A Systematic Review of Closed-Incision Negative-Pressure Wound Therapy for Hepato-Pancreato-Biliary Surgery: Updated Evidence, Context, and Clinical Implications
Abstract
1. Introduction
2. Materials and Methods
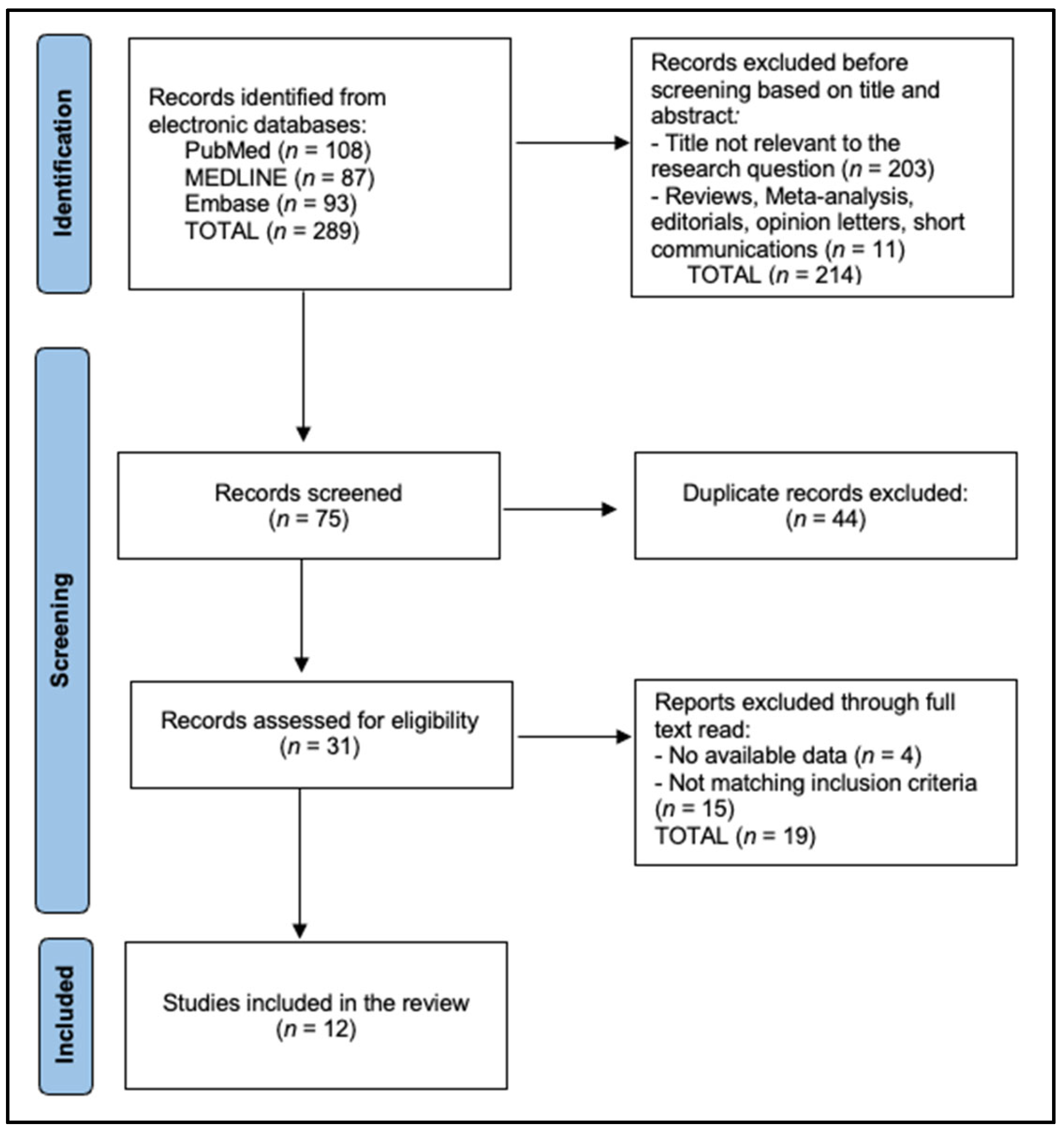
2.1. Search Strategy and Protocol
2.2. Eligibility Criteria
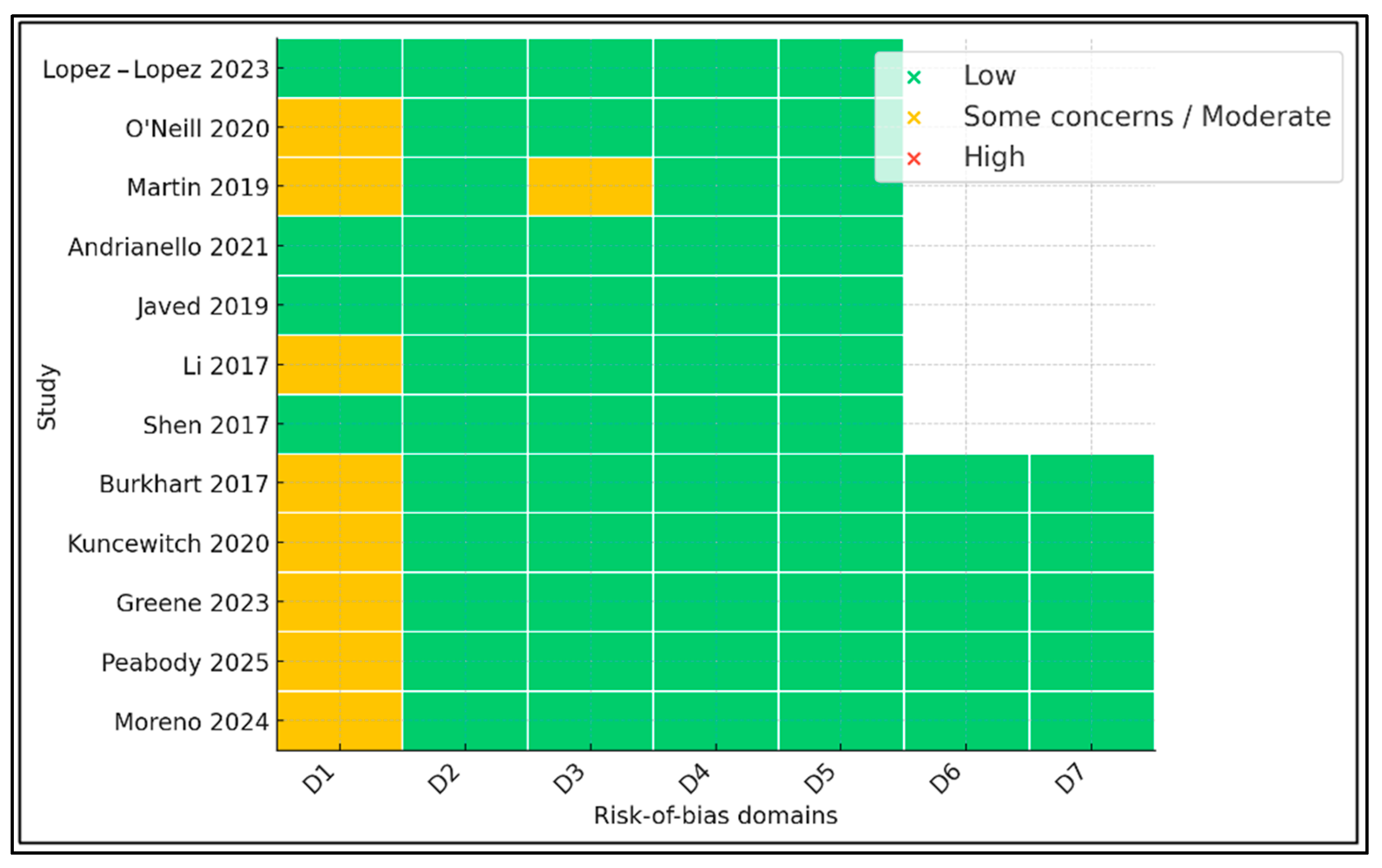
2.3. Data Extraction and Risk-of-Bias Assessment
2.4. Outcome Definitions
2.5. Statistical Analysis
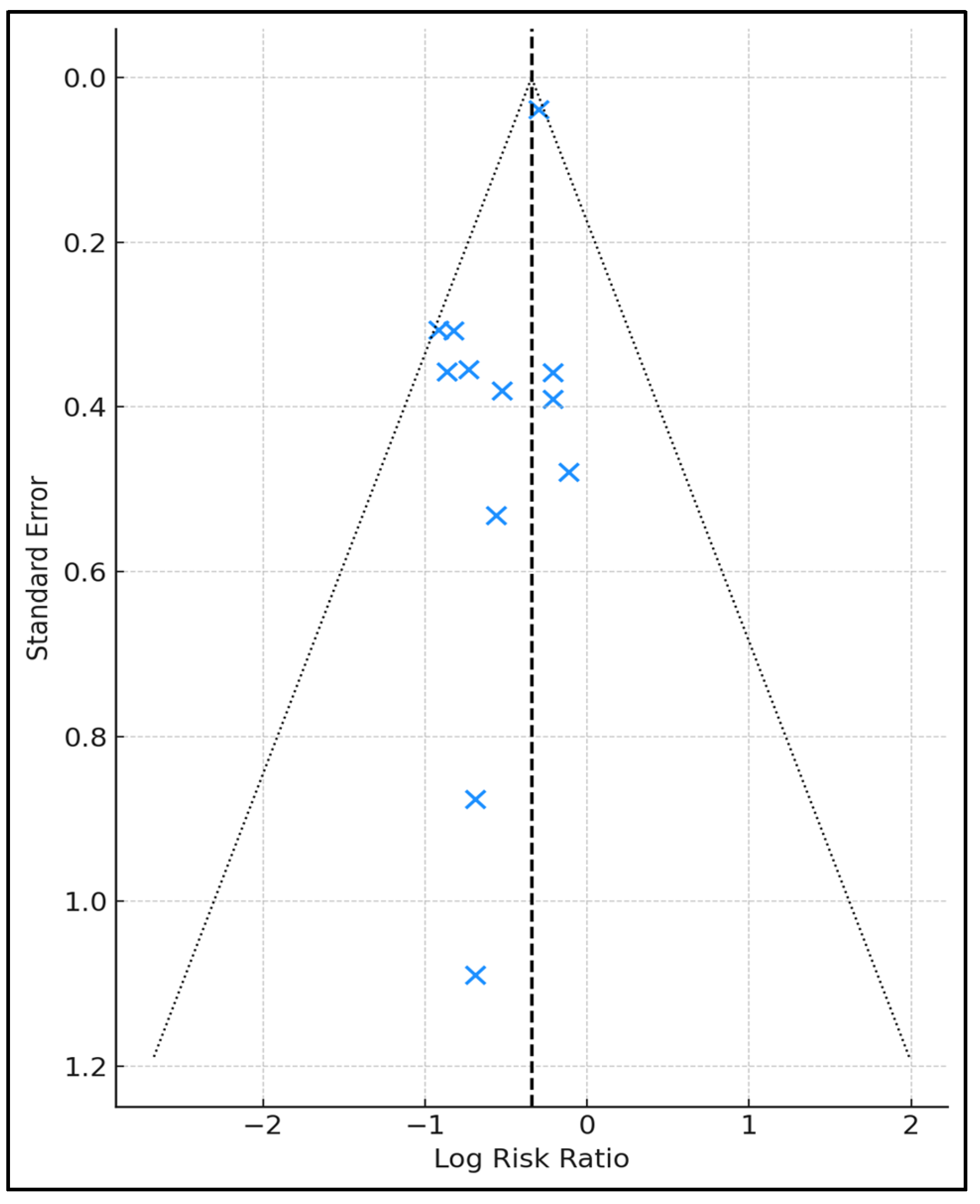
3. Results
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gillespie, B.M.; Harbeck, E.; Rattray, M.; Liang, R.; Walker, R.; Latimer, S.; Thalib, L.; Andersson, A.E.; Griffin, B.; Ware, R.; et al. Worldwide incidence of surgical site infections in general surgical patients: A systematic review and meta-analysis of 488,594 patients. Int. J. Surg. 2021, 95, 106136. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.E.; Sheen, A.J.; Whitehead, K.A. A systematic review on the incidence and risk factors of surgical site infections following hepatopancreatobiliary (HPB) surgery. AIMS Bioeng. 2022, 9, 123–144. [Google Scholar] [CrossRef]
- Moreno Elola-Olaso, A.; Davenport, D.L.; Hundley, J.C.; Daily, M.F.; Gedaly, R. Predictors of surgical site infection after liver resection: A multicentre analysis using National Surgical Quality Improvement Program data. HPB 2012, 14, 136–141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zaver, V.; Kankanalu, P. Negative Pressure Wound Therapy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK576388 (accessed on 14 July 2025).
