Ultrasound-Guided Deep Parasternal Intercostal Plane Block in Off-Pump Cardiac Arterial Bypass Surgery: A Retrospective Cohort Single Center Study
Abstract
1. Introduction
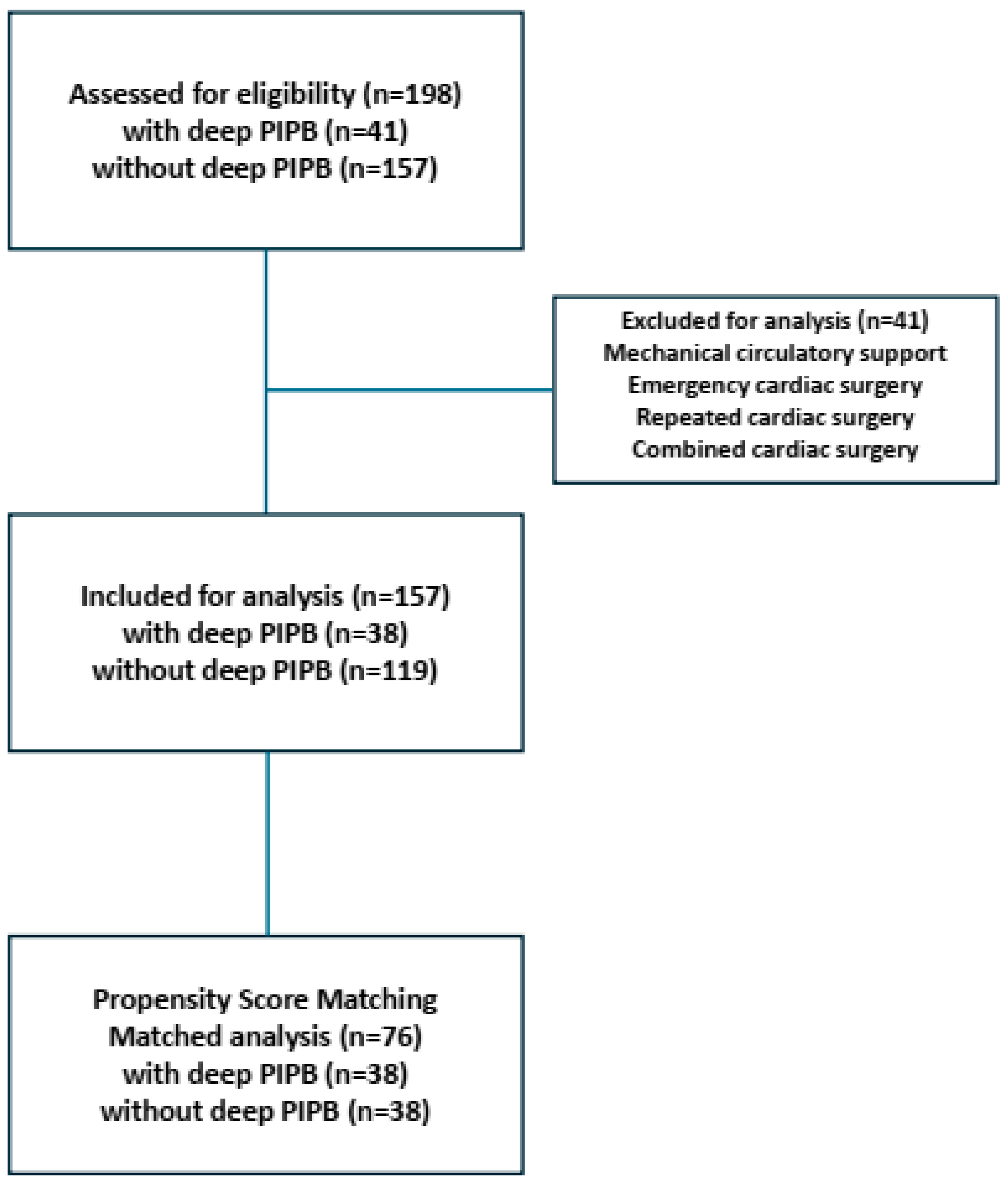
2. Materials and Methods
2.1. Design and Patient Collective
2.2. Data Collection of Electronic Health Records
2.3. Deep PIPB Performance and Postoperative Pain Management
2.4. Outcome Endpoints and Independent Variables
2.5. Statistical Analysis
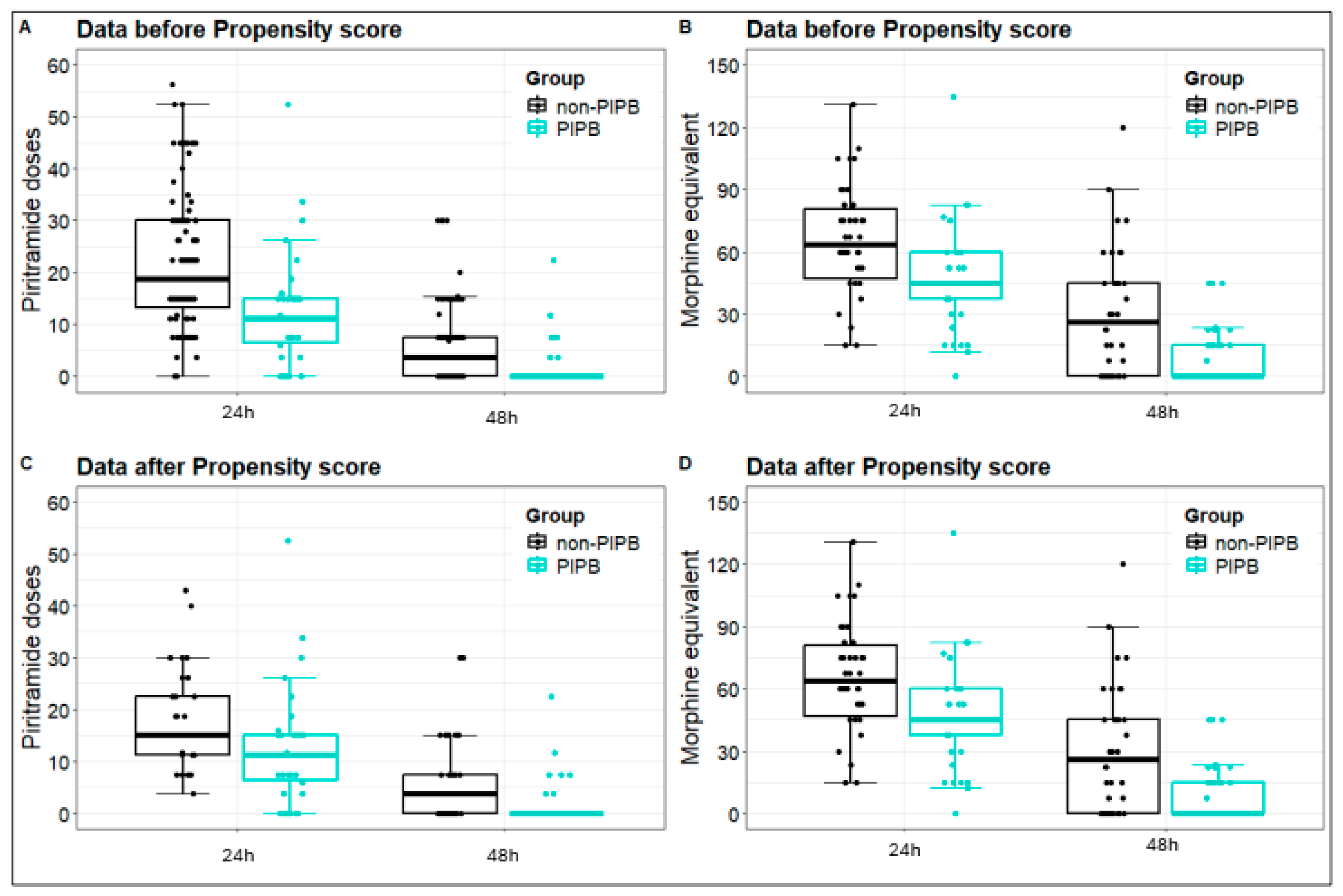
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ERACS | Enhanced Recovery After Cardiac Surgery |
| ME | Morphine Equivalent |
| LOS | Length of Stay |
| BMI | Body Mass Index |
| ICU | Intensive Care Unit |
| PIPB | Parasternal Intercostal Plane Block |
| OPCAB | Off-Pump Cardiac Artery Bypass |
| NRS | Numeric Rating Scale |
| BPS | Behavior Pain Scale |
| H | Hours |
References
- Lahtinen, P.; Kokki, H.; Hynynen, M. Pain after cardiac surgery: A prospective cohort study of 1-year incidence and intensity. Anesthesiology 2006, 105, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Kwanten, L.E.; O’Brien, B.; Anwar, S. Opioid-Based Anesthesia and Analgesia for Adult Cardiac Surgery: History and Narrative Review of the Literature. J. Cardiothorac. Vasc. Anesth. 2019, 33, 808–816. [Google Scholar] [CrossRef]
- Maddali, M.M.; Kurian, E.; Fahr, J. Extubation time, hemodynamic stability, and postoperative pain control in patients undergoing coronary artery bypass surgery: An evaluation of fentanyl, remifentanil, and nonsteroidal antiinflammatory drugs with propofol for perioperative and postoperative management. J. Clin. Anesth. 2006, 18, 605–610. [Google Scholar] [CrossRef]
- Fayaz, M.K.; Abel, R.J.; Pugh, S.C.; Hall, J.E.; Djaiani, G.; Mecklenburgh, J.S. Opioid-sparing effects of diclofenac and paracetamol lead to improved outcomes after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2004, 18, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Mehta, Y.; Arora, D. Benefits and risks of epidural analgesia in cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Lamy, A.; Devereaux, P.J.; Prabhakaran, D.; Taggart, D.P.; Hu, S.; Paolasso, E.; Straka, Z.; Piegas, L.S.; Akar, A.R.; Jain, A.R.; et al. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N. Engl. J. Med. 2013, 368, 1179–1188. [Google Scholar] [CrossRef]
