The Effect of Aerobic Training on Healthy Small Airways—A Forced Oscillation Technique Approach to Optimize Long Term Care in COPD
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intervention
- Standing trunk rotations holding baton with synchronized profound respiratory efforts
- Chest expansion exercises with 0.5 kg dumbbells (lateral lifts)
- Kettlebell Halo rotation exercise
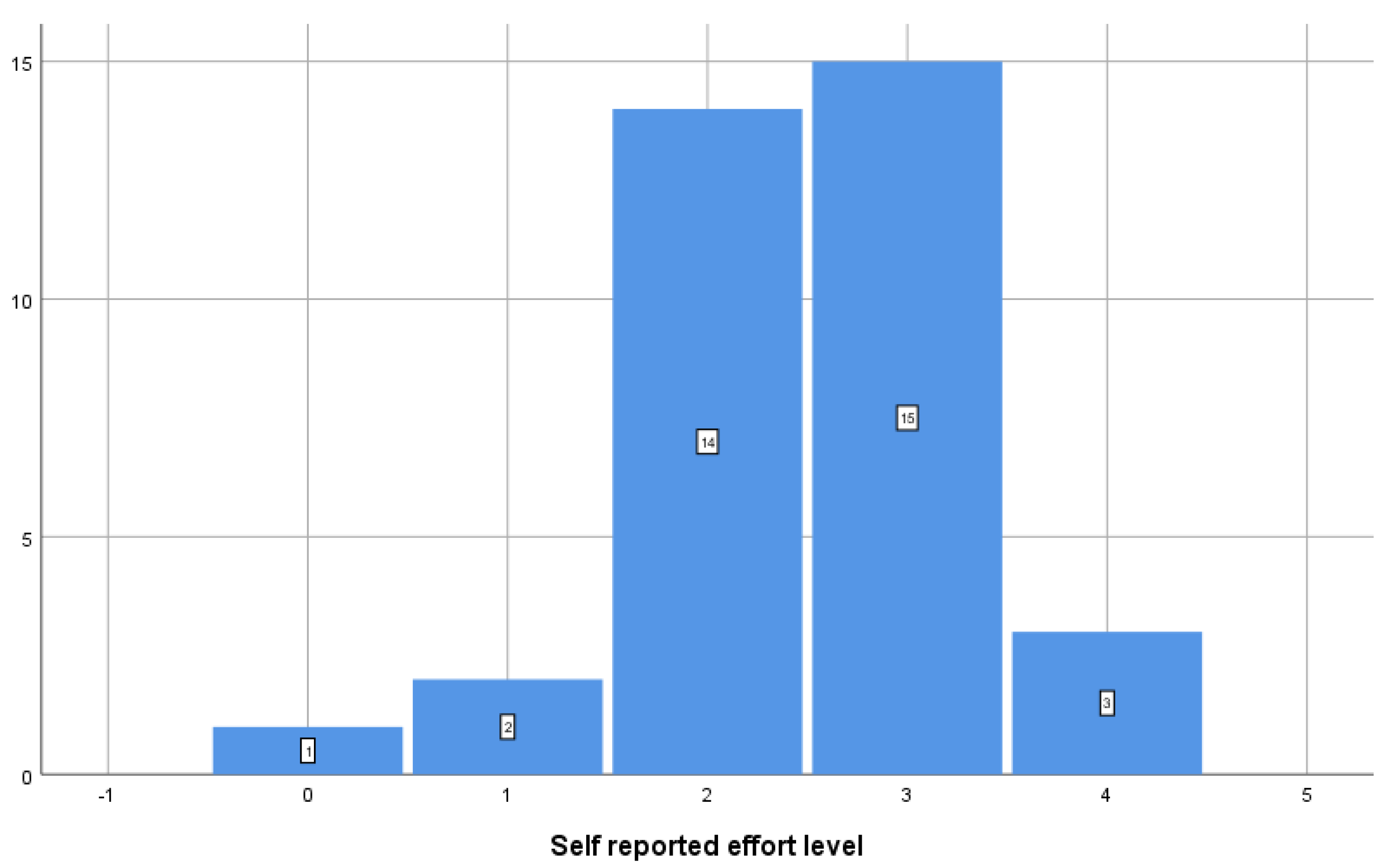
3. Results
4. Discussion
4.1. Main Findings
4.2. Ventilatory Parameters
4.3. Potential Physiological Mechanisms
4.4. Post Hoc Analysis
4.5. Extrapolating Results to a COPD-Centered Rehabilitation Program
4.6. Limits and Caveats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COPD | Chronic obstructive pulmonary disease |
| FOT | Force oscillation technique |
| IOS | Impulse oscillometry |
| FEV1 | First second of forced expiration |
| FVC | Forced vital capacity |
| R5Hz | Respiratory resistance |
| X5Hz | Lung reactance |
| D5-20% | peripheral airway resistance |
| AX | Reactance area |
| Fres. | Resonant frequency |
References
- Vogelmeier, C.F.; Román-Rodríguez, M.; Singh, D.; Han, M.K.; Rodríguez-Roisin, R.; Ferguson, G.T. Goals of COPD treatment: Focus on symptoms and exacerbations. Respir. Med. 2020, 166, 105938. [Google Scholar] [CrossRef]
- Pyszora, A.; Lewko, A. Non-pharmacological Management in Palliative Care for Patients with Advanced COPD. Front. Cardiovasc. Med. 2022, 9, 907664. [Google Scholar] [CrossRef]
- Knaut, C.; Bonfanti Mesquita, C.; Dourado, V.Z.; De Godoy, I.; Tanni, S.E. Evaluation of Inflammatory Markers in Patients Undergoing a Short-Term Aerobic Exercise Program While Hospitalized Due to Acute Exacerbation of COPD. Int. J. Inflamm. 2020, 2020, 6492720. [Google Scholar] [CrossRef]
- Ibrahim, C.J.; Probandari, A.N.; Susanto, Y.S.; Aphridasari, J. Optimal Intensity of Aerobic Exercise Training for Patient with Chronic Obstructive Pulmonary Disease (COPD): Systematic Review and Meta-Analysis. Respir. Sci. 2023, 3, 116–131. [Google Scholar] [CrossRef]
- Qiao, Z.; Kou, Z.; Zhang, J.; Lv, D.; Cui, X.; Li, D.; Jiang, T.; Yu, X.; Liu, K. Optimal Intensity and Type of Lower Limb Aerobic Training for Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Network Meta-Analysis of RCTs. Ther. Adv. Respir. Dis. 2025, 19, 17534666251323190. [Google Scholar] [CrossRef]
- Bickel, S.; Popler, J.; Lesnick, B.; Eid, N. Impulse Oscillometry: Interpretation and practical applications. Chest 2014, 146, 841–847. [Google Scholar] [CrossRef]