- Groenen, H.; Jalalzadeh, H.; Buis, D.R.; Dreissen, Y.E.M.; Goosen, J.H.M.; Griekspoor, M.; Harmsen, W.J.; IJpma, F.F.A.; van der Laan, M.J.; Schaad, R.R.; et al. Incisional negative pressure wound therapy for the prevention of surgical site infection: An up-to-date meta-analysis and trial sequential analysis. EClinicalMedicine 2023, 62, 102105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuuli, M.G.; Liu, J.; Tita, A.T.N.; Longo, S.; Trudell, A.; Carter, E.B.; Shanks, A.; Woolfolk, C.; Caughey, A.B.; Warren, D.K.; et al. Effect of Prophylactic Negative Pressure Wound Therapy vs Standard Wound Dressing on Surgical-Site Infection in Obese Women After Cesarean Delivery: A Randomized Clinical Trial. JAMA 2020, 324, 1180–1189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nanashima, A.; Arai, J.; Oyama, S.; Ishii, M.; Abo, T.; Wada, H.; Takagi, K.; Tsuchiya, T.; Nagayasu, T. Associated factors with surgical site infections after hepatectomy: Predictions and countermeasures by a retrospective cohort study. Int. J. Surg. 2014, 12, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Brennfleck, F.W.; Linsenmeier, L.; Junger, H.H.G.; Schmidt, K.M.; Werner, J.M.; Woehl, D.; Zeman, F.; Mutzbauer, I.; Hutchinson, J.A.; Geissler, E.K.; et al. Negative pressure wound therapy (NPWT) on closed incisions to prevent surgical site infection in high-risk patients in hepatopancreatobiliary surgery: Study protocol for a randomized controlled trial-the NP-SSI trial. Trials 2020, 21, 918. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Lopez-Lopez, V.; Hiciano-Guillermo, A.; Martinez-Alarcon, L.; Delegido, A.; Alconchel, F.; Pons, J.A.; Fernández, J.Á.; Ríos, A.; Rodríguez, J.M.; Miura, K.; et al. Postoperative negative-pressure incision therapy after liver transplant (PONILITRANS study): A randomized controlled trial. Surgery 2023, 173, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.H.; Martin, R.C.G., 2nd. Negative-pressure wound therapy does not reduce superficial SSI in pancreatectomy and hepatectomy procedures. J. Surg. Oncol. 2020, 122, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.G.; O’Neill, C.H. Negative-pressure therapy for hepatectomy and pancreatectomy: A randomized trial for surgical site infection prevention. HPB 2019, 21, S26–S27. [Google Scholar] [CrossRef]
- Andrianello, S.; Landoni, L.; Bortolato, C.; Iudici, L.; Tuveri, M.; Pea, A.; De Pastena, M.; Malleo, G.; Bonamini, D.; Manzini, G.; et al. Negative pressure wound therapy for prevention of surgical site infection in patients at high risk after clean-contaminated major pancreatic resections: A single-center, phase 3, randomized clinical trial. Surgery 2021, 169, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.A.; Teinor, J.; Wright, M.; Ding, D.; Burkhart, R.A.; Hundt, J.; Cameron, J.L.; Makary, M.A.; He, J.; Eckhauser, F.E.; et al. Negative Pressure Wound Therapy for Surgical-site Infections: A Randomized Trial. Ann. Surg. 2019, 269, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, R.A.; Javed, A.A.; Ronnekleiv-Kelly, S.; Wright, M.J.; Poruk, K.E.; Eckhauser, F.; Makary, M.A.; Cameron, J.L.; Wolfgang, C.L.; He, J.; et