Comparative Analysis of Single-Stage vs. Multiple-Stage Interventions in the Management of Subarachnoid Hemorrhage in Patients with Multiple Intracranial Aneurysms
Abstract
1. Introduction
2. Methods
3. Results
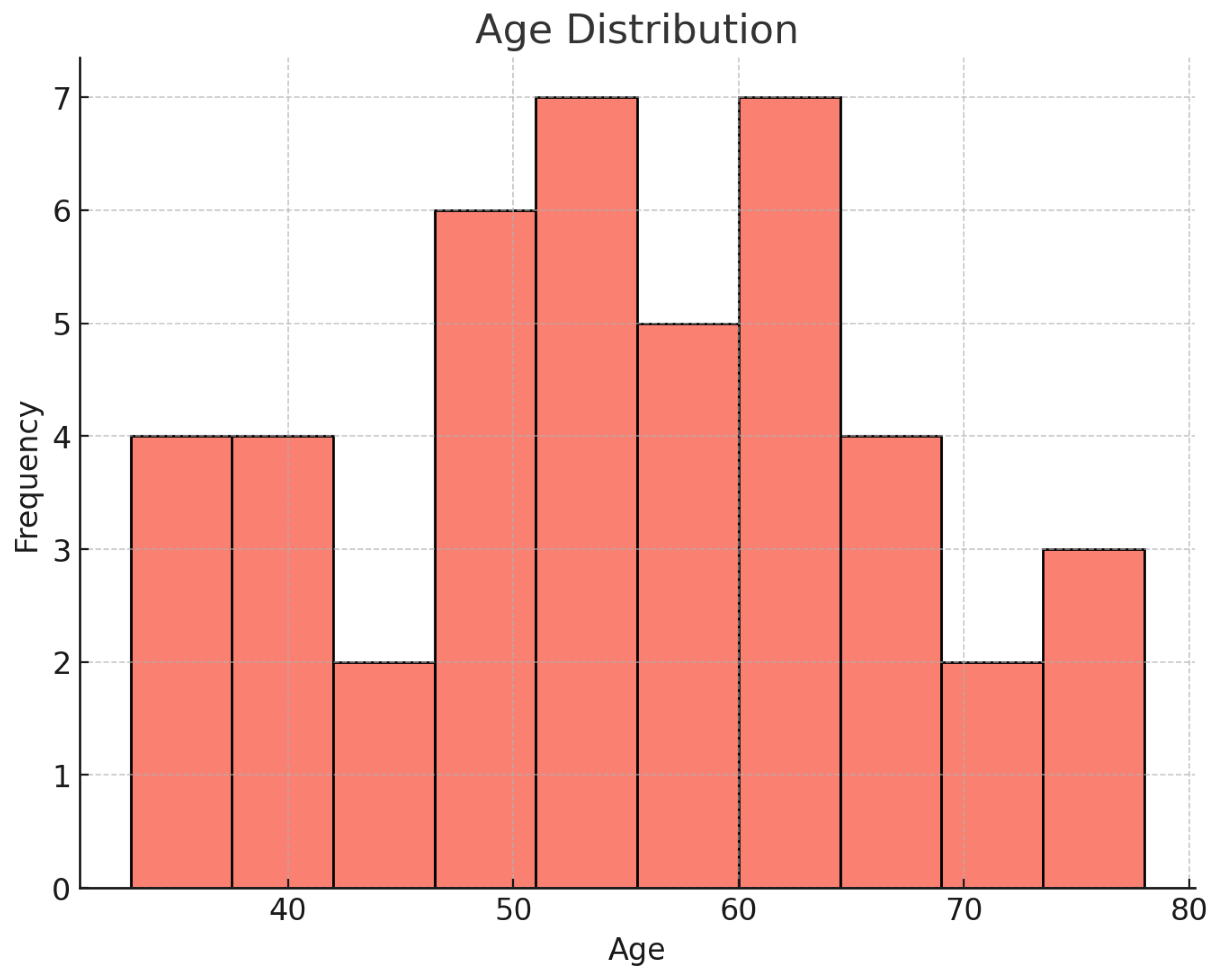
3.1. Patient Demographics, Risk Factors, and Symptoms
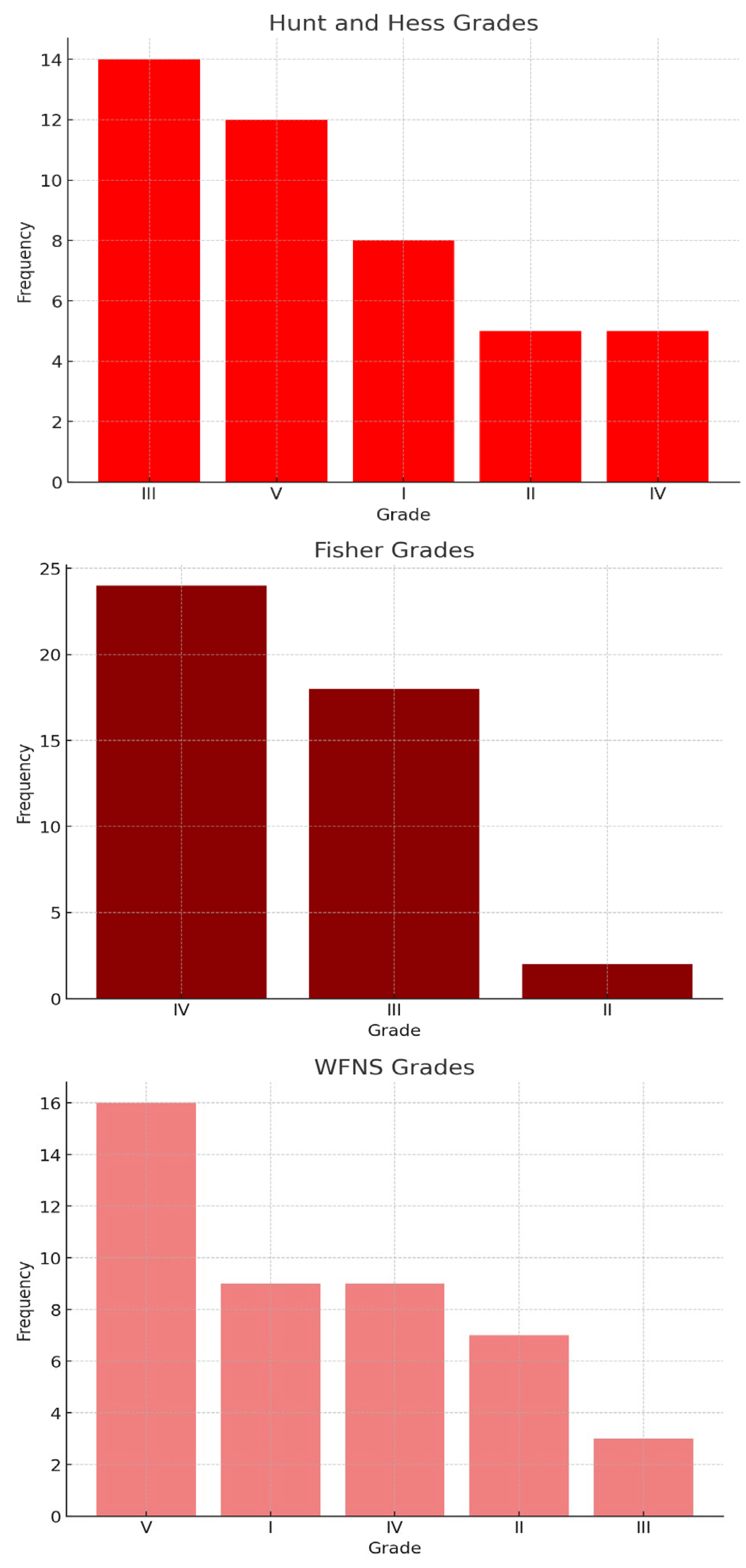
3.2. Severity of SAH
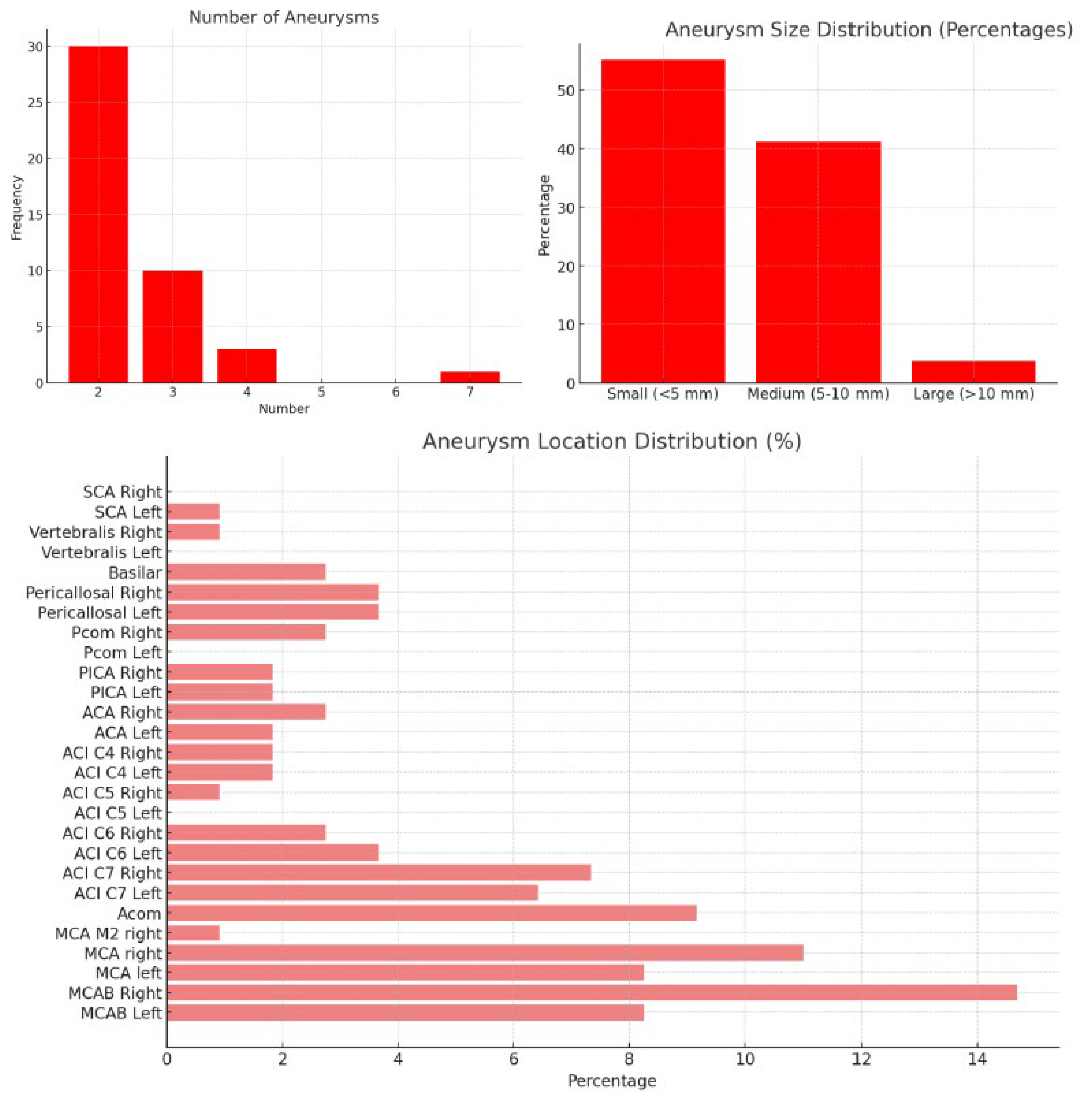
3.3. Aneurysm Characteristics
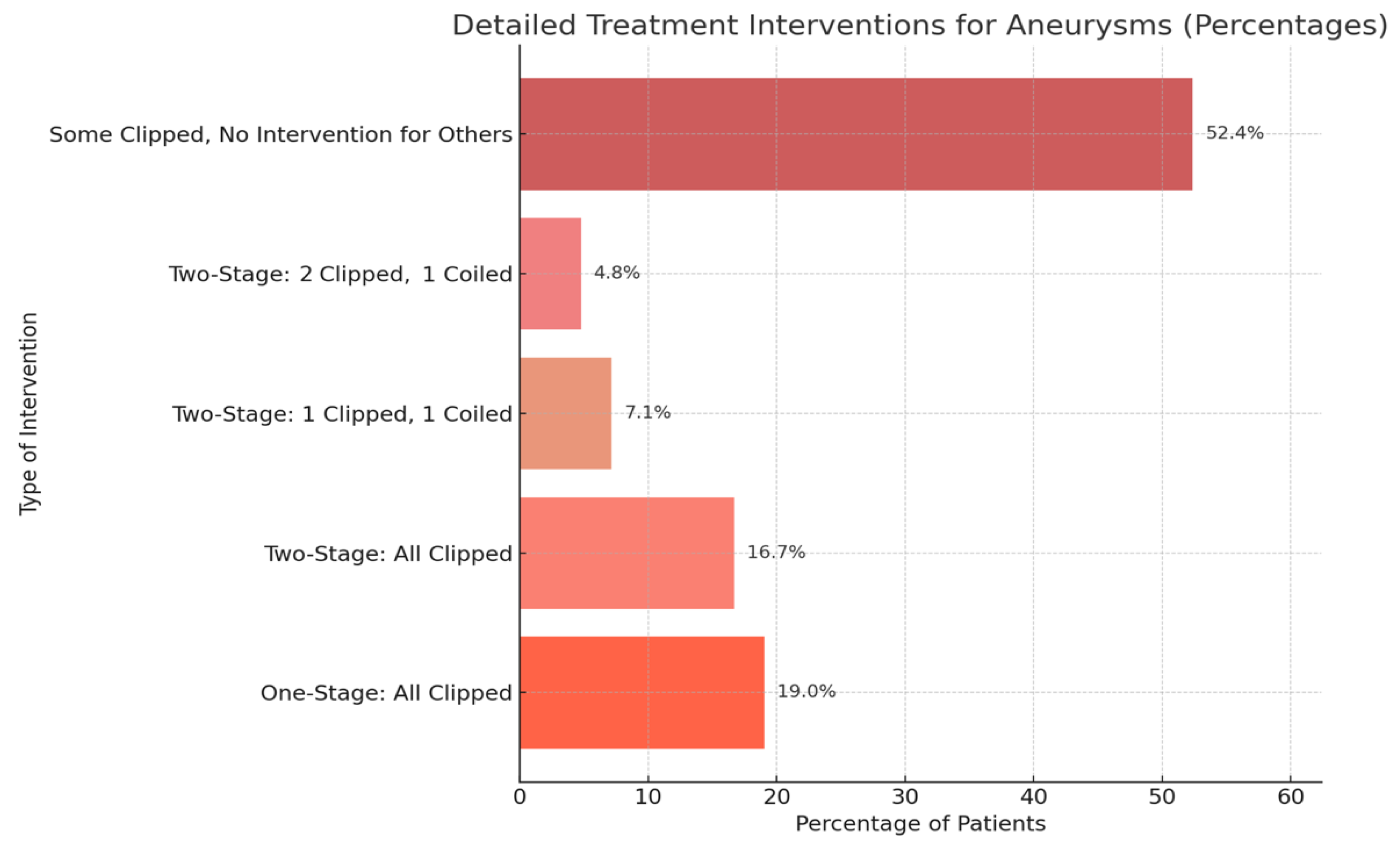
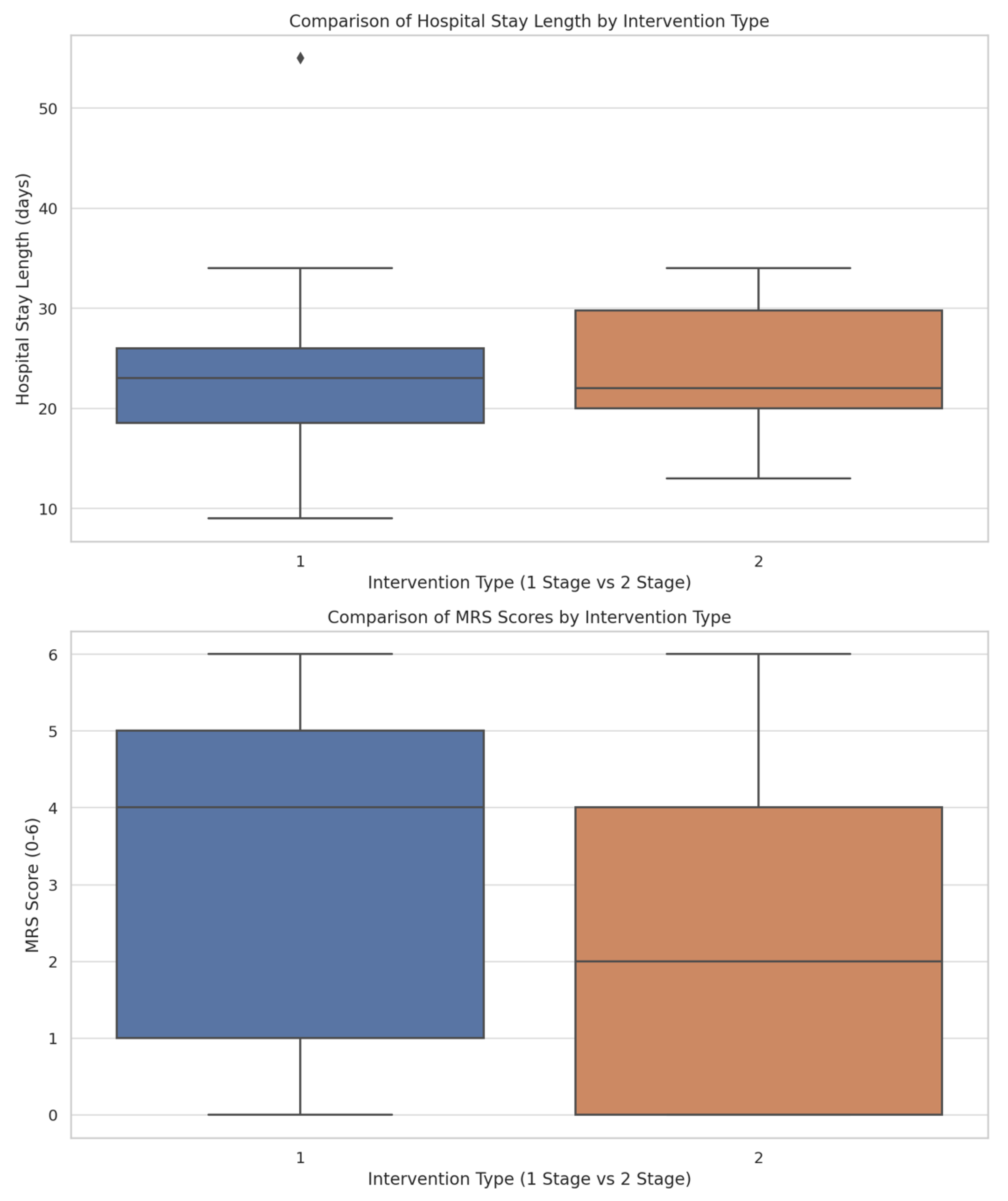
3.4. Treatment Stages, Interventions, and Clinical Outcomes
4. Discussion
4.1. Patient Demographics, Risk Factors, and Symptoms
4.2. Severity of SAH
4.3. Aneurysm Characteristics
4.4. Treatment Stages, Interventions, and Clinical Outcomes
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef]
- Lv, B.; Lan, J.-X.; Si, Y.-F.; Ren, Y.-F.; Li, M.-Y.; Guo, F.-F.; Tang, G.; Bian, Y.; Wang, X.-H.; Zhang, R.-J.; et al. Epidemiological trends of subarachnoid hemorrhage at global, regional, and national level: A trend analysis study from 1990 to 2021. Mil. Med. Res. 2024, 11, 46. [Google Scholar] [CrossRef]
- Nwafor, D.C.; Brichacek, A.L.; Rallo, M.S.; Bidwai, N.; Marsh, R.A. Subarachnoid hemorrhage: New insights on pathogenesis. Front. Stroke 2023, 2, 1110506. [Google Scholar] [CrossRef]
- Hirokazu, K. Hemodynamics in Intracranial Aneurysm Formation. In Hemodynamics of the Human Body; Anil, T., Ed.; IntechOpen: Rijeka, Yugoslavia, 2024; p. Ch. 3. [Google Scholar]
- Signorelli, F.; Gory, B.; Riva, R.; Labeyrie, P.E.; Pelissou-Guyotat, I.; Turjman, F. Hemodynamics, inflammation, vascular remodeling, and the development and rupture of intracranial aneurysms: A review. Neuroimmunol. Neuroinflammation 2015, 2, 59–67. [Google Scholar] [CrossRef]