- Frantz, S.; Nihlén, U.; Dencker, M.; Engström, G.; Löfdahl, C.G.; Wollmer, P. Impulse oscillometry may be of value in detecting early manifestations of COPD. Respir. Med. 2012, 106, 1116–1123. [Google Scholar] [CrossRef]
- Kleinhendler, E.; Rosman, M.; Fireman, E.; Freund, O.; Gershman, I.; Pumin, I.; Perluk, T.; Tiran, B.; Unterman, A.; Bar-Shai, A. Impulse Oscillometry as an Alternative Lung Function Test for Hospitalized Adults. Respir. Care 2024, 69, 415–421. [Google Scholar] [CrossRef]
- Barreto, M.; Veneroni, C.; Caiulo, M.; Evangelisti, M.; Pompilio, P.P.; Mazzuca, M.C.; Raponi, G.; Pagani, J.; Parisi, P. Within-Breath Oscillometry for Identifying Exercise-Induced Bronchoconstriction in Pediatric Patients Reporting Symptoms with Exercise. Front. Pediatr. 2024, 11, 1324413. [Google Scholar] [CrossRef]
- Schumm, B.; Bremer, S.; Knödlseder, K.; Schönfelder, M.; Hain, R.; Semmler, L.; Lorenz, E.; Wackerhage, H.; Kähler, C.J.; Jörres, R. Indices of Airway Resistance and Reactance from Impulse Oscillometry Correlate with Aerosol Particle Emission in Different Age Groups. Sci. Rep. 2024, 14, 4644. [Google Scholar] [CrossRef]
- Meigh, N.J.; Keogh, J.W.L.; Schram, B.; Hing, W.A. Kettlebell training in clinical practice: A scoping review. BMC Sports Sci. Med. Rehabil. 2019, 11, 19. [Google Scholar] [CrossRef]
- Peters, C.M.; Dempsey, J.A.; Hopkins, S.R.; Sheel, A.W. Is the Lung Built for Exercise? Advances and Unresolved Questions. Med. Sci. Sports Exerc. 2023, 55, 2143–2159. [Google Scholar] [CrossRef]
- Xu, J.; Sun, X.; Zhu, H.; Cao, Y.; Pudasaini, B.; Yang, W.; Liu, J.; Guo, J. Long-term variability of impulse oscillometry and spirometry in stable COPD and asthma. Respir. Res. 2022, 23, 262. [Google Scholar] [CrossRef]
- Bates, J.H.T. Systems physiology of the airways in health and obstructive pulmonary disease. Wires Mech. Dis. 2016, 8, 423–437. [Google Scholar] [CrossRef]
- Goldman, M.D. Clinical Application of Forced Oscillation. Pulm. Pharmacol. Ther. 2001, 14, 341–350. [Google Scholar] [CrossRef]
- Melo-Silva, C.A.; Nunes, W.M.C.; Nascimento, E.S.P.; Guerra, E.M.; Roza, M.R.; Silva-Costa, S.; Machado-Silva, W.; Avelar, G.G.; Nóbrega, O.T.; Amado, V.M. Modulating respiratory mechanics and inflammation in hepatopulmonary syndrome: Aerobic exercise as a therapeutic strategy. Respir. Physiol. Neurobiol. 2025, 335, 104410. [Google Scholar] [CrossRef]