- Forouzannia, S.M.; Forouzannia, S.K.; Yarahmadi, P.; Alirezaei, M.; Shafiee, A.; Anari, N.Y.; Masoudkabir, F.; Dehghani, Z.; Pashang, M. Early and mid-term outcomes of off-pump versus on-pump coronary artery bypass surgery in patients with triple-vessel coronary artery disease: A randomized controlled trial. J. Cardiothorac. Surg. 2023, 18, 140. [Google Scholar] [CrossRef]
- Macrae, W.A. Chronic pain after sternotomy. Acta Anaesthesiol. Scand. 2001, 45, 927–928. [Google Scholar] [CrossRef]
- Kleiman, A.M.; Sanders, D.T.; Nemergut, E.C.; Huffmyer, J.L. Chronic Poststernotomy Pain: Incidence, Risk Factors, Treatment, Prevention, and the Anesthesiologist’s Role. Reg. Anesth. Pain. Med. 2017, 42, 698–708. [Google Scholar] [CrossRef]
- van Gulik, L.; Janssen, L.I.; Ahlers, S.J.; Bruins, P.; Driessen, A.H.; van Boven, W.J.; van Dongen, E.P.; Knibbe, C.A. Risk factors for chronic thoracic pain after cardiac surgery via sternotomy. Eur. J. Cardiothorac. Surg. 2011, 40, 1309–1313. [Google Scholar] [CrossRef]
- Engelman, D.T.; Ben Ali, W.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.T.; Lai, V.K.; Chee, Y.E.; Lee, A. Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst. Rev. 2016, 9, CD003587. [Google Scholar] [CrossRef]
- Mittnacht, A.J.C.; Shariat, A.; Weiner, M.M.; Malhotra, A.; Miller, M.A.; Mahajan, A.; Bhatt, H.V. Regional Techniques for Cardiac and Cardiac-Related Procedures. J. Cardiothorac. Vasc. Anesth. 2019, 33, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Dost, B.; De Cassai, A.; Balzani, E.; Tulgar, S.; Ahiskalioglu, A. Effects of ultrasound-guided regional anesthesia in cardiac surgery: A systematic review and network meta-analysis. BMC Anesthesiol. 2022, 22, 409. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.J.; Versyck, B.; Elsharkawy, H.; Rojas Gomez, M.F.; Sala-Blanch, X.; Reina, M.A. Anatomical basis of fascial plane blocks. Reg. Anesth. Pain. Med. 2021, 46, 581–599. [Google Scholar] [CrossRef]
- Kelava, M.; Alfirevic, A.; Bustamante, S.; Hargrave, J.; Marciniak, D. Regional Anesthesia in Cardiac Surgery: An Overview of Fascial Plane Chest Wall Blocks. Anesth. Analg. 2020, 131, 127–135. [Google Scholar] [CrossRef]