- Sundström, J.; Söderholm, M.; Söderberg, S.; Alfredsson, L.; Andersson, M.; Bellocco, R.; Björck, M.; Broberg, P.; Eriksson, M.; Forsberg, B.; et al. Risk factors for subarachnoid haemorrhage: A nationwide cohort of 950 000 adults. Leuk. Res. 2019, 48, 2018–2025. [Google Scholar] [CrossRef]
- van den Berg, R. Imaging and Management in Subarachnoid Hemorrhage. In Clinical Neuroradiology: The ESNR Textbook; Barkhof, F., Jäger, H., Thurnher, M., Rovira, À., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–27. [Google Scholar]
- Mascia, L.; Mazzeo, A.T.; Caccia, S. Critical Care Management of Subarachnoid Hemorrhage (SAH). In Practical Trends in Anesthesia and Intensive Care 2017; Chiumello, D., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 147–151. [Google Scholar]
- Nguyen, T.A.; Mai, T.D.; Vu, L.D.; Dao, C.X.; Ngo, H.M.; Hoang, H.B.; Tran, T.A.; Pham, T.Q.; Pham, D.T.; Nguyen, M.H.; et al. Validation of the accuracy of the modified World Federation of Neurosurgical Societies subarachnoid hemorrhage grading scale for predicting the outcomes of patients with aneurysmal subarachnoid hemorrhage. PLoS ONE 2023, 18, e0289267. [Google Scholar] [CrossRef] [PubMed]
- de Winkel, J.; Cras, T.Y.; Dammers, R.; van Doormaal, P.J.; van der Jagt, M.; Dippel, D.W.; Roozenbeek, B. Early predictors of functional outcome in poor-grade aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. BMC Neurol. 2022, 22, 239. [Google Scholar] [CrossRef]
