Prognostic Value of Inflammation and Nutrition-Based Scores in Low-Risk Myelodysplastic Syndrome: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Partitioning
2.2. Model Training and Validation
2.3. Performance Evaluation
2.4. Statistical Interpretation
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Nutrition-Based and Inflammation Scores Analysis
3.3. Univariate and Multivariate Analysis of Nutrition-Based and Inflammation Scores
3.4. Multivariate Analysis of Nutrition-Based and Inflammation Scores
3.5. Survival Analysis
4. Discussion
5. Conclusions
6. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood J. Am. Soc. Hematol. 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Kovtonyuk, L.V.; Fritsch, K.; Feng, X.; Manz, M.G.; Takizawa, H. Inflamm-Aging of Hematopoiesis, Hematopoietic Stem Cells, and the Bone Marrow Microenvironment. Front. Immunol. 2016, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in aging, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm—Aging as Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood J. Am. Soc. Hematol. 1997, 89, 2079–2088. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood J. Am. Soc. Hematol. 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Malcovati, L.; Strupp, C.; Ambaglio, I.; Kuendgen, A.; Zipperer, E.; Travaglino, E.; Invernizzi, R.; Pascutto, C.; Lazzarino, M.; et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica 2011, 96, 441–449. [Google Scholar] [CrossRef]
- Sarici, A.; Ar, M.C.; Yokus, O.; Ongoren, S.; Ayer, M.; Altindal, S.; Koker, H.T.; Kuzu, O.F. The impact of transfusion burden and comorbidities on the prognosis of patients with myelodysplastic syndromes. Transfus. Apher. Sci. 2020, 59, 102845. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Shan, J.; Faderl, S.; Cortes, J.; Ravandi, F.; Borthakur, G.; Wierda, W.G.; Pierce, S.; Estey, E.; Liu, J.; et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia 2008, 22, 538–543. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Malcovati, L.; Boveri, E.; Travaglino, E.; Pietra, D.; Pascutto, C.; Passamonti, F.; Invernizzi, R.; Castello, A.; Magrini, U.; et al. Clinical relevance of bone marrow fibrosis and CD34-positive cell clusters in primary myelodysplastic syndromes. J. Clin. Oncol. 2009, 27, 754–762. [Google Scholar] [CrossRef]
- Kasprzak, A.; Assadi, C.; Nachtkamp, K.; Rudelius, M.; Haas, R.; Giagounidis, A.; Götze, K.; Gattermann, N.; Germing, U. Monocytosis at the time of diagnosis has a negative prognostic impact in myelodysplastic syndromes with less than 5% bone marrow blasts. Ann. Hematol. 2023, 102, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Komrokji, R.S.; Corrales-Yepez, M.; Kharfan-Dabaja, M.A.; Al Ali, N.H.; Padron, E.; Rollison, D.E.; Pinilla-Ibarz, J.; Zhang, L.; Epling-Burnette, P.K.; Lancet, J.E.; et al. Hypoalbuminemia is an independent prognostic factor for overall survival in myelodysplastic syndromes. Am. J. Hematol. 