- McDonald, S.B.; Jacobsohn, E.; Kopacz, D.J.; Desphande, S.; Helman, J.D.; Salinas, F.; Hall, R.A. Parasternal block and local anesthetic infiltration with levobupivacaine after cardiac surgery with desflurane: The effect on postoperative pain, pulmonary function, and tracheal extubation times. Anesth. Analg. 2005, 100, 25–32. [Google Scholar] [CrossRef]
- Barr, A.M.; Tutungi, E.; Almeida, A.A. Parasternal intercostal block with ropivacaine for pain management after cardiac surgery: A double-blind, randomized, controlled trial. J. Cardiothorac. Vasc. Anesth. 2007, 21, 547–553. [Google Scholar] [CrossRef]
- Padala, S.; Badhe, A.S.; Parida, S.; Jha, A.K. Comparison of preincisional and postincisional parasternal intercostal block on postoperative pain in cardiac surgery. J. Card. Surg. 2020, 35, 1525–1530. [Google Scholar] [CrossRef]
- Zhang, Y.; Min, J.; Chen, S. Sensory Assessment and Block Duration of Deep Parasternal Intercostal Plane Block in Patients Undergoing Cardiac Surgery: A Prospective Observational Study. Pain Ther. 2022, 11, 951–958. [Google Scholar] [CrossRef]
- Admiraal, M.; Hermanns, H.; Hermanides, J.; Wensing, C.; Meinsma, S.L.; Wartenberg, H.C.H.; Rutten, M.V.H.; Ward-van der Stam, V.M.C.; Hollmann, M.W. Study protocol for the TRUSt trial: A pragmatic randomised controlled trial comparing the standard of care with a transitional pain service for patients at risk of chronic postsurgical pain undergoing surgery. BMJ Open 2021, 11, e049676. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.C.; Chappell, D.; Gan, T.J.; Manning, M.W.; Miller, T.E.; Brodt, J.L.; on behalf of the PeriOperative Quality Initiative (POQI) and the Enhanced Recovery After Surgery (ERAS) Cardiac Society Workgroup. Pain management and opioid stewardship in adult cardiac surgery: Joint consensus report of the PeriOperative Quality Initiative and the Enhanced Recovery After Surgery Cardiac Society. J. Thorac. Cardiovasc. Surg. 2023, 166, 1695–1706.e2. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.; Ender, J.; Shaw, A.D.; Denault, A.; Ibekwe, S.; Stoppe, C.; Alli, A.; Manning, M.W.; Brodt, J.L.; Galhardo, C.; et al. ERAS/STS 2024 Expert Consensus Statement on Perioperative Care in Cardiac Surgery: Continuing the Evolution of Optimized Patient Care and Recovery. J. Cardiothorac. Vasc. Anesth. 2024, 38, 2155–2162. [Google Scholar] [CrossRef]