- Andic, C.; Aydemir, F.; Kardes, O.; Gedikoglu, M.; Akin, S. Single-stage endovascular treatment of multiple intracranial aneurysms with combined endovascular tech-niques: Is it safe to treat all at once? J. Neurointerv. Surg. 2017, 9, 1069–1074. [Google Scholar] [CrossRef]
- Asiltürk, M.; Abdallah, A. Clinical outcomes of multiple aneurysms microsurgical clipping: Evaluation of 90 patients. Neurol. i Neurochir. Polska 2018, 52, 15–24. [Google Scholar] [CrossRef]
- Dunn, G.P.; Nahed, B.V.; Walcott, B.P.; Jung, H.; Tierney, T.S.; Ogilvy, C.S. Dual ipsilateral craniotomies through a single incision for the surgical management of multiple intracranial aneurysms. World Neurosurg. 2012, 77, 502–506. [Google Scholar] [CrossRef]
- Guo, S.; Xing, Y. Surgical treatment of multiple intracranial aneurysms. Turk. Neurosurg. 2014, 24, 208–213. [Google Scholar]
- Hadjiathanasiou, A.; Schuss, P.; Brandecker, S.; Welchowski, T.; Schmid, M.; Vatter, H.; Güresir, E. Multiple aneurysms in subarachnoid hemorrhage—Identification of the ruptured aneurysm, when the bleeding pattern is not self-explanatory—Development of a novel prediction score. BMC Neurol. 2020, 20, 70. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Cho, W.-S.; Pang, C.H.; Choi, Y.H.; Bae, J.W.; Ha, E.J.; Lee, S.H.; Kim, K.M.; Kang, H.-S.; Kim, J.E. Treatment outcomes of 1-stage clipping of multiple unruptured intracranial aneurysms via keyhole approaches. J. Neurosurg. 2022, 136, 475–484. [Google Scholar] [CrossRef]