2012, 87, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Uliel, N.; Segal, G.; Perri, A.; Turpashvili, N.; Kassif Lerner, R.; Itelman, E. Low ALT, a marker of sarcopenia and frailty, is associated with shortened survival amongst myelodysplastic syndrome patients: A retrospective study. Medicine 2023, 102, e33659. [Google Scholar] [CrossRef]
- Shi, C.; Gong, S.; Niu, T.; Li, T.; Wu, A.; Zheng, X.; Yang, S.; Ouyang, G.; Mu, Q. The Prognostic Value of Pretherapy Peripheral Blood Inflammatory Indices in Myelodysplastic Syndromes. Front. Oncol. 2022, 12, 877981. [Google Scholar] [CrossRef]
- Shi, C.; Gong, S.; Wu, A.; Niu, T.; Wu, N.; Zhang, Y.; Ouyang, G.; Mu, Q. Hyperfibrinogenemia as a Poor Prognostic Indicator in Myelodysplastic Syndrome. Cancer Manag. Res. 2022, 14, 1857–1865. [Google Scholar] [CrossRef]
- Ikeya, T.; Shibutani, M.; Maeda, K.; Sugano, K.; Nagahara, H.; Ohtani, H.; Hirakawa, K. Maintenance of the nutritional prognostic index predicts survival in patients with unresectable metastatic colorectal cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 307–313. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, B.; Lu, T.; Li, D.; Li, T.; Shen, Z.; He, C.; Wang, Y.; Li, B.; Zhang, H.; et al. Value of the prognostic nutritional index (PNI) in patients with newly diagnosed, CD5-positive diffuse large B-cell lymphoma: A multicenter retrospective study of the Huaihai Lymphoma Working Group. Cancer 2022, 128, 3487–3494. [Google Scholar]
- Mirili, C.; Paydas, S.; Kapukaya, T.K.; Yilmaz, A. Systemic immune-inflammation index predicting survival outcome in patients with classical Hodgkin lymphoma. Biomark. Med. 2019, 13, 1565–1575. [Google Scholar] [CrossRef]
- Chang, Y.J.; Xu, L.P.; Liu, D.H.; Liu, K.Y.; Han, W.; Chen, Y.H.; Chen, H.; Wang, J.Z.; Zhang, X.H.; Zhao, X.Y.; et al. Platelet engraftment in patients with hematologic malignancies following unmanipulated haploidentical blood and marrow transplantation: Effects of CD34+ cell dose and disease status. Biol. Blood Marrow Transplant. 2009, 15, 632–638. [Google Scholar] [CrossRef]
- Lee, S.F.; Ng, T.Y.; Wong, F.C.S. The Value of Prognostic Nutritional Index in Follicular Lymphoma. Am. J. Clin. Oncol. 2019, 42, 202–207. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, A.; Zheng, X.; Fu, X.; Zheng, H.; Gao, H.; Wang, J.; Liang, X.; Tian, T.; Ruan, Z.; et al. Prognostic Nutritional Index identifies risk of early progression and survival outcomes in Advanced Non-small Cell Lung Cancer patients treated with PD-1 inhibitors. J. Cancer 2021, 12, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Imbesi, S.; Musolino, C.; Allegra, A.; Saija, A.; Morabito, F.; Calapai, G.; Gangemi, S. Oxidative stress in oncohematologic diseases: An update. Expert Rev. Hematol. 