al. The use of negative pressure wound therapy to prevent post-operative surgical site infections following pancreaticoduodenectomy. HPB 2017, 19, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Kuncewitch, M.P.; Blackham, A.U.; Clark, C.J.; Dodson, R.M.; Russell, G.B.; Levine, E.A.; Shen, P. Effect of Negative Pressure Wound Therapy on Wound Complications Post-Pancreatectomy. Am. Surg. 2019, 85, 1–7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greene, B.; Lagrotteria, A.; Tsang, M.E.; Jayaraman, S. Closed incision nega tive pressure wound therapy following pancreaticoduodenectomy for prevention of surgical site infections in high-risk patients. Can. J. Surg. 2023, 66, E507–E512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, P.Y.; Yang, D.; Liu, D.; Sun, S.J.; Zhang, L.Y. Reducing Surgical Site Infection with Negative-Pressure Wound Therapy After Open Abdominal Surgery: A Prospective Randomized Controlled Study. Scand. J. Surg. 2017, 106, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Blackham, A.U.; Lewis, S.; Clark, C.J.; Howerton, R.; Mogal, H.D.; Dodson, R.M.; Russell, G.B.; Levine, E.A. Phase II Randomized Trial of Negative-Pressure Wound Therapy to Decrease Surgical Site Infection in Patients Undergoing Laparotomy for Gastrointestinal, Pancreatic, and Peritoneal Surface Malignancies. J. Am. Coll. Surg. 2017, 224, 726–737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peabody, J.; Jatana, S.; Verhoeff, K.; Shapiro, A.M.J.; Bigam, D.L.; Anderson, B.; Dajani, K. Impact of Negative Pressure Wound Therapy on Outcomes Following Pancreaticoduodenectomy: An NSQIP Analysis of 14,044 Patients. Surg. Tech. Dev. 2025, 14, 8. [Google Scholar] [CrossRef]
- Moreno Gijón, M.; Suárez Sánchez, A.; de Santiago Álvarez, I.; Rodicio Miravalles, J.L.; Amoza Pais, S.; Rodríguez Uría, R.; Sanz Navarro, S.; Díaz Vico, T.; Turienzo Santos, E.; Sanz Álvarez, L. The efficacy of negative-pressure wound therapy (NPWT) in the prevention of surgical site occurrences in open abdominal surgery: A randomized clinical trial. Surgery 2025, 178, 108920. [Google Scholar] [CrossRef] [PubMed]
- Daliu, P.; Bogdan, I.; Rosca, O.; Licker, M.; Stanga, L.C.; Hogea, E.; Vaduva, D.B.; Muntean, D. Fungal Pulmonary Coinfections in COVID-19: Microbiological Assessment, Inflammatory Profiles, and Clinical Outcomes. Biomedicines 2025, 13, 864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, M.S.; Moore, Z.; Patton, D.; McNamara, D.; O’Connor, T.; Avsar, P. A systematic review of patient risk factors for complications following stoma formation among adults undergoing colorectal surgery. Int. J. Colorectal Dis. 2023, 38, 238, Erratum in Int. J. Colorectal Dis. 2023, 38, 255. https://doi.org/10.1007/s00384-023-04549-9. [Google Scholar] [CrossRef] [PubMed]
- Stanga, L.C.; Vaduva, D.M.B.; Grigoras, M.L.; Nussbaum, L.A.; Gurgus, D.; Strat, L.; Zamfir, A.S.; Poroch, V.; Folescu, R. Nosocomial Infections Distribution and Impact in Medical Units. Rev. Chim. 2019, 70, 2265–2268. [Google Scholar] [CrossRef]
