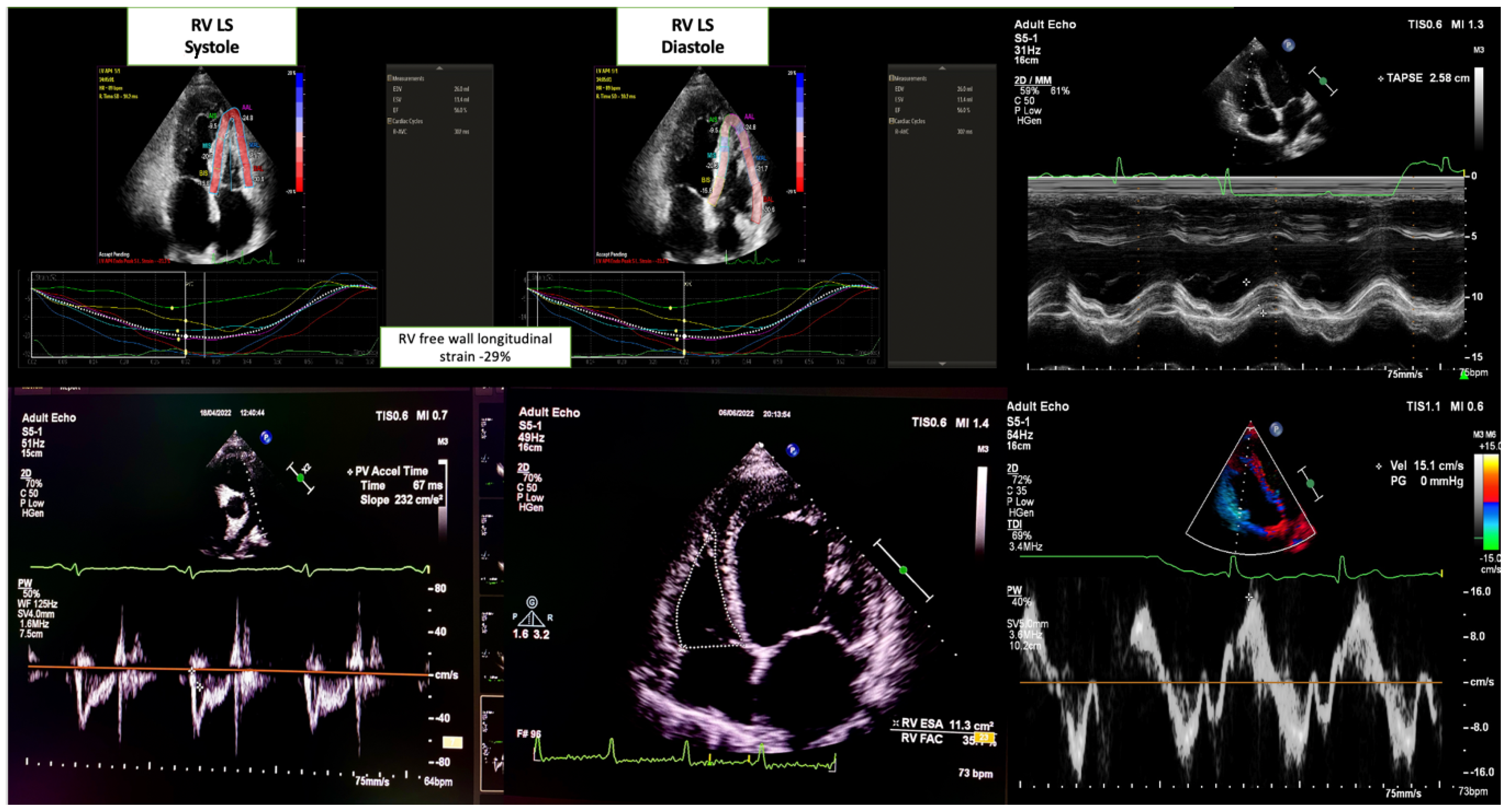
2.3. Echocardiographic Evaluation
- Left atrium (LA): diameter at end systole along the parasternal long axis.