2013, 6, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C.; Thomassen, M.; Hasselbalch Riley, C.; Kjær, L.; Stauffer Larsen, T.; Jensen, M.K.; Bjerrum, O.W.; Kruse, T.A.; Skov, V. Whole blood transcriptional profiling reveals deregulation of oxidative and antioxidative defence genes in myelofibrosis and related neoplasms. Potential implications of downregulation of Nrf2 for genomic instability and disease progression. PLoS ONE 2014, 9, e112786. [Google Scholar] [CrossRef] [PubMed]
- Ghoti, H.; Amer, J.; Winder, A.; Rachmilewitz, E.; Fibach, E. Oxidative stress in red blood cells, platelets and polymorphonuclear leukocytes from patients with myelodysplastic syndrome. Eur. J. Haematol. 2007, 79, 463–467. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Cortesão, E.; Oliveiros, B.; Alves, V.; Espadana, A.I.; Rito, L.; Magalhães, E.; Lobão, M.J.; Pereira, A.; Nascimento Costa, J.M.; et al. Oxidative stress and mitochondrial dysfunction play a role in myelodysplastic syndrome development, diagnosis, and prognosis: A pilot study. Free Radic Res. 2015, 49, 1081–1094. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, H.; Huang, C.; Lei, Y. Cancer drug resistance: Redox resetting renders a way. Oncotarget 2016, 7, 42740–42761. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Y.; Ye, H.; Gao, F.; Li, Z.; Qin, S. The prognostic value of preoperative laboratory data indicators in patients with esophageal carcinoma: An observational study. Medicine 2024, 103, e38477. [Google Scholar] [CrossRef]
- Qian, J.Y.; Hao, Y.; Yu, H.H.; Wu, L.L.; Liu, Z.Y.; Peng, Q.; Li, Z.X.; Li, K.; Liu, Y.E.; Wang, R.R.; et al. A Novel Systematic Oxidative Stress Score Predicts the Survival of Patients with Early-Stage Lung Adenocarcinoma. Cancers 2023, 15, 1718. [Google Scholar] [CrossRef]
- Schneider, M.; Rolfs, C.; Trumpp, M.; Winter, S.; Fischer, L.; Richter, M.; Menger, V.; Nenoff, K.; Grieb, N.; Metzeler, K.H.; et al. Activation of distinct inflammatory pathways in subgroups of LR-MDS. Leukemia 2023, 37, 1709–1718. [Google Scholar] [CrossRef]
- Basiorka, A.A.; McGraw, K.L.; Eksioglu, E.A.; Chen, X.; Johnson, J.; Zhang, L.; Zhang, Q.; Irvine, B.A.; Cluzeau, T.; Sallman, D.A.; et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood J. Am. Soc. Hematol. 2016, 128, 2960–2975. [Google Scholar] [CrossRef]