- Hong, T.; Wang, Y. Unilateral approach to clip bilateral multiple intracranial aneurysms. Surg. Neurol. 2009, 72 (Suppl. S1), S23–S28, discussion S28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, W.; Chang, H.; Shen, Y.; Ma, C.; Mao, L.; Li, Z.; Lu, H. Endovascular treatment of multiple intracranial aneurysms in patients with subarachnoid hemorrhage: One or multiple sessions? Front. Neurol. 2023, 14, 1196725. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, V.; Bederson, J.B.; Sehba, F.A.; Gallyas, F. Gender influences the initial impact of subarachnoid hemorrhage: An experimental investigation. PLoS ONE 2013, 8, e80101. [Google Scholar] [CrossRef]
- Shaikh, N.; Chanda, A.; Nawaz, S.; Alkubaisi, A.; Alyafei, A.; Ganaw, A.E.A.; Malmstrom, M.F. Aneurysmal Subarachnoid Haemorrhage: Epidemiology, Aetiology, and Pathophysiology. In Management of Subarachnoid Hemorrhage; Ganaw, A.E.A., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–11. [Google Scholar]
- Schöller, K.; Massmann, M.; Markl, G.; Kunz, M.; Fesl, G.; Brückmann, H.; Pfefferkorn, T.; Tonn, J.-C.; Schichor, C. Aneurysmal subarachnoid hemorrhage in elderly patients: Long-term outcome and prognostic factors in an interdisciplinary treatment approach. J. Neurol. 2013, 260, 1052–1060. [Google Scholar] [CrossRef]
- Nasr, D.M.; Fugate, J.; Brown, R.D. The Genetics of Cerebral Aneurysms and Other Vascular Malformations. In Stroke Genetics; Sharma, P., Meschia, J.F., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 53–78. [Google Scholar]
- Brown, R.D.; Broderick, J.P. Unruptured intracranial aneurysms: Epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014, 13, 393–404. [Google Scholar] [CrossRef]