- Iacob, M.S.; Kundnani, N.R.; Sharma, A.; Meche, V.; Ciobotaru, P.; Bedreag, O.; Sandesc, D.; Dragan, S.R.; Papurica, M.; Stanga, L.C. Multifactorial Risk Stratification in Patients with Heart Failure, Chronic Kidney Disease, and Atrial Fibrillation: A Comprehensive Analysis. Life 2025, 15, 786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Denti, F.C.; Guerra, E.; Caroppo, F.; Abruzzese, P.; Alessi, F.; Barone, F.; Bernardino, P.; Bergamini, M.; Bernardo, M.C.; Bosio, G.; et al. Outcomes of a Risk-Stratified Protocol for Preventing Peristomal Skin Complications in Patients with an Ostomy: A Cohort Study. Nurs. Rep. 2025, 15, 179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahebally, S.M.; McKevitt, K.; Stephens, I.; Fitzpatrick, F.; Deasy, J.; Burke, J.P.; McNamara, D. Negative Pressure Wound Therapy for Closed Laparotomy Incisions in General and Colorectal Surgery: A Systematic Review and Meta-analysis. JAMA Surg. 2018, 153, e183467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- SUNRRISE Trial Study Group; Atherton, K.; Brown, J.; Clouston, H.; Coe, P.; Duarte, R.; Dudi-Venkata, N.N.; Duff, S.; Egoroff, N.; Fish, R.; et al. Negative Pressure Dressings to Prevent Surgical Site Infection After Emergency Laparotomy: The SUNRRISE Randomized Clinical Trial. JAMA 2025, 333, 853–863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seth, I.; Gibson, D.; Lim, B.; Cevik, J.; Bulloch, G.; Xie, Y.; Marcaccini, G.; Rozen, W.M.; Cuomo, R. Advancements, applications, and safety of negative pressure wound therapy: A comprehensive review of its impact on wound outcomes. Plast. Aesthet. Res. 2024, 11, 29. [Google Scholar] [CrossRef]
- Marckmann, M.; Henriksen, N.A.; Krarup, P.M.; Helgstrand, F.; Vester-Glowinski, P.; Christoffersen, M.W.; Jensen, K.K. PROphylactic closed incision Negative-PRESSure treatment in open incisional hernia repair: Protocol for a multicenter randomized trial (PROPRESS study). Contemp. Clin. Trials Commun. 2024, 38, 101256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ceppa, E.P.; Kim, R.C.; Niedzwiecki, D.; Lowe, M.E.; Warren, D.A.; House, M.G.; Nakeeb, A.; Zani, S.; Moyer, A.N.; Blazer, D.G., 3rd; et al. Closed Incision Negative Pressure Therapy to Reduce Surgical Site Infection in High-Risk Gastrointestinal Surgery: A Randomized Controlled Trial. J. Am. Coll. Surg. 2023, 236, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Oishi, T.; Nakamura, F.; Okuno, N.; Tamagaki, K.; Nakamura, Y.; Sakuramoto, K.; Kuwagata, Y. Effect of closed negative pressure wound therapy after laparotomy for gastrointestinal perforation: A single-center observational study. Acute Med. Surg. 2025, 12, e70069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chung, J.N.C.; Ali, O.; Hawthornthwaite, E.; Watkinson, T.; Blyth, U.; McKigney, N.; Harji, D.P.; Griffiths, B. Closed Incision Negative Pressure Wound Therapy Is Associated with Reduced Surgical Site Infection after Emergency Laparotomy: A Propensity Matched-Cohort Analysis. Surgery 2021, 170, 1568–1573. [Google Scholar] [CrossRef]