- Sallman, D.A.; List, A. The central role of inflammatory signaling in the pathogenesis of myelodysplastic syndromes. Blood J. Am. Soc. Hematol. 2019, 133, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Horstman, I.M.; Vinke, P.C.; Suazo-Zepeda, E.; Hiltermann, T.J.N.; Heuvelmans, M.A.; Corpeleijn, E.; de Bock, G.H. The association of nutritional and inflammatory biomarkers with overall survival in patients with non-small-cell lung cancer treated with immune checkpoint inhibitors. Thorac. Cancer 2024, 15, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Mozas, P.; Rivero, A.; Rivas-Delgado, A.; Nadeu, F.; Giné, E.; Delgado, J.; Villamor, N.; Campo, E.; Pérez-Galán, P.; Magnano, L.; et al. The Prognostic Nutritional Index (PNI) is an independent predictor of overall survival in older patients with follicular lymphoma. Leuk. Lymphoma 2022, 63, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Ma, J.; Fei, L.; Chen, Q.; Tao, S.; He, Z.; Wang, C.; Yu, L. Clinical significance of prognostic nutritional index in myelodysplastic syndrome. Hematology 2023, 28, 2161209. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Liu, J.; Yu, Y.; Liu, Y.; Peng, Q.; Liu, H.; Guan, X. A feedback loop: Interactions between Inflammatory Signals and Clonal Hematopoiesis in Cardiovascular Disease. Mol. Biol. Rep. 2021, 48, 3785–3798. [Google Scholar] [CrossRef]

| Variables | n = 175 (%) |
|---|---|
| Age (years) | |
| Median (min–max) | 68 (29–88) |
| ≤60 | 50 (28.6) |
| 61–70 | 62 (35.4) |
| >70 | 63 (36.0) |
| Gender | |
| Male | 87 (49.7) |
| Female | 88 (50.3) |
| MDS subtypes | |
| Hypocellular MDS | 6 (3.4) |
| MDS-5Q syndrome | 6 (3.4) |
| MDS-EB1 | 15 (8.6) |
| MDS-MLD | 89 (50.9) |
| MDS-SLD | 59 (33.7) |
| Cytogenetic profile | |
| Del (5q) | 6 (3.4) |
| Del (20q) | 3 (1.8) |
| Del (11q) | 4 (2.2) |
| Del (7q) | 4 (2.2) |
| Trisomy 8 | 5 (2.9) |
| Normal Karyotype | 153 (87.5) |
| IPSS score | |
| Median (min–max) | 0.5 (0–1) |
| Low | 87 (49.7) |
| Intermediate-1 | 88 (50.3) |
| R-IPSS score | |
| Median (min–max) | 2.5 (0–4.5) |
| Very low | 28 (16) |
| Low | 113 (64.6) |
| Intermediate | 34 (19.4) |
| Transfusion dependence | |
| None | 108 (61.7) |
| Present | 67 (38.3) |
| Treatment modality | |
| ESA | 123 (70.3) |
| Lenalidomide | 6 (3.4) |
| Azacitidine | 18 (10.3) |
| IST | 4 (2.2) |
| Transfusion Only | 24 (13.8) |
| AML conversion | |
| None | 166 (94.9) |
| Present | 9 (5.1) |
| Mortality | |
| Alive | 59 (33.7) |
| Deceased | 116 (66.3) |
| Cardiovascular death | |
| None | 53 (45.7) |
| Present | 63 (54.3) |
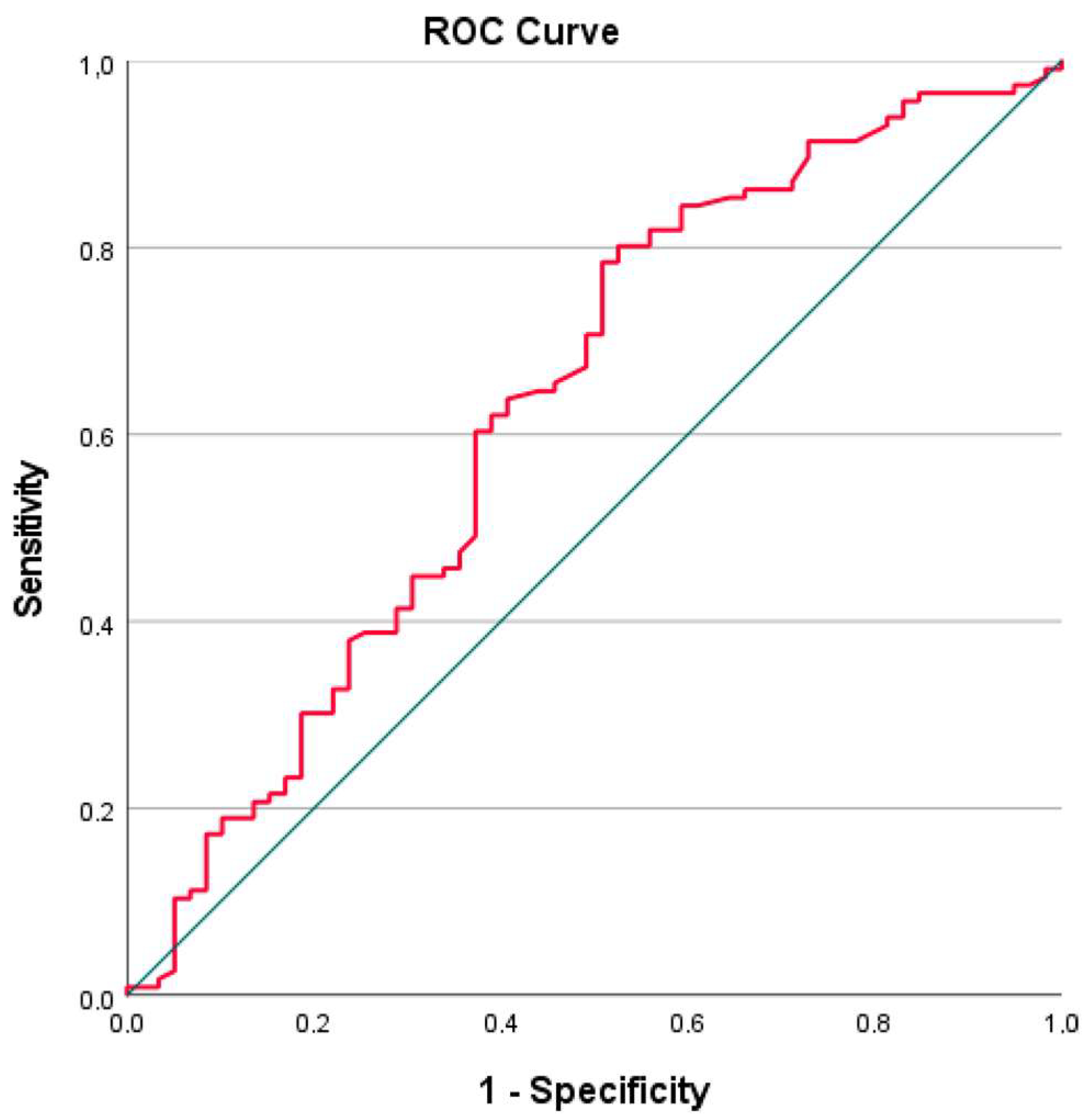
| AUC | 95% CI | Cut-Off | Sensitivity | Specificity | p-Value | |
|---|---|---|---|---|---|---|
| PNI | 0.629 | 0.538–0.720 | 47.47 | 60 | 63 | 0.005 |
| PNI | 0.629 | 0.538–0.720 | 44 | 37.07 | 76.27 | 0.005 |
| Variables, Median (Min–Max) | PNI ≤ 47.47 (n = 92) | PNI > 47.47 (n = 83) | Total (n = 175) | p |
|---|---|---|---|---|
| Gender | ||||
| Female | 44 | 44 | 88 | 0.493 x2 |
| Male | 48 | 39 | 87 | |
| Age (years) | 69 (31–88) | 66 (29–83) | 68 (29–88) | 0.052 m |
| MDS subtype | ||||
| Hypoplastic MDS | 6 | 0 | 6 | |
| 5-Q syndrome | 2 | 4 | 6 | |
| MDS-EB-1 | 9 | 6 | 15 | 0.139 x2 |