- Knekt, P. Risk factors for subarachnoid hemorrhage in a longitudinal population study. J. Clin. Epidemiol. 1991, 44, 933–939. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Wang, J.; Zhang, L.; Li, C. The Biological Effects of Smoking on the Formation and Rupture of Intracranial Aneurysms: A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 13, 862916. [Google Scholar] [CrossRef]
- van der Steen, W.E.; Leemans, E.L.; van den Berg, R.; Roos, Y.B.; Marquering, H.A.; Verbaan, D.; Majoie, C.B. Radiological scales predicting delayed cerebral ischemia in subarachnoid hemorrhage: Systematic review and meta-analysis. Neuroradiology 2019, 61, 247–256. [Google Scholar] [CrossRef]
- Bosel, J. What Do We Mean by Poor-Grade Aneurysmal Subarachnoid Hemorrhage and What Can We Do? Neurocrit. Care 2016, 25, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Teksam, M.; McKinney, A.; Casey, S.; Asis, M.; Kieffer, S.; Truwit, C.L. Multi-section ct angiography for detection of cerebral aneurysms. AJNR Am. J. Neuroradiol. 2004, 25, 1485–1492. [Google Scholar]
- Loumiotis, I.; Wagenbach, A.; Brown, R.D.; Lanzino, G. Small (<10-mm) incidentally found intracranial aneurysms, Part 1: Reasons for detection, demographics, location, and risk factors in 212 consecutive patients. Neurosurg. Focus 2011, 31, E3. [Google Scholar] [CrossRef] [PubMed]
- van der Kamp, L.T.; Rinkel, G.J.; Verbaan, D.; van den Berg, R.; Vandertop, W.P.; Murayama, Y.; Vergouwen, M.D. Risk of Rupture After Intracranial Aneurysm Growth. JAMA Neurol. 2021, 78, 1228–1235. [Google Scholar] [CrossRef]
- Brinjikji, W.; Zhu, Y.-Q.; Lanzino, G.; Cloft, H.; Murad, M.; Wang, Z.; Kallmes, D. Risk Factors for Growth of Intracranial Aneurysms: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2016, 37, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Putman, C.; Sheridan, M.; Cebral, J. Hemodynamic patterns of anterior communicating artery aneurysms: A possible association with rupture. Am. J. Neuroradiol. 2009, 30, 297–302. [Google Scholar] [CrossRef]
