Assessment of Serum suPAR Levels in Patients with Group 1 and Group 4 Pulmonary Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Statistical Analysis
3. Results
3.1. Clinical, Laboratory, and Echocardiographic Parameters
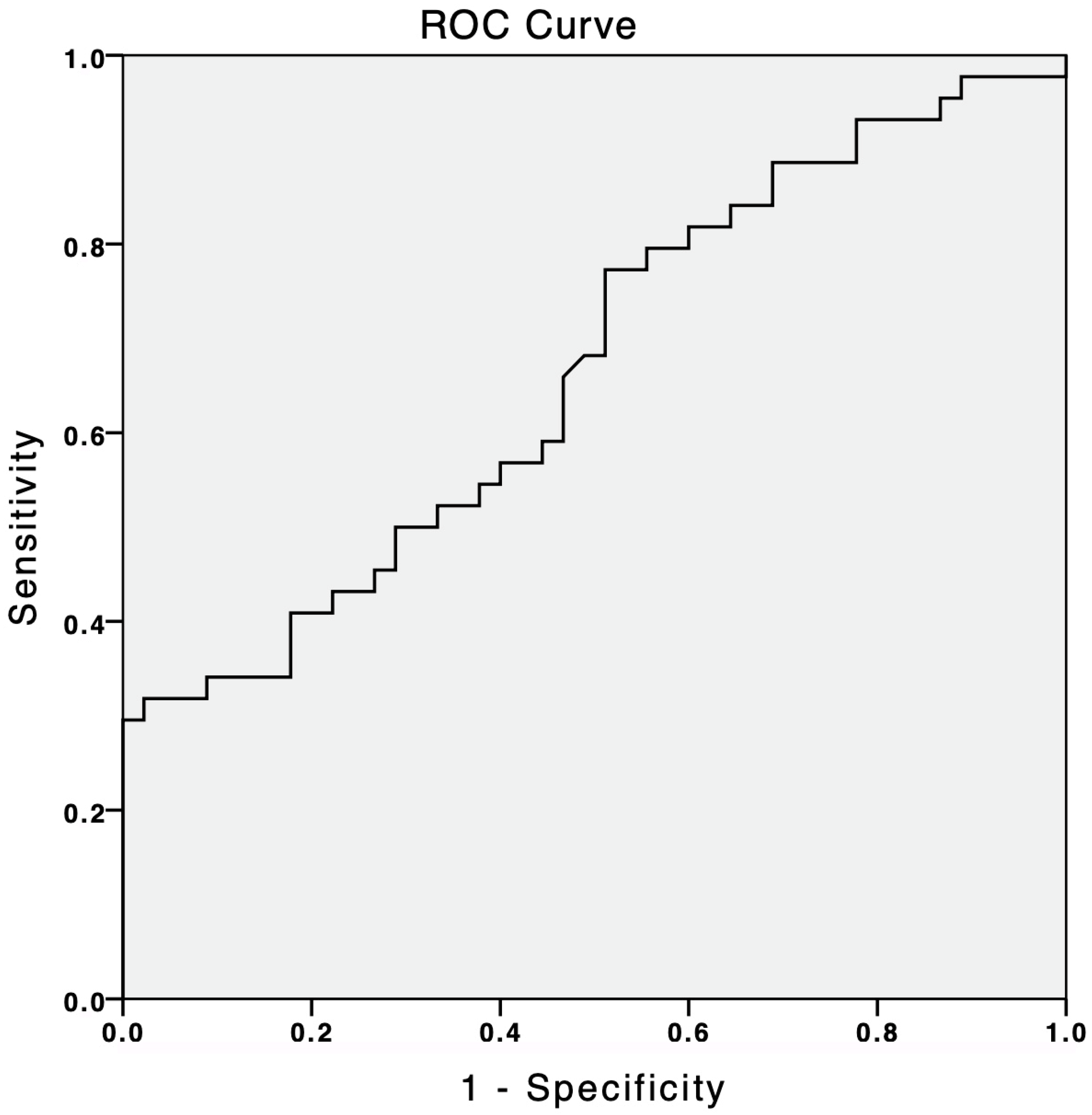
3.2. suPAR
3.3. Comparison of Patients with Group 1 and Group 4 PH
4. Discussion
4.1. Future Perspectives
- suPAR levels and long-term prognosis of patients with PH;
- The effects of PAH-specific treatments on suPAR levels;
- The effects of the inhibition of suPAR on the hemodynamic profile and long-term prognosis of PH;
- suPAR levels should be investigated in other subgroups of PH (Groups 2, 3, and 5);
- Large-scale multicenter studies are needed to clarify the cut-off suPAR levels, similar to NT-proBNP, for use in risk assessment and prediction of long-term mortality;
- After clarifying the cut-off values for suPAR in patients with PH, these values must be validated in an independent control group. On the other hand, investigating the effects of suPAR levels on the prediction power of the REVEAL 2.0 risk score should be valuable.
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pulido, T.; Adzerikho, I.; Channick, R.N.; Delcroix, M.; Galie, N.; Ghofrani, H.A.; Jansa, P.; Jing, Z.C.; Le Brun, F.O.; Mehta, S.; et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Yaylali, Y.T.; Basarici, I.; Kilickiran Avci, B.; Meric, M.; Sinan, U.Y.; Senol, H.; Kucukoglu, M.S.; Ongen, Z. Risk assessment and survival of patients with pulmonary hypertension: Multicenter experience in Turkey. Anatol. J. Cardiol. 2019, 21, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Sinan, U.Y.; Demir, R.; Canbolat, I.P.; Palabiyik, M.; Kaya, A.; Kucukoglu, M.S. Pulmonary hypertension experience in an expert university hospital. Anatol. J. Cardiol. 2018, 20, 35–40. [Google Scholar] [CrossRef]
- Price, L.C.; Wort, S.J.; Perros, F.; Dorfmuller, P.; Huertas, A.; Montani, D.; Cohen-Kaminsky, S.; Humbert, M. Inflammation in pulmonary arterial hypertension. Chest 2012, 141, 210–221. [Google Scholar] [CrossRef]
- Savai, R.; Pullamsetti, S.S.; Kolbe, J.; Bieniek, E.; Voswinckel, R.; Fink, L.; Scheed, A.; Ritter, C.; Dahal, B.K.; Vater, A.; et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 897–908. [Google Scholar] [CrossRef]
- Stacher, E.; Graham, B.B.; Hunt, J.M.; Gandjeva, A.; Groshong, S.D.; McLaughlin, V.V.; Jessup, M.; Grizzle, W.E.; Aldred, M.A.; Cool, C.D.; et al. Modern age pathology of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 261–272. [Google Scholar] [CrossRef]
- Sahara, M.; Sata, M.; Morita, T.; Nakamura, K.