| MDS-SLD | 46 | 43 | 89 | |
| MDS-MLD | 29 | 30 | 59 | |
| Leukocytes, /µL | 3895 (500–12,000) | 4950 (2200–10,800) | 4560 (500–12,000) | <0.001 m |
| Lymphocytes, /µL | 955 (250–2510) | 1890 (862–5100) | 1400 (250–5100) | <0.001 m |
| Monocytes, /µL | 349.5 (20–4300) | 452 (26–1190) | 415 (20–4300) | 0.154 m |
| Neutrophils, /µL | 2355 (330–10,000) | 2450 (150–8350) | 2400 (150–10,000) | 0.893 m |
| Hemoglobin, g/dL | 8.75 ± 1.58 | 9.27 ± 1.49 | 9 (4.0–12.8) | 0.029 t |
| MCV, fL | 91.5 (64–117) | 96 (62–120) | 93 (62–120) | 0.020 m |
| Platelets, ×109 | 136 (5.3–457) | 233 (5.9–714) | 165 (5.9–714) | <0.001 m |
| IPSS | 0.5 (0–1) | 0 (0–1) | 0.5 (0–1) | 0.109 m |
| R-IPSS | 2.5 (1–4.5) | 2 (0–4.5) | 2.5 (0–4.5) | 0.013 m |
| Transfusion dependence | ||||
| None | 54 | 54 | 108 | 0.387 x2 |
| Present | 38 | 29 | 67 | |
| Ferritin, µg/L | 292 (5–6800) | 312 (4.5–2412) | 297 (4.5–6800) | 0.948 m |
| LDH, U/L | 223 (114–983) | 202 (114–612) | 205 (114–983) | 0.232 m |
| Albumin, g/L | 37 (10–43) | 43 (35–49) | 40 (10–49) | <0.001 m |
| Total protein, g/L | 68 (10–84) | 72 (61–85) | 70 (10–85) | <0.001 m |
| Cholesterol, mg/dL | 143 (85–363) | 161 (97–275) | 158 (85–363) | 0.117 m |
| Total bilirubin, mg/d | 0.67 (0.15–5.2) | 0.66 (0.12–2.65) | 0.66 (0.12–5.2) | 0.815 m |
| Creatinine, mg/dL | 0.91 (0.5–7) | 0.80 (0.5–1.72) | 0.84 (0.5–7) | 0.007 m |
| BUN, mg/dL | 21 (7–81) | 18 (9–45) | 20 (7–81) | 0.006 m |
| LDH/albumin | 6.22 (0–44.68) | 4.72 (0–15.3) | 5.26 (0–44.68) | <0.001 m |
| LDH/lymphocytes | 0.22 (0–1.89) | 0.11 (0–0.35) | 0.14 (0–1.89) | <0.001 m |
| SIRI | 0.92 (0.03–18.5) | 0.54 (0–4.07) | 0.68 (0–18.5) | 0.005 m |
| NSII | 51.0 (1.2–612) | 156 (11.2–3360) | 81.4 (1.2–3360) | <0.001 m |
| SII | 326.5 (20.2–2935) | 248.8 (2.11–2700) | 297.5 (2.11–2935) | 0.201 m |
| LMR | 2.97 (0.06–38.5) | 4.49 (1.36–160) | 3.71 (0.06–160) | <0.001 m |
| PLR | 132.7 (5.1–808) | 105.3 (2.11–432) | 118.6 (2.11–808) | 0.029 m |
| NLR | 2.82 (0.37–15.6) | 1.39 (0.03–4.99) | 1.83 (0.03–15.6) | <0.001 m |
| SOS | 184.8 (102–847.7) | 157 (79.6–514) | 168 (79.6–847.7) | 0.038 m |
| AML Transformation | ||||
| Present | 5 | 4 | 9 | 0.854 x2 |
| None | 87 | 79 | 166 | |
| Final status | ||||
| Alive | 22 | 37 | 59 | 0.004 x2 |
| Deceased | 70 | 46 | 116 | |
| OS (months) | 45.46 (33.2–57.6) | 75.1 (56.6–93.5) | 63.1 (49.4–76.7) | <0.001 k |
| AUC | 95% CI | Cut-Off | Sensitivity | Specificity | p-Value | |
|---|---|---|---|---|---|---|
| PNI | 0.543 | 0.395–0.690 | 47.14 | 55 | 51 | 0.668 |
| Univariate Cox Regression | Multivariate Cox Regression | |||||
|---|---|---|---|---|---|---|
| Risk Factors | HR | 95% CI | p | HR | 95% CI | p-value |
| Age | 1.046 | 1.027–1.066 | <0.001 | 1.057 | 1.036–1.078 | <0.001 |
| Gender | 0.575 | 0.397–0.832 | 0.003 | 0.529 | 0.359–0.779 | 0.001 |