- Guo, H.; Liu, J.-F.; Li, C.-H.; Wang, J.-W.; Li, H.; Gao, B.-L. Greater hemodynamic stresses initiate aneurysms on major cerebral arterial bifurcations. Front. Neurol. 2023, 14, 1265484. [Google Scholar] [CrossRef]
- Katsuki, M.; Wada, N.; Yamamoto, Y. Single-stage clipping with bifrontal and bilateral frontotemporal craniotomies for subarachnoid hemorrhage with multiple cerebral aneurysms using Sugita head holding system: A case report. Surg. Neurol. Int. 2020, 11, 76. [Google Scholar] [CrossRef]
- Umezawa, K.; Kimura, S.; Kurosaki, K.; Takegami, T.; Ogita, S.; Okamoto, T.; Takahashi, T. Treatment of Multiple Intracranial Aneurysms with 1-stage Clipping. Surg. Cereb. Stroke 2018, 46, 31–39. [Google Scholar] [CrossRef][Green Version]
- Renowden, S.; Nelson, R. Management of incidental unruptured intracranial aneurysms. Prac. Neurol. 2020, 20, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, A.; Oushy, S.; Lu, A.; Ramsay, I.A.; Young, J.; Sanikommu, S.; Rinaldo, L.; Orscelik, A.; Shrigiri, S.; Savastano, L.E.; et al. Microsurgical clipping of ruptured basilar artery perforator aneurysms in the endovascular era: A single-center experience. Neurochirurgie 2025, 71, 101669. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, J.S. Outcome of ruptured anterior communicating artery aneurysm treatment compared between surgical clipping and endovascular coiling: A single-center analysis. Medicine 2022, 101, e30754. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, H.; Orscelik, A.; Elawady, S.S.; Sowlat, M.-M.; Cunningham, C.M.; Al Kasab, S.; Uchida, K.; Yoshimura, S.; Spiotta, A.M. Endovascular Coiling of Ruptured Tiny Saccular Intracranial Aneurysms: A Systematic Review and Meta-Analysis. World Neurosurg. 2024, 187, E414–E446. [Google Scholar] [CrossRef]


| a | |||
| Category | Metric | Count | Percentage |
| Patient Demographics | Female | 35 | 68.18% |
| Male | 9 | 20.45% | |
| Age (mean) | 54.5 ± 12.0 | ||
| Age (Range) | 33–78 | ||
| Family History | Yes | 17 | 38.64% |
| No | 19 | 43.18% | |
| Unknown | 8 | 18.18% | |
| Risk Factors | Arterial Hypertension | 21 | 47.73% |
| None | 17 | 38.64% | |
| Smoking | 8 | 18.18% | |
| Coronary Heart Disease | 7 | 15.91% | |
| Migraine | 2 | 4.55% | |
| Depression | 1 | 2.27% | |
| Diabetes Mellitus | 1 | 2.27% | |
| Hyperlipidemia | 1 | 2.27% | |
| Obesity | 1 | 2.27% | |
| Symotoms | Headache | 35 | 79.55% |
| Nausea | 16 | 36.36% | |
| Vomiting | 13 | 29.55% | |
| Neck Pain | 9 | 20.45% | |
| Seizure | 7 | 15.91% | |
| Loss of Consciousness | 7 | 15.91% | |
| Coma | 6 | 13.64% | |
| Dysarthria | 6 | 13.64% | |
| Decreased Consciousness | 3 | 6.82% | |
| Syncope | 4 | 9.09% | |
| Hemiparesis | 1 | 2.27% | |
| Motor Weakness | 1 | 2.27% | |
| Retrograde Amnesia | 1 | 2.27% | |
| Right Hemiplegia | 1 | 2.27% | |
| Urination | 1 | 2.27% | |
| b | |||
| Aneurysm Sizes | - Small (<5 mm) | 59 | 55.14% |
| - Medium (5–10 mm) | 44 | 41.12% | |
| - Large (>10 mm) | 4 | 3.74% | |
| Top Aneurysm Locations | MCAB Right | 16 | 14.95% |
| MCA Right | 12 | 11.21% | |
| Acom | 10 | 9.35% | |
| Radiological Features | Intracerebral Hemorrhage | 24 | 54.55% |