- Darley, D.R.; Nilsen, K.; Vazirani, J.; Borg, B.M.; Levvey, B.; Snell, G.; Plit, M.L.; Tonga, K.O. Airway oscillometry parameters in baseline lung allograft dysfunction: Associations from a multicenter study. J. Heart Lung Transplant. 2023, 42, 767–777. [Google Scholar] [CrossRef]
- Catalán, J.S.; Lalmolda, C.; Hernández-Voth, A.; Blanco, M.C.; Murphy, P.; Gonzalez-Ramos, L.; Florez-Solarana, P.; Lloret-Puig, B.; Lujan, M. Thoracoabdominal Asynchrony in Very Severe COPD: Clinical and Functional Correlates During Exercise. Arch. Bronconeumol. 2025, in press. [Google Scholar] [CrossRef]
- Polkey, M.I.; Qiu, Z.-H.; Zhou, L.; Zhu, M.-D.; Wu, Y.-X.; Chen, Y.-Y.; Ye, S.-P.; He, Y.-S.; Jiang, M.; He, B.-T.; et al. Tai Chi and Pulmonary Rehabilitation Compared for Treatment-Naive Patients with COPD. Chest 2018, 153, 1116–1124. [Google Scholar] [CrossRef]
- Guo, Y.; Li, M.; Xie, C.; Liu, X.; Chen, Y.; Yang, J.; Wu, Y.; Chen, S.; Wang, S.; Lin, J. Effect of the cervical and thoracic “Daoyin” training on posture and pulmonary function in patients with upper crossed syndrome: A randomized controlled trial. BMC Complement. Med. Ther. 2025, 25, 41. [Google Scholar] [CrossRef]
- Janyacharoen, T.; Thayon, M.; Bushong, W.; Jaikla, N.; Sawanyawisuth, K. Effects of resistance exercise on cardiopulmonary factors in sedentary individuals. J. Phys. Ther. Sci. 2016, 28, 213–217. [Google Scholar] [CrossRef]
- Marcal, A.; Rodrigues, R.; Nascimento, V.; Da Silva Grigoletto, M.; CSousa, E.; Odilon Abrahin, O. Single- and multiple-set resistance training improves skeletal and respiratory muscle strength in elderly women. Clin. Interv. Aging 2014, 9, 1775–1782. [Google Scholar] [CrossRef]
- Han, J.; Park, S.; Kim, Y.; Choi, Y.; Lyu, H. Effects of forward head posture on forced vital capacity and respiratory muscles activity. J. Phys. Ther. Sci. 2016, 28, 128–131. [Google Scholar] [CrossRef]
- Dimitriadis, Z.; Kapreli, E.; Strimpakos, N.; Oldham, J. Respiratory weakness in patients with chronic neck pain. Man. Ther. 2013, 18, 248–253. [Google Scholar] [CrossRef]
- Wirth, B.; Amstalden, M.; Perk, M.; Boutellier, U.; Humphreys, B.K. Respiratory dysfunction in patients with chronic neck pain—Influence of thoracic spine and chest mobility. Man. Ther. 2014, 19, 440–444. [Google Scholar] [CrossRef]
- Al-Alwan, A.; Bates, J.H.T.; Chapman, D.G.; Kaminsky, D.A.; DeSarno, M.J.; Irvin, C.G.; Dixon, A.E. The Nonallergic Asthma of Obesity. A Matter of Distal Lung Compliance. Am. J. Respir. Crit. Care Med. 2014, 189, 1494–1502. [Google Scholar] [CrossRef]