| IPSS | 3.020 | 1.710–5.333 | <0.001 | |||
| R-IPSS | 1.469 | 1.215–1.777 | <0.001 | 1.532 | 1.242–1.889 | <0.001 |
| Transfusion dependence | 2.101 | 1.456–3.031 | <0.001 | |||
| Treatment Modality (HMA vs. Other) | 0.911 | 0.511–1.624 | 0.752 | |||
| AML Transformation | 3.265 | 1.623–6.568 | <0.001 | 6.381 | 2.966–13.727 | <0.001 |
| Leukocyte, /µL | 1.000 | 1.000–1.000 | 0.032 | |||
| Lymphocyte, /µL | 1.000 | 0.999–1.000 | 0.011 | |||
| Hemoglobin, g/dL | 0.846 | 0.753–0.950 | 0.005 | |||
| MCV, fL | 1.016 | 1.000–1.033 | 0.049 | |||
| Platelet, ×109 | 1.000 | 1.000–1.000 | 0.018 | |||
| LDH, U/L | 1.002 | 1.000–1.004 | 0.026 | |||
| Albumin, g/L | 0.952 | 0.926–0.979 | <0.001 | |||
| Ferritin, µg/L | 1.000 | 1.000–1.000 | 0.024 | |||
| PNI | 0.957 | 0.937–0.979 | <0.001 | 0.954 | 0.931–0.978 | <0.001 |
| NLR | 1.085 | 1.000–1.177 | 0.049 | |||
| SIRI | 1.087 | 1.015–1.164 | 0.016 | |||
| SOS | 1.003 | 1.001–1.005 | 0.009 | 1.003 | 1.001–1.006 | 0.002 |
| LDH/Lymphocytes | 2.277 | 1.259–4.118 | 0.007 | |||
| LDH/Albumin | 1.076 | 1.031–1.123 | <0.001 | |||
| Fold | AUC | 95% CI | p-Value | Mortality Rate |
|---|---|---|---|---|
| Fold 1 | 0.76 | 0.59–0.92 | 0.002 | 67.6% |
| Fold 2 | 0.76 | 0.59–0.94 | 0.004 | 65.7% |
| Fold 3 | 0.55 | 0.34–0.76 | 0.663 | 66.7% |
| Fold 4 | 0.85 | 0.72–0.98 | <0.001 | 65.7% |
| Fold 5 | 0.63 | 0.44–0.82 | 0.181 | 65.7% |
| Overall (Mean ± SD) | 0.71 ± 0.12 | 66.3 ± 0.8% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ersal, T.; Özkocaman, V.; Çubukçu, S.; Güllü Koca, T.; Hunutlu, F.Ç.; Yavuz, Ş.; Elgün, E.; Ocakoğlu, G.; Özkalemkaş, F. Prognostic Value of Inflammation and Nutrition-Based Scores in Low-Risk Myelodysplastic Syndrome: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 4751. https://doi.org/10.3390/jcm14134751
Ersal T, Özkocaman V, Çubukçu S, Güllü Koca T, Hunutlu FÇ, Yavuz Ş, Elgün E, Ocakoğlu G, Özkalemkaş F. Prognostic Value of Inflammation and Nutrition-Based Scores in Low-Risk Myelodysplastic Syndrome: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(13):4751. https://doi.org/10.3390/jcm14134751
Chicago/Turabian StyleErsal, Tuba, Vildan Özkocaman, Sinem Çubukçu, Tuba Güllü Koca, Fazıl Çağrı Hunutlu, Şeyma Yavuz, Ezel Elgün, Gökhan Ocakoğlu, and Fahir Özkalemkaş. 2025. "Prognostic Value of Inflammation and Nutrition-Based Scores in Low-Risk Myelodysplastic Syndrome: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 13: 4751. https://doi.org/10.3390/jcm14134751
APA StyleErsal, T., Özkocaman, V., Çubukçu, S., Güllü Koca, T., Hunutlu, F. Ç., Yavuz, Ş., Elgün, E., Ocakoğlu, G., & Özkalemkaş, F. (2025). Prognostic Value of Inflammation and Nutrition-Based Scores in Low-Risk Myelodysplastic Syndrome: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(13), 4751. https://doi.org/10.3390/jcm14134751