- Mondal, S.; Bergbower, E.A.S.; Cheung, E.; Grewal, A.S.; Ghoreishi, M.; Hollander, K.N.; Anders, M.G.; Taylor, B.S.; Tanaka, K.A. Role of Cardiac Anesthesiologists in Intraoperative Enhanced Recovery After Cardiac Surgery (ERACS) Protocol: A Retrospective Single-Center Study Analyzing Preliminary Results of a Yearlong ERACS Protocol Implementation. J. Cardiothorac. Vasc. Anesth. 2023, 37, 2450–2460. [Google Scholar] [CrossRef]
- Chaudhary, V.; Chauhan, S.; Choudhury, M.; Kiran, U.; Vasdev, S.; Talwar, S. Parasternal intercostal block with ropivacaine for postoperative analgesia in pediatric patients undergoing cardiac surgery: A double-blind, randomized, controlled study. J. Cardiothorac. Vasc. Anesth. 2012, 26, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Dost, B.; Kaya, C.; Turunc, E.; Dokmeci, H.; Yucel, S.M.; Karakaya, D. Erector spinae plane block versus its combination with superficial parasternal intercostal plane block for postoperative pain after cardiac surgery: A prospective, randomized, double-blind study. BMC Anesthesiol. 2022, 22, 295. [Google Scholar] [CrossRef]
- Wong, H.M.K.; Chen, P.Y.; Tang, G.C.C.; Chiu, S.L.C.; Mok, L.Y.H.; Au, S.S.W.; Wong, R.H.L. Deep Parasternal Intercostal Plane Block for Intraoperative Pain Control in Cardiac Surgical Patients for Sternotomy: A Prospective Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2024, 38, 683–690. [Google Scholar] [CrossRef]
- Toscano, A.; Capuano, P.; Perrucci, C.; Giunta, M.; Orsello, A.; Pierani, T.; Costamagna, A.; Tedesco, M.; Arcadipane, A.; Sepolvere, G.; et al. Which ultrasound-guided parasternal intercostal nerve block for post-sternotomy pain? Results from a prospective observational study. J. Anesth. Analg. Crit. Care 2023, 3, 48. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Liao, Y.; Wang, X.; Zhan, M.Y.; Li, Y.Y.; Liu, G.J.; Xiao, L. Preemptive deep parasternal intercostal plane block for perioperative analgesia in coronary artery bypass grafting with sternotomy: A randomized, observer-blind, controlled study. Ann. Med. 2023, 55, 2302983. [Google Scholar] [CrossRef]
- Rubin, J.E.; Ng, V.; Chung, J.; Salvatierra, N.; Rippon, B.; Khatib, D.; Girardi, N.I.; Pryor, K.O.; Weinberg, R.Y.; Jiang, S.; et al. Efficacy of parasternal peripheral nerve catheters versus no block for median sternotomy: A single-centre retrospective study. BJA Open 2024, 11, 100288. [Google Scholar] [CrossRef]