- Pulmonary valve: Assessed using continuous wave (CW) and pulsed wave (PW) Doppler on the parasternal short axis. Pulmonary acceleration time was measured using PW Doppler across the pulmonary valve.
- LV systolic function: Calculated using the modified Simpson biplane method by manually tracing the apical four- and two-chamber views.
- RV systolic function: Evaluated using the apical four-chamber view. The key parameters were as follows:
- Tricuspid annular plane systolic excursion (TAPSE) measured via M-mode.
- Peak systolic tricuspid annular velocity (RV TDI S′) was obtained using tissue Doppler imaging.
- RV fractional area change (RV FAC) was calculated as the percentage of RV end-diastolic and end-systolic area changes.
- Systolic pulmonary artery pressure was estimated using the peak velocity gradient of tricuspid regurgitation flow, right atrial pressure derived from the inferior vena cava dimensions, and respiratory variability.
2.4. Statistical Analysis
Comparisons Between Groups
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magrì, D.; De Martino, F.; Moscucci, F.; Agostoni, P.; Sciomer, S. Anemia and iron deficiency in heart failure: Clinical and prognostic role. Heart Fail. Clin. 2019, 15, 359–369. [Google Scholar] [CrossRef]
- Anand, I.S.; Gupta, P. Anemia and iron deficiency in heart failure: Current concepts and emerging therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef]
- Alnuwaysir, R.I.; Hoes, M.F.; van Veldhuisen, D.J.; van der Meer, P.; Grote Beverborg, N. Iron deficiency in heart failure: Mechanisms and pathophysiology. J. Clin. Med. 2021, 11, 125. [Google Scholar] [CrossRef]
- Zhang, H.; Jamieson, K.L.; Grenier, J.; Nikhanj, A.; Tang, Z.; Wang, F.; Wang, S.; Seidman, J.G.; Seidman, C.E.; Thompson, R. Myocardial iron deficiency and mitochondrial dysfunction in advanced heart failure in humans. J. Am. Heart Assoc. 2022, 11, e022853. [Google Scholar] [CrossRef]
- Loncar, G.; Obradovic, D.; Thiele, H.; von Haehling, S.; Lainscak, M. Iron deficiency in heart failure. ESC Heart Fail. 2021, 8, 2368–2379. [Google Scholar] [CrossRef]