- Munro, S.P.; Dearden, A.; Joseph, M.; O’Donoghue, J.M. Reducing donor-site complications in DIEP flap breast reconstruction with closed incisional negative pressure therapy: A cost-benefit analysis. J. Plast. Reconstr. Aesthet. Surg. 2023, 78, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kerivan, L.T.; Vilain, K.A.; Hill, T.M.; Guidry, C.A. Closed Incisional Negative Pressure Wound Therapy is Cost-Effective at Reducing Superficial Surgical Site Infections. Surg. Infect. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Swinford, A.; Martucci, J.; Wang, J.; Wlodarczyk, J.R.; Gupta, A.; Cologne, K.G.; Koller, S.E.; Hsieh, C.; Duldulao, M.P. Mechanically powered negative pressure dressing reduces surgical site infection after stoma reversal. Surg. Open Sci. 2025, 23, 69–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller-Mikolajczyk, C.; Beach, K.; Silverman, R.; Cooper, M. The Evolution of Commercial Negative Pressure Wound Therapy Systems over the Past Three Decades. Adv. Wound Care 2024, 13, 375–390. [Google Scholar] [CrossRef] [PubMed]
| # | First Author (Year) | Country | Design | Operation(s) | n (NPWT/Control) | Device | NPWT Duration (d) |
|---|---|---|---|---|---|---|---|
| 1 | López-López 2023 [10] | Spain | RCT | Liver transplant | 108 (54/54) | Prevena™ | 4 |
| 2 | O’Neill 2020 [11] | USA | RCT | Hepatectomy ± pancreatectomy | 40 (20/20) | PICO™ | 7 |
| 3 | Martin 2019 [12] | USA | RCT (abstract) | Hepatectomy ± pancreatectomy | 60 (30/30) | Prevena™ | 5 |
| 4 | Andrianello 2021 [13] | Italy | RCT | Pancreatic resection (high-risk) | 100 (50/50) | PICO™ | 7 |
| 5 | Javed 2019 [14] | USA | RCT | Pancreaticoduodenectomy | 120 (60/60) | Prevena™ | 5 |
| 6 | Burkhart 2017 [15] | ||||||
| 7 | Kuncewitch 2020 [16] | USA | Cohort | Pancreatectomy | 98 (48/50) | PICO™ | 7 |
| 8 | Greene 2023 [17] | Canada | Cohort | Pancreaticoduodenectomy | 175 (61/114) | iVAC® | 5 |
| 9 | Li 2017 [18] | China | RCT | Open abdominal (incl. hepatectomy 16%) | 130 (65/65) | PICO™ | 3 |
| 10 | Shen 2017 [19] | USA | RCT | Laparotomy for GI/HPB tumors | 112 (56/56) | Prevena™ | 4 |
| 11 | Peabody 2025 [20] | USA | NSQIP analysis | Pancreatectomy (national) | 14,044 (2812/11,232) | Mixed | NR |
| 12 | Moreno 2024 [21] | Spain | RCT | Laparotomy (25% liver) | 275 (147/128) | PICO™ | 4 |
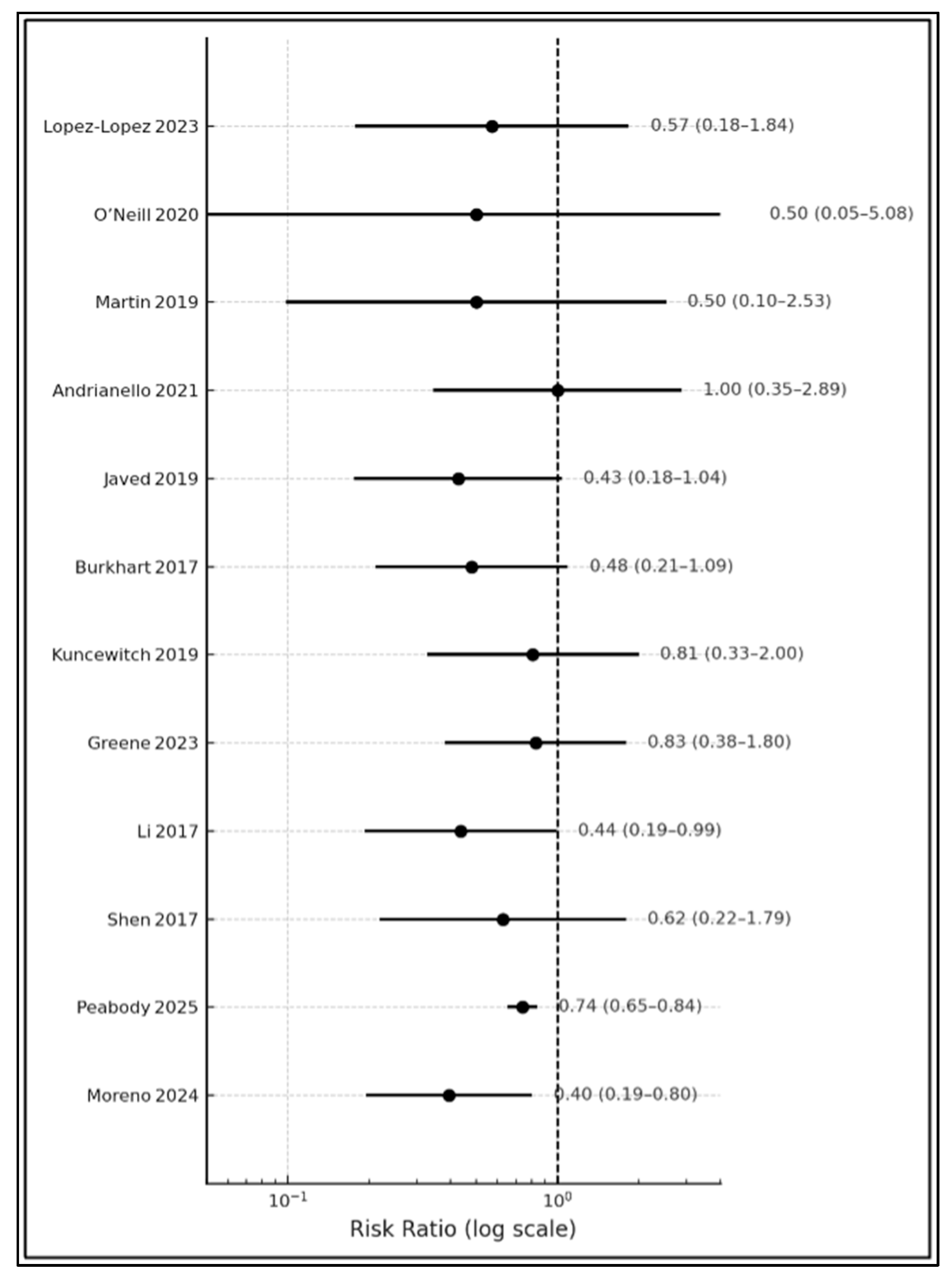
| # | Study | Superficial SSI % (NPWT vs. Control) | Deep/Organ SSI % | Relative Risk (95% CI) |
|---|---|---|---|---|
| 1 | López-López 2023 [10] | 7.4 vs. 13.0 | 20.4 vs. 22.2 | 0.57 (0.20–1.61) |
| 2 | O’Neill 2020 [11] | 5.0 vs. 10.0 | 10.0 vs. 20.0 | 0.50 (0.06–4.31) |
| 3 | Martin 2019 [12] | 6.7 vs. 13.3 | NR | 0.50 (0.09–2.79) |
| 4 | Andrianello 2021 [13] | 10.9 vs. 12.2 | 46.7 vs. 43.8 | 0.89 (0.35–2.29) |
| 5 | Javed 2019 [14] | 9.8 vs. 23.3 | 29.5 vs. 37.0 | 0.42 (0.21–0.85) |
| 6 | Burkhart 2017 [15] | 12.0 vs. 25.0 | 18.0 vs. 21.0 | 0.48 (0.24–0.96) |
| 7 | Kuncewitch 2020 [16] | 14.6 vs. 18.0 | 22.9 vs. 26.0 | 0.81 (0.40–1.63) |
| 8 | Greene 2023 [17] | 13.0 vs. 16.0 | 26.0 vs. 29.0 | 0.81 (0.38–1.75) |
| 9 | Li 2017 [18] | 11.0 vs. 25.0 | NR | 0.44 (0.24–0.80) |
| 10 | Shen 2017 [19] | 8.9 vs. 15.0 | 31.0 vs. 36.0 | 0.59 (0.28–1.24) |
| 11 | Peabody 2025 [20] | 9.1 vs. 12.3 | 15.5 vs. 18.7 | 0.74 (0.69–0.80) |