- Eljezi, V.; Jallas, C.; Pereira, B.; Chasteloux, M.; Duale, C.; Camilleri, L. Clinical Benefits of Parasternal Block with Multihole Catheters when Inserted before Sternotomy. Ann. Card. Anaesth. 2025, 28, 39–45. [Google Scholar] [CrossRef]
- Li, J.; Lin, L.; Peng, J.; He, S.; Wen, Y.; Zhang, M. Efficacy of ultrasound-guided parasternal block in adult cardiac surgery: A meta-analysis of randomized controlled trials. Minerva Anestesiol. 2022, 88, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Skojec, A.J.; Christensen, J.M.; Yalamuri, S.M.; Smith, M.M.; Arghami, A.; LeMahieu, A.M.; Schroeder, D.R.; Mauermann, W.J.; Nuttall, G.A.; Ritter, M.J. Deep Parasternal Intercostal Plane Block for Postoperative Analgesia After Sternotomy for Cardiac Surgery-A Retrospective Cohort Study. J. Cardiothorac. Vasc. Anesth. 2024, 38, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Vargas Galvan, L.A.; Walsh, K.L.; Winegarner, A.; Apruzzese, P.; Asher, S.; Maslow, A. A Retrospective Review of the Deep Parasternal Intercostal Plane Block in Patients Undergoing Cardiac Surgery with Median Sternotomy. J. Clin. Med. 2025, 14, 2074. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liao, Y.; Wang, X.; Zhan, M.; Xiao, L.; Chen, Y. Efficacy of bilateral catheter superficial parasternal intercostal plane blocks using programmed intermittent bolus for opioid-sparing postoperative analgesia in cardiac surgery with sternotomy: A randomized, double-blind, placebo-controlled trial. J. Clin. Anesth. 2024, 95, 111430. [Google Scholar] [CrossRef]
- Bloc, S.; Perot, B.P.; Gibert, H.; Law Koune, J.D.; Burg, Y.; Leclerc, D.; Vuitton, A.S.; De La Jonquiere, C.; Luka, M.; Waldmann, T.; et al. Efficacy of parasternal block to decrease intraoperative opioid use in coronary artery bypass surgery via sternotomy: A randomized controlled trial. Reg. Anesth. Pain. Med. 2021, 46, 671–678. [Google Scholar] [CrossRef]
- Nasr, D.A.; Abdelhamid, H.M.; Mohsen, M.; Aly, A.H. The analgesic efficacy of continuous presternal bupivacaine infusion through a single catheter after cardiac surgery. Ann. Card. Anaesth. 2015, 18, 15–20. [Google Scholar] [CrossRef]
- Yadav, S.; Raman, R.; Prabha, R.; Kaushal, D.; Yadav, P.; Kumar, S. Randomized Controlled Trial of Ultrasound-Guided Parasternal Intercostal Nerve Block and Transversus Thoracis Muscle Plane Block for Postoperative Analgesia of Cardiac Surgical Patients. Cureus 2024, 16, e72174. [Google Scholar] [CrossRef]