- Beale, A.; Carballo, D.; Stirnemann, J.; Garin, N.; Agoritsas, T.; Serratrice, J.; Kaye, D.; Meyer, P.; Carballo, S. Iron deficiency in acute decompensated heart failure. J. Clin. Med. 2019, 8, 1569. [Google Scholar] [CrossRef]
- Palau, P.; Llàcer, P.; Domínguez, E.; Tormo, J.P.; Zakarne, R.; Mollar, A.; Martínez, A.; Miñana, G.; Santas, E.; Almenar, L. Iron deficiency and short-term adverse events in patients with decompensated heart failure. Clin. Res. Cardiol. 2021, 110, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Enjuanes, C.; Klip, I.T.; Bruguera, J.; Cladellas, M.; Ponikowski, P.; Banasiak, W.; Van Veldhuisen, D.J.; Van Der Meer, P.; Jankowska, E.A.; Comín-Colet, J. Iron deficiency and health-related quality of life in chronic heart failure: Results from a multicenter European study. Int. J. Cardiol. 2014, 174, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Santas, E.; Miñana, G.; Cardells, I.; Palau, P.; Llàcer, P.; Fácila, L.; Almenar, L.; López-Lereu, M.P.; Monmeneu, J.V.; Sanchis, J. Short-term changes in left and right systolic function following ferric carboxymaltose: A substudy of the Myocardial-IRON trial. ESC Heart Fail. 2020, 7, 4222–4230. [Google Scholar] [CrossRef] [PubMed]
- Martens, P. The effect of iron deficiency on cardiac function and structure in heart failure with reduced ejection fraction. Card. Fail. Rev. 2022, 8, e06. [Google Scholar] [CrossRef]
- Miñana, G.; Santas, E.; de la Espriella, R.; Núñez, E.; Lorenzo, M.; Núñez, G.; Valero, E.; Bodí, V.; Chorro, F.J.; Sanchis, J. Right ventricular function and iron deficiency in acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 406–414. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography: Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J.-Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, J.-H. Strain analysis of the right ventricle using two-dimensional echocardiography. J. Cardiovasc. Imaging 2018, 26, 111–124. [Google Scholar] [CrossRef]