| 12 | Moreno 2024 [21] | 6.8 vs. 17.2 | 19.0 vs. 23.4 | 0.40 (0.22–0.73) |
| # | Study | Median LOS d (NPWT vs. Control) | Re-Operation % | 90-Day Mortality % | Device-Related Complications % |
|---|---|---|---|---|---|
| 1 | López-López 2023 [10] | 13 vs. 14 | 1.9 vs. 5.6 | 1.9 vs. 3.7 | 0 |
| 2 | O’Neill 2020 [11] | 8 vs. 9 | 5 vs. 5 | 0 vs. 0 | 0 |
| 3 | Martin 2019 [12] | NR | NR | NR | 0 |
| 4 | Andrianello 2021 [13] | 11 vs. 12 | 2 vs. 4 | 2 vs. 4 | 0 |
| 5 | Javed 2019 [14] | 9 vs. 11 | 3 vs. 6 | 0 vs. 2 | 1.7 (seroma) |
| 6 | Burkhart 2017 [15] | 10 vs. 13 | 4 vs. 8 | 2 vs. 2 | 0 |
| 7 | Kuncewitch 2020 [16] | 9 vs. 10 | 4 vs. 10 | 0 vs. 2 | 0 |
| 8 | Greene 2023 [17] | 11 vs. 12 | 5 vs. 7 | 2 vs. 3 | 0 |
| 9 | Li 2017 [18] | 7 vs. 10 | 2 vs. 5 | 0 vs. 1 | 0 |
| 10 | Shen 2017 [19] | 10 vs. 11 | 4 vs. 6 | 1 vs. 2 | 0 |
| 11 | Peabody 2025 [20] | NR | 3.5 vs. 4.1 | 1.2 vs. 1.5 | NR |
| 12 | Moreno 2024 [21] | 9 vs. 12 | 3 vs. 5 | 0.7 vs. 1.6 | 0 |
| Outcome (30 Days) | Participants (Studies) | Relative Effect (95% CI) | Absolute Effect | Certainty | Explanation |
|---|---|---|---|---|---|
| Superficial SSI | 15,212 (12) | RR 0.71 (0.63–0.79) | 126 → 90 per 1000 | Moderate | Downgraded 1× for imprecision |
| Deep/organ-space SSI | 14,456 (9) | RR 0.93 (0.79–1.09) | 188 → 175 per 1000 | Low | Imprecision + bias |
| Length of stay | 9135 (10) | MD −1.7 days (−2.5 to −0.9) | – | Low | Inconsistency |
| Re-operation | 9987 (11) | RR 0.56 (0.39–0.80) | 61 → 34 per 1000 | Low | Bias + imprecision |
| 90-day mortality | 12,604 (9) | RR 0.94 (0.69–1.28) | 21 → 20 per 1000 | Low | Imprecision |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feier, C.V.I.; Gaborean, V.; Faur, I.F.; Vonica, R.C.; Faur, A.M.; Rus, V.I.; Dragan, B.S.; Muntean, C. A Systematic Review of Closed-Incision Negative-Pressure Wound Therapy for Hepato-Pancreato-Biliary Surgery: Updated Evidence, Context, and Clinical Implications. J. Clin. Med. 2025, 14, 5191. https://doi.org/10.3390/jcm14155191
Feier CVI, Gaborean V, Faur IF, Vonica RC, Faur AM, Rus VI, Dragan BS, Muntean C. A Systematic Review of Closed-Incision Negative-Pressure Wound Therapy for Hepato-Pancreato-Biliary Surgery: Updated Evidence, Context, and Clinical Implications. Journal of Clinical Medicine. 2025; 14(15):5191. https://doi.org/10.3390/jcm14155191
Chicago/Turabian StyleFeier, Catalin Vladut Ionut, Vasile Gaborean, Ionut Flaviu Faur, Razvan Constantin Vonica, Alaviana Monique Faur, Vladut Iosif Rus, Beniamin Sorin Dragan, and Calin Muntean. 2025. "A Systematic Review of Closed-Incision Negative-Pressure Wound Therapy for Hepato-Pancreato-Biliary Surgery: Updated Evidence, Context, and Clinical Implications" Journal of Clinical Medicine 14, no. 15: 5191. https://doi.org/10.3390/jcm14155191
APA StyleFeier, C. V. I., Gaborean, V., Faur, I. F., Vonica, R. C., Faur, A. M., Rus, V. I., Dragan, B. S., & Muntean, C. (2025). A Systematic Review of Closed-Incision Negative-Pressure Wound Therapy for Hepato-Pancreato-Biliary Surgery: Updated Evidence, Context, and Clinical Implications. Journal of Clinical Medicine, 14(15), 5191. https://doi.org/10.3390/jcm14155191