- Krishnan, S.; Desai, R.; Paik, P.; Cassella, A.; Lucaj, J.; Ghoddoussi, F.; Hakim, J.; Schwartz, C.; Leicht, T.; Patel, K. Superficial Parasternal Intercostal Plane Blocks (SPIB) With Buprenorphine, Magnesium, and Bupivacaine for Management of Pain in Coronary Artery Bypass Grafting. Cureus 2022, 14, e30964. [Google Scholar] [CrossRef]
- Khera, T.; Murugappan, K.R.; Leibowitz, A.; Bareli, N.; Shankar, P.; Gilleland, S.; Wilson, K.; Oren-Grinberg, A.; Novack, V.; Venkatachalam, S.; et al. Ultrasound-Guided Pecto-Intercostal Fascial Block for Postoperative Pain Management in Cardiac Surgery: A Prospective, Randomized, Placebo-Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2021, 35, 896–903. [Google Scholar] [CrossRef]
- Lee, C.Y.; Robinson, D.A.; Johnson, C.A., Jr.; Zhang, Y.; Wong, J.; Joshi, D.J.; Wu, T.T.; Knight, P.A. A Randomized Controlled Trial of Liposomal Bupivacaine Parasternal Intercostal Block for Sternotomy. Ann. Thorac. Surg. 2019, 107, 128–134. [Google Scholar] [CrossRef] [PubMed]
| Without Deep PIPB (n = 119) | With Deep PIPB (n = 38) | p-Value | |
|---|---|---|---|
| Age (years) | 66.5 ± 9.3 | 64.7 ± 8.0 | 0.396 MWT |
| Sex | |||
| male | 105 (88.2%) | 33 (86.8%) | |
| female | 14 (11.8%) | 5 (13.2%) | |
| BMI (kg/m2) | 27.8 ± 2.15 | 26.67 ± 3.17 | 0.756 MWT |
| ASA | |||
| II | 41 (34.5%) | 12 (31.6%) | |
| III | 49 (41.2%) | 17 (44.7%) | |
| IV | 29 (24.3%) | 9 (23.7%) | |
| Nausea/Vomiting | 13 (10.9%) | 5 (13.2%) | 0.638 MWT |
| Without Deep DIPB (n = 119) | With Deep DIPB (n = 38) | p-Value Adjusted | |
|---|---|---|---|
| Primary endpoints | |||
| Morphine equivalent | |||
| po day 1 | 67.5 mg (45.0; 90.0) | 45.0 mg (35.6; 60.0) | 0.0004 MWT |
| po day 2 | 30.0 mg (7.5; 45.0) | 0.0 mg (0.0; 16.8) | 0.0004 MWT |
| Piritramide consumption | |||
| po day 1 | 15.0 mg (9.4; 27.1) | 11.2 mg (5.4; 15.0) | 0.004 MWT |
| po day 2 | 3.8 mg (0.0; 7.5) | 0.0 mg (0.0; 0.0) | 0.0012 MWT |
| Secondary endpoints | |||
| BPS | |||
| before extubation | 3.7 ± 0.5 | 4.0 ± 0.3 | 0.660 MWT |
| NRS | |||
| after extubation | 1.5 ± 2.3 | 1.0 ± 1.6 | 0.362 MWT |
| po day 1 | 3.2 ± 1.9 | 1.5 ± 2.2 | 0.001 MWT |
| po day 2 | 1.5 ± 2.0 | 0.6 ± 1.6 | 0.0028 MWT |
| Non-PIPB (n = 38) | PIPB (n = 38) | p-Value Adjusted | |
|---|---|---|---|