- Kobak, K.A.; Radwańska, M.; Dzięgała, M.; Kasztura, M.; Josiak, K.; Banasiak, W.; Ponikowski, P.; Jankowska, E.A. Structural and functional abnormalities in iron-depleted heart. Heart Fail. Rev. 2019, 24, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.J.; Luo, A.; Park, K.C.; Loonat, A.A.; Lakhal-Littleton, S.; Robbins, P.A.; Swietach, P. Iron-deficiency anemia reduces cardiac contraction by downregulating RyR2 channels and suppressing SERCA pump activity. JCI Insight 2019, 4, e125618. [Google Scholar] [CrossRef] [PubMed]
- Beck-da-Silva, L.; Piardi, D.; Soder, S.; Rohde, L.E.; Pereira-Barretto, A.C.; de Albuquerque, D.; Bocchi, E.; Vilas-Boas, F.; Moura, L.Z.; Montera, M.W. IRON-HF study: A randomized trial to assess the effects of iron in heart failure patients with anemia. Int. J. Cardiol. 2013, 168, 3439–3442. [Google Scholar] [CrossRef] [PubMed]
- Avni, T.; Leibovici, L.; Gafter-Gvili, A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: Systematic review and meta-analysis. Eur. J. Heart Fail. 2012, 14, 423–429. [Google Scholar] [CrossRef]
- Meyer, P.; Filippatos, G.S.; Ahmed, M.I.; Iskandrian, A.E.; Bittner, V.; Perry, G.J.; White, M.; Aban, I.B.; Mujib, M.; Dell’Italia, L.J. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation 2010, 121, 252–258. [Google Scholar] [CrossRef]
- Bosch, L.; Lam, C.S.; Gong, L.; Chan, S.P.; Sim, D.; Yeo, D.; Jaufeerally, F.; Leong, K.T.G.; Ong, H.Y.; Ng, T.P. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1664–1671. [Google Scholar] [CrossRef]
- Raina, A.; Meeran, T. Right ventricular dysfunction and its contribution to morbidity and mortality in left ventricular heart failure. Curr. Heart Fail. Rep. 2018, 15, 94–105. [Google Scholar] [CrossRef]
- Das, P.; Thandavarayan, R.A.; Watanabe, K.; Velayutham, R.; Arumugam, S. Right ventricular failure: A comorbidity or a clinical emergency? Heart Fail. Rev. 2022, 27, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Hwang, S.-J.; Lin, G.; Redfield, M.M.; Borlaug, B.A. Right heart dysfunction in heart failure with preserved ejection fraction. Eur. Heart J. 2014, 35, 3452–3462. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.A.; Sanchez, C.; Beussink, L.; Daruwalla, V.; Freed, B.H.; Selvaraj, S.; Shah, S.J. Lack of association between anemia and intrinsic left ventricular diastolic function or cardiac mechanics in heart failure with preserved ejection fraction. Am. J. Cardiol. 2018, 122, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Oni, O.; Adebiyi, A.; Aje, A.; Akingbola, T. Right ventricular function assessment in sickle cell anaemia patients using echocardiography. Haematol. Int. J. 2019, 1, 136. [Google Scholar]
- Barbosa, M.M.; Vasconcelos, M.C.M.; Ferrari, T.C.A.; Fernandes, B.M.; Passaglia, L.G.; Silva, C.M.; Nunes, M.C.P. Assessment of ventricular function in adults with sickle cell disease: Role of two-dimensional speckle-tracking strain. J. Am. Soc. Echocardiogr. 2014, 27, 1216–1222. [Google Scholar] [CrossRef]
- Maeder, M.T.; Khammy, O.; Dos Remedios, C.; Kaye, D.M. Myocardial and systemic iron depletion in heart failure: Implications for anemia accompanying heart failure. J. Am. Coll. Cardiol. 2011, 58, 474–480. [Google Scholar] [CrossRef]
- Zhang, H.; Zhabyeyev, P.; Wang, S.; Oudit, G.Y. Role of iron metabolism in heart failure: From iron deficiency to iron overload. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 1925–1937. [Google Scholar] [CrossRef]
- Engin, K.; Sinan, U.Y.; Arslan, S.; Kucukoglu, M.S. The effect of iron deficiency on RV function in acute decompensated heart failure patients. Eur. Heart J. 2023, 44 (Suppl. S2), ehad655.929. [Google Scholar] [CrossRef]
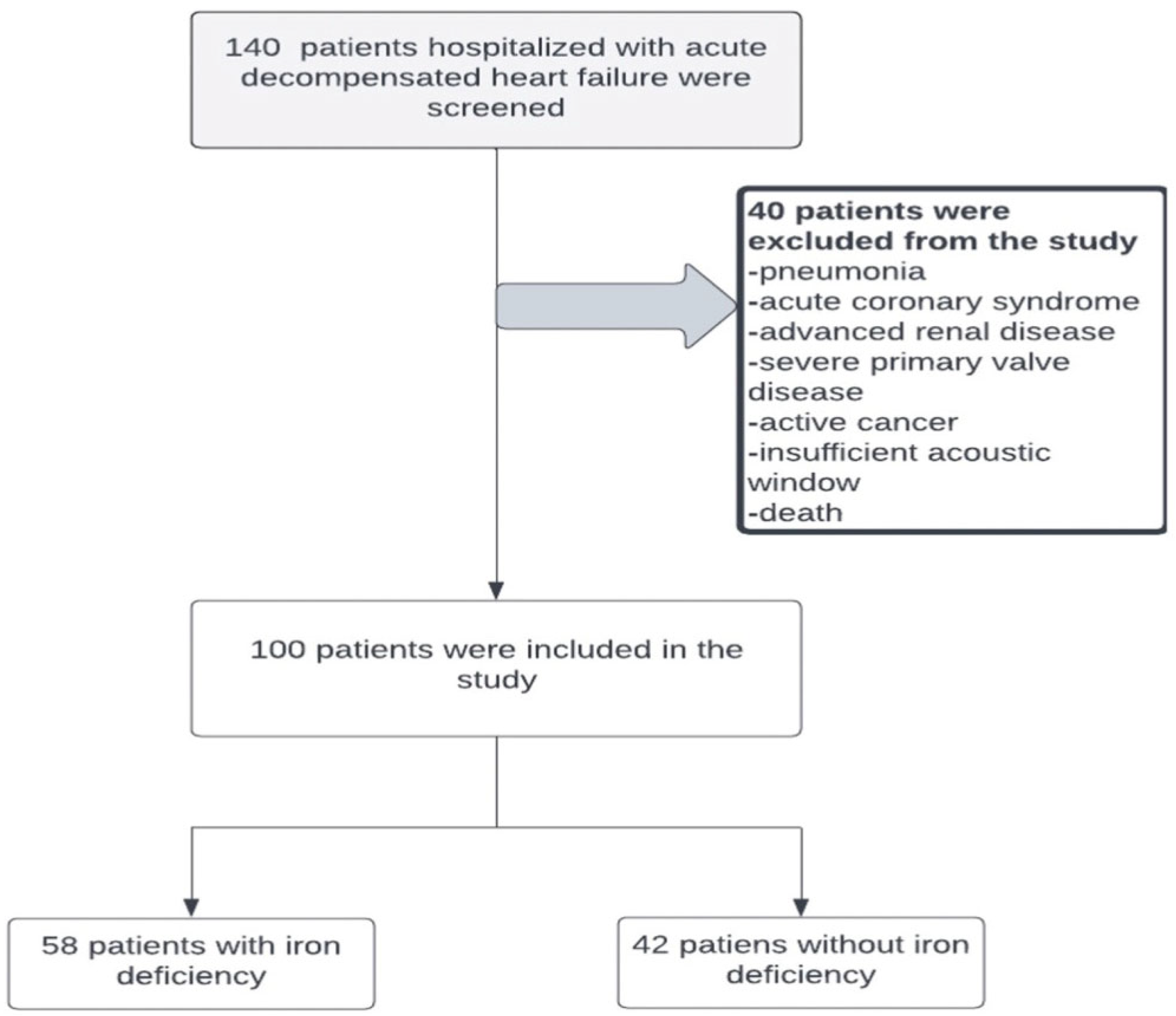
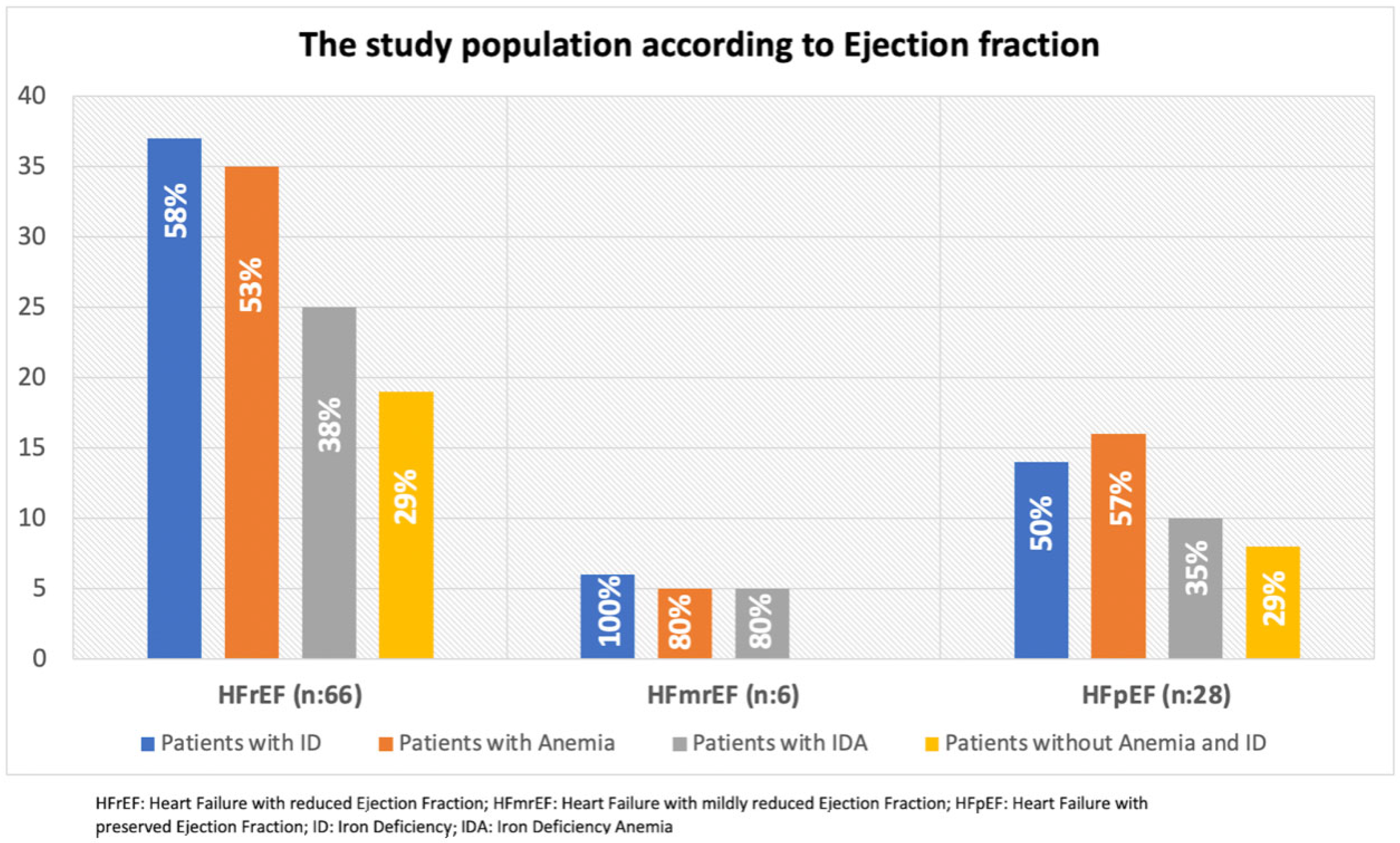
| Variable | Iron Deficiency (−) (n = 42) | Iron Deficiency (+) (n = 58) | p-Value |
|---|---|---|---|
| Age (years) | 68 (56–85) | 70 (54–87) | 0.65 |
| Gender (male), n (%) | 29 (69.0) | 35 (60.3) | 0.40 |
| Hypertension, n (%) | 33 (78.6) | 47 (81.0) | 0.80 |
| Diabetes mellitus, n (%) | 19 (45.2) | 33 (56.9) | 0.31 |
| Ischaemic heart disease, n (%) | 27 (64.3) | 44 (75.9) | 0.26 |
| Asthma/COPD, n (%) | 4 (9.5) | 14 (24.1) | 0.07 |
| Diuretic treatment prior to admission, n (%) | 40 (95.2) | 57 (98.3) | 0.57 |
| Heart rate (b.p.m.) | 76 (60–120) | 84 (55–115) | 0.38 |
| SBP (mmHg) | 130 (90–160) | 127 (100–155) | 0.67 |
| Severe TR, n (%) | 6 (14.3) | 15 (25.9) | 0.21 |
| Haemoglobin (g/dL) | 13.5 (10.5–16.0) | 11.6 (9.1–14.8) | <0.01 * |