| Hydrocephalus | 21 | 47.73% | |
| Outcome Measures | ||||
|---|---|---|---|---|
| Measure | Mean | SD | Min | Max |
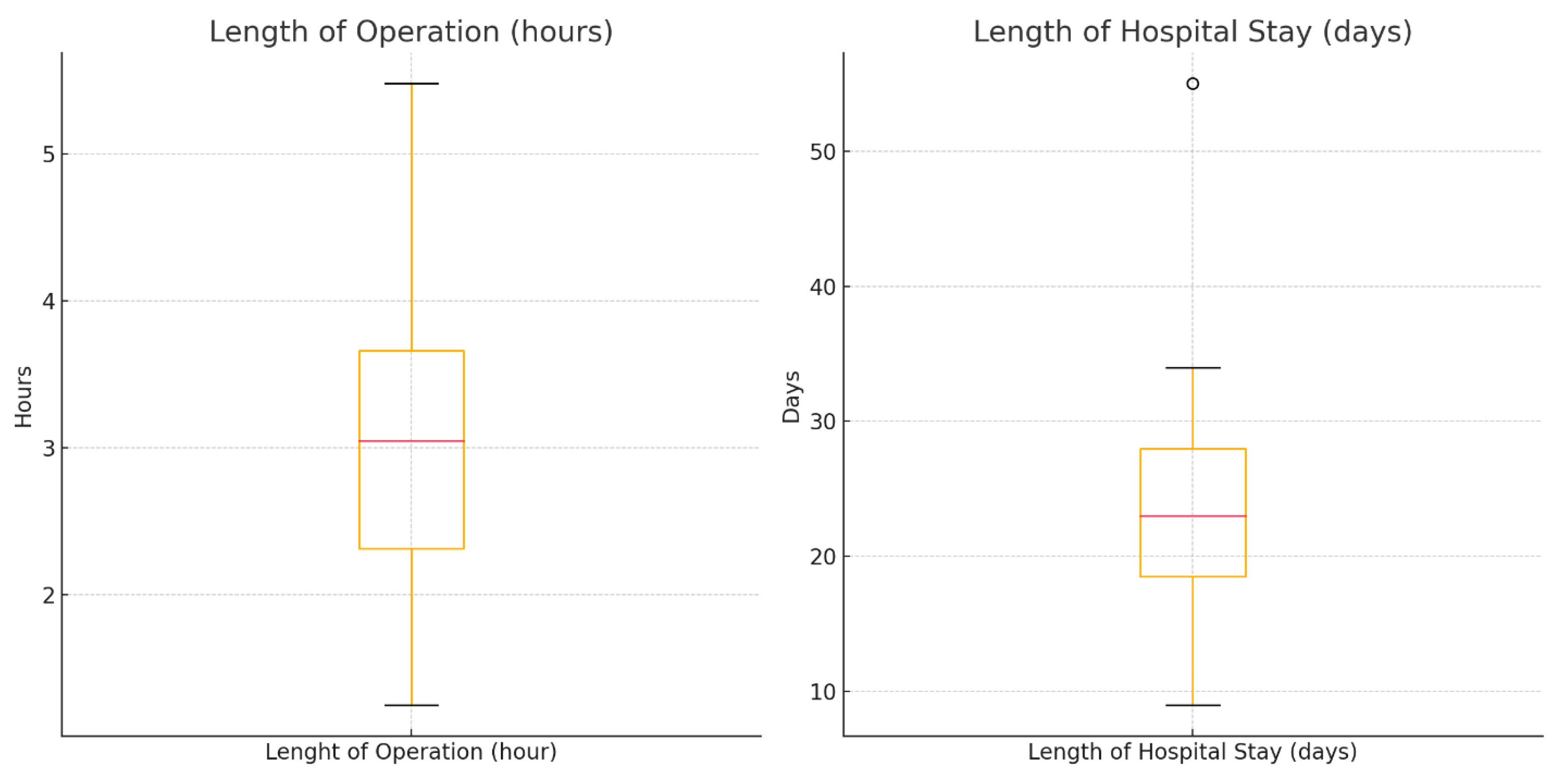
| Length of Operation (hours) | 3.09 | 1.01 | 1.25 | 5.48 |
| Length of Hospital Stay (days) | 23.19 | 8.56 | 9.00 | 55.00 |
| Measure | Yes | No | ||
| Post-op Complications (%) | 77.27% | 22.73% | ||
| Vasospasm (%) | 72.73% | 27.27% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atallah, O.; Alrefaie, K.; Badary, A. Comparative Analysis of Single-Stage vs. Multiple-Stage Interventions in the Management of Subarachnoid Hemorrhage in Patients with Multiple Intracranial Aneurysms. J. Clin. Med. 2025, 14, 4705. https://doi.org/10.3390/jcm14134705
Atallah O, Alrefaie K, Badary A. Comparative Analysis of Single-Stage vs. Multiple-Stage Interventions in the Management of Subarachnoid Hemorrhage in Patients with Multiple Intracranial Aneurysms. Journal of Clinical Medicine. 2025; 14(13):4705. https://doi.org/10.3390/jcm14134705
Chicago/Turabian StyleAtallah, Oday, Khadeja Alrefaie, and Amr Badary. 2025. "Comparative Analysis of Single-Stage vs. Multiple-Stage Interventions in the Management of Subarachnoid Hemorrhage in Patients with Multiple Intracranial Aneurysms" Journal of Clinical Medicine 14, no. 13: 4705. https://doi.org/10.3390/jcm14134705
APA StyleAtallah, O., Alrefaie, K., & Badary, A. (2025). Comparative Analysis of Single-Stage vs. Multiple-Stage Interventions in the Management of Subarachnoid Hemorrhage in Patients with Multiple Intracranial Aneurysms. Journal of Clinical Medicine, 14(13), 4705. https://doi.org/10.3390/jcm14134705