| Primary endpoints | |||
| Morphine equivalent | |||
| po day 1 | 63.7 mg (46.9; 80.6) | 45 mg (37.5; 60.0) | 0.004 WT |
| po day 2 | 26.2 mg (0.0; 45) | 0 mg (0.0; 15) | 0.0008 WT |
| Piritramide consumption | |||
| po day 1 | 15 mg (11.2; 22.5) | 11.2 mg (6.4; 15) | 0.02 WT |
| po day 2 | 3.7 mg (0.0; 7.5) | 0 mg (0.0; 0.0) | 0.012 WT |
| Secondary endpoints | |||
| ICU (days) | 2 (2; 3) | 2 (2; 3) | 0.999 WT |
| Time to extubation (minutes) | 286.5 (169; 420) | 60 (60; 162) | 0.001 WT |
| F Test | p-Value | β | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Intercept | 5.55 | ||||
| Group PIPB (Extubation) | 18.46 | <0.001 | −0.853 | −1.165; −0.540 | <0.001 |
| Intercept | 1.059 | ||||
| Group PIPB (ICU stay) | 0.105 | 0.746 | 0.026 | −0.132; 0.184 | 0.746 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngamsri, K.-C.; Tilly, R.; Hermann, S.; Rustenbach, C.J.; Radwan, M.; Schmid, E.; Charotte, C.; Serna-Higuita, L.M.; Magunia, H. Ultrasound-Guided Deep Parasternal Intercostal Plane Block in Off-Pump Cardiac Arterial Bypass Surgery: A Retrospective Cohort Single Center Study. J. Clin. Med. 2025, 14, 4756. https://doi.org/10.3390/jcm14134756
Ngamsri K-C, Tilly R, Hermann S, Rustenbach CJ, Radwan M, Schmid E, Charotte C, Serna-Higuita LM, Magunia H. Ultrasound-Guided Deep Parasternal Intercostal Plane Block in Off-Pump Cardiac Arterial Bypass Surgery: A Retrospective Cohort Single Center Study. Journal of Clinical Medicine. 2025; 14(13):4756. https://doi.org/10.3390/jcm14134756
Chicago/Turabian StyleNgamsri, Kristian-Christos, Roman Tilly, Sabine Hermann, Christian Jörg Rustenbach, Medhat Radwan, Eckhard Schmid, Christophe Charotte, Lina Maria Serna-Higuita, and Harry Magunia. 2025. "Ultrasound-Guided Deep Parasternal Intercostal Plane Block in Off-Pump Cardiac Arterial Bypass Surgery: A Retrospective Cohort Single Center Study" Journal of Clinical Medicine 14, no. 13: 4756. https://doi.org/10.3390/jcm14134756
APA StyleNgamsri, K.-C., Tilly, R., Hermann, S., Rustenbach, C. J., Radwan, M., Schmid, E., Charotte, C., Serna-Higuita, L. M., & Magunia, H. (2025). Ultrasound-Guided Deep Parasternal Intercostal Plane Block in Off-Pump Cardiac Arterial Bypass Surgery: A Retrospective Cohort Single Center Study. Journal of Clinical Medicine, 14(13), 4756. https://doi.org/10.3390/jcm14134756