| Haematocrit (%) | 40.2 (31.8–49.0) | 34.7 (26.4–45.1) | <0.01 * |
| Serum iron (μg/dL) | 50 (34–128) | 35 (16–198) | <0.01 * |
| Ferritin (ng/dL) | 156.6 (112–954) | 61.9 (10.3–169) | <0.01 * |
| TSAT (%) | 26.3 (17.7–60.1) | 14.5 (4.7–78.1) | <0.01 * |
| WBC (×103/mL) | 8.2 (4.5–64) | 8.1 (4.4–12) | 0.25 |
| CRP (mg/dL) | 5.0 (0.4–19) | 6.7 (0.6–32.4) | 0.23 |
| Creatinine (mg/dL) | 1.2 (0.5–1.9) | 1.1 (0.6–2.0) | 0.98 |
| eGFR (mL/min/1.73 m2) | 60.1 (30.5–120) | 53.4 (32.7–120) | 0.44 |
| Serum sodium (mmol/L) | 139.2 (130–145) | 138.1 (129–145) | 0.55 |
| Serum potassium (mmol/L) | 4.3 (3.0–5.3) | 4.5 (3.2–5.4) | 0.56 |
| ALT (U/L) | 20.5 (6–46) | 17 (3–43) | 0.08 |
| NT-proBNP (pg/mL) | 4500 (1000–20,000) | 3925 (1500–35,000) | 0.79 |
| Troponin (ng/mL) | 0.021 (0.007–0.05) | 0.018 (0.003–0.07) | 0.60 |
| Total cholesterol (mg/dL) | 142 (72–234) | 140 (91–298) | 0.68 |
| LDL-cholesterol (mg/dL) | 84 (34–156) | 75 (39–219) | 0.70 |
| HDL-cholesterol (mg/dL) | 40 (24–63) | 39.5 (21–93) | 0.46 |
| Echocardiographic findings | |||
| LVEF (%) | 32.5 (20–60) | 36 (18–60) | 0.91 |
| LAD (mm) | 48 (40–66) | 46.5 (42–61) | 0.59 |
| TAPSE (mm) | 17.2 (13–22.8) | 15.6 (12–22.9) | 0.05 |
| RV FAC (%) | 38.3 (24–54) | 36.4 (21–53) | 0.21 |
| RV-LS (%) | −18.2 (8.6–27.2) | −14.7 (6.3–26.8) | 0.005 * |
| Pulmonary acceleration time (ms) | 97.8 (60–140) | 88.8 (55–130) | 0.05 |
| PASP (mmHg) | 45 (30–100) | 50 (28–80) | 0.32 |
| RV TDI S’ (cm/s) | 10.7 (4.2–15.7) | 9.4 (5.3–17.0) | 0.09 |
| Parameter | Anaemia (−) ID (+) (n = 17) | Anaemia (+) ID (+) (n = 41) | p -Value |
Anaemia (−) ID (−) (n = 27) |
Anaemia (−) ID (+) (n = 17) | p -Value |
|---|---|---|---|---|---|---|
| LVEF (%) | 35 (18–60) | 38 (18–60) | 0.41 | 30 (20–60) | 35 (18–60) | 0.42 |
| LAD (mm) | 49 (44–60) | 46 (42–61) | 0.12 | 48 (40–61) | 49 (44–60) | 0.36 |
| TAPSE (mm) | 18 (12–22) | 15.2 (12–22.9) | 0.08 | 17.3 (14.1–22.8) | 18 (12–22) | 0.90 |
| FAC (%) | 38.6 (20–50) | 36 (20–53) | 0.39 | 38 (18.5–50) | 38.6 (20.7–50) | 0.09 |
| RV-LS (%) | −16.2 (6.3–26.8) | −14.7 (6.5–25.8) | 0.66 | −20.4 (−9.9–27) | −16.2 (6.3–26.8) | 0.05 * |