- van Huisstede, A.; Rudolphus, A.; Cabezas, M.C.; Biter, L.U.; van de Geijn, G.-J.; Taube, C.; Hiemstra, P.S.; Braunstahl, G.-J.; van Schadewijk, A. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax 2015, 70, 659–667. [Google Scholar] [CrossRef]
- Hakala, K.; Stenius-Aarniala, B.; Sovija, A. Effects of Weight Loss on Peak Flow Variability, Airways Obstruction, and Lung Volumes in Obese Patients with Asthma. Chest 2000, 118, 1315–1321. [Google Scholar] [CrossRef]
- Dixon, A.E.; Poynter, M.E.; Garrow, O.J.; Kaminsky, D.A.; Tharp, W.G.; Bates, J.H.T. Peripheral Airway Dysfunction in Obesity and Obese Asthma. Chest 2023, 163, 753–762. [Google Scholar] [CrossRef]
- Sánchez-Peña, E.; Rodríguz-Valdés, S.A.; Donoso-Riveros, D.F.; Escobar-Cabello, M.; Del Sol, M.; Valenzuela-Aedo, F.; Lizama-Pérez, R.; Muñoz-Cofrém, R. Implications of Airway Resistance and Conductance on the Respiratory Rate in individuals with Various Nutritional States Exposed To Exercise. J. Multidiscip. Healthc. 2024, 17, 4353–4362. [Google Scholar] [CrossRef]
- Zhang, Y.; Tanabe, N.; Sato, S.; Shiraishi, Y.; Maetani, T.; Sakamoto, R.; Sato, A.; Muro, S.; Hirai, T. Longitudinal changes in respiratory reactance in patients with COPD: Associations with longitudinal change in air-trapping, exacerbations, and mortality. Respir. Physiol. Neurobiol. 2024, 322, 104216. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Garcia-Aymerich, J.; Lange, P.; Benet, M.; Schnohr, P.; Antó, J.M. Regular Physical Activity Modifies Smoking-related Lung Function Decline and Reduces Risk of Chronic Obstructive Pulmonary Disease: A Population-based Cohort Study. Am. J. Respir. Crit. Care Med. 2007, 175, 458–463. [Google Scholar] [CrossRef]
- Smith, J.R.; Cross, T.J.; Van Iterson, E.H.; Johnson, B.D.; Olson, T.P. Resistive and elastic work of breathing in older and younger adults during exercise. J. Appl. Physiol. 2018, 125, 190–197. [Google Scholar] [CrossRef]
- Piorunek, T.; Kostrzewska, M.; Cofta, S.; Batura-Gabryel, H.; Andrzejczak, P.; Bogdański, P.; Wysocka, E. Impulse Oscillometry in the Diagnosis of Airway Resistance in Chronic Obstructive Pulmonary Disease. In Allergens and Airway Hyperreactivity; Advances in Experimental Medicine and Biology; Pokorski, M., Ed.; Springer International Publishing: Cham, Switzerland, 2014; Volume 838, pp. 47–52. Available online: https://link.springer.com/10.1007/5584_2014_49 (accessed on 20 February 2025).
| Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|
| Age (years) | 20 | 63 | 32.4 | 10.5 |
| Body mass index | 16 | 31 | 24.2 | 3.3 |
| Gender | Number | Percentage | ||
| Female | 9 | 25.7% | ||
| Male | 26 | 74.2% |
| Mean | Std. Deviation | Mean Difference | 95% Confidence Interval of the Difference | p Value | ||
|---|---|---|---|---|---|---|
| R5Hz initial (kPa/L/s) | 0.27 | 0.10 | 0.03 | 0.00 | 0.06 | 0.05 |
| R5Hz post effort (kPa/L/s) | 0.24 | 0.07 | ||||
| X5Hz initial (kPa/L/s) | −0.06 | 0.04 | −0.01 | −0.05 | 0.03 | 0.60 |
| X5Hz post effort (kPa/L/s) | −0.05 | 0.09 | ||||
| Fres initial (1/s) | 10.51 | 3.55 | 0.11 | −0.71 | 0.93 | 0.79 |
| Fres post effort (1/s) | 10.40 | 3.21 | ||||
| AX initial (kPa/L) | 0.21 | 0.25 | 0.02 | −0.03 | 0.08 | 0.36 |
| AX post effort (kPa/L) | 0.19 | 0.18 | ||||
| D5-20% initial (%) | 7.62 | 8.59 | 0.84 | −2.04 | 3.71 | 0.56 |
| D5-20% post effort (%) | 6.78 | 7.41 | ||||
| Mean | Std. Deviation | Mean Difference | 95% Confidence Interval of the Difference | p Value | ||
|---|---|---|---|---|---|---|
| R5Hz initial (kPa/L/s) | 0.25 | 0.10 | 0.02 | −0.02 | 0.06 | 0.26 |
| R5Hz post effort (kPa/L/s) | 0.23 | 0.07 | ||||
| X5Hz initial (kPa/L/s) | −0.04 | 0.04 | 0.02 | 0.00 | 0.04 | 0.04 |
| X5Hz post effort (kPa/L/s) | −0.06 | 0.03 | ||||
| Fres initial (1/s) | 9.44 | 3.05 | 0.00 | −0.57 | 0.58 | 0.99 |
| Fres post effort (1/s) | 9.43 | 2.64 | ||||
| AX initial (kPa/L) | 0.17 | 0.23 | 0.01 | −0.05 | 0.06 | 0.84 |
| AX post effort (kPa/L) | 0.16 | 0.15 | ||||
| D5-20% initial (%) | 4.85 | 7.08 | −0.12 | −2.67 | 2.43 | 0.92 |
| D5-20% post effort (%) | 4.96 | 6.83 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavarache, I.E.; Cernomaz, T.A.; Grosu-Creangă, I.A.; Trofor, A. The Effect of Aerobic Training on Healthy Small Airways—A Forced Oscillation Technique Approach to Optimize Long Term Care in COPD. J. Clin. Med. 2025, 14, 4755. https://doi.org/10.3390/jcm14134755
Stavarache IE, Cernomaz TA, Grosu-Creangă IA, Trofor A. The Effect of Aerobic Training on Healthy Small Airways—A Forced Oscillation Technique Approach to Optimize Long Term Care in COPD. Journal of Clinical Medicine. 2025; 14(13):4755. https://doi.org/10.3390/jcm14134755
Chicago/Turabian StyleStavarache, Ioan Emanuel, Tudor Andrei Cernomaz, Ionela Alina Grosu-Creangă, and Antigona Trofor. 2025. "The Effect of Aerobic Training on Healthy Small Airways—A Forced Oscillation Technique Approach to Optimize Long Term Care in COPD" Journal of Clinical Medicine 14, no. 13: 4755. https://doi.org/10.3390/jcm14134755
APA StyleStavarache, I. E., Cernomaz, T. A., Grosu-Creangă, I. A., & Trofor, A. (2025). The Effect of Aerobic Training on Healthy Small Airways—A Forced Oscillation Technique Approach to Optimize Long Term Care in COPD. Journal of Clinical Medicine, 14(13), 4755. https://doi.org/10.3390/jcm14134755