| Pulmonary acceleration time (ms) | 92 (50–116) | 88 (56–132) | 0.91 | 96.9 ± 22.1 | 86.9 ± 21.1 | 0.20 |
| PASP (mmHg) | 50 (30–80) | 50 (28–70) | 0.72 | 45 (30–100) | 50 (30–80) | 0.68 |
| RV TDI S’ (cm/s) | 10.3 (5.3–16.3) | 9.1 (6.4–17) | 0.78 | 12.2 (4.2–15.4) | 10.3 (5.3–16.3) | 0.21 |
| Variable | Unstandardised Coefficient (B) | Std. Error | Beta | p -Value | 95% CI |
|---|---|---|---|---|---|
| Constant | −0.961 | 7.507 | 0.89 | −15.872 to 13.950 | |
| Haemoglobin (HGB) | 0.652 | 0.248 | 0.252 | 0.01 * | 0.160 to 1.145 |
| LVEF (%) | 0.158 | 0.035 | 0.429 | <0.01 * | 0.088 to 0.228 |
| eGFR (mL/min/1.73 m2) | −0.010 | 0.66 | |||
| Heart rate (bpm) | −0.046 | 0.12 | |||
| DBP (mmHg) | 0.008 | 0.90 | |||
| Troponin (ng/mL) | 5.444 | 0.87 | |||
| Ferritin (ng/mL) | 0.007 | 0.15 | |||
| LAD (mm) | 0.126 | 0.19 |
| Parameter | N | Correlation Coefficient (r) | Standard Error (SE) | p -Value |
|---|---|---|---|---|
| Ferritin vs. RV-LS | 99 | 0.21 | - | 0.04 * |
| Ferritin vs. pulmonary acceleration time (ms) | 99 | 0.25 | - | 0.02 * |
| Haemoglobin vs. RV-LS | 99 | 0.32 | - | 0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engin, K.; Sinan, U.Y.; Arslan, S.; Kucukoglu, M.S. Effect of Iron Deficiency on Right Ventricular Strain in Patients Diagnosed with Acute Heart Failure. J. Clin. Med. 2025, 14, 5188. https://doi.org/10.3390/jcm14155188
Engin K, Sinan UY, Arslan S, Kucukoglu MS. Effect of Iron Deficiency on Right Ventricular Strain in Patients Diagnosed with Acute Heart Failure. Journal of Clinical Medicine. 2025; 14(15):5188. https://doi.org/10.3390/jcm14155188
Chicago/Turabian StyleEngin, Kemal, Umit Yasar Sinan, Sukru Arslan, and Mehmet Serdar Kucukoglu. 2025. "Effect of Iron Deficiency on Right Ventricular Strain in Patients Diagnosed with Acute Heart Failure" Journal of Clinical Medicine 14, no. 15: 5188. https://doi.org/10.3390/jcm14155188
APA StyleEngin, K., Sinan, U. Y., Arslan, S., & Kucukoglu, M. S. (2025). Effect of Iron Deficiency on Right Ventricular Strain in Patients Diagnosed with Acute Heart Failure. Journal of Clinical Medicine, 14(15), 5188. https://doi.org/10.3390/jcm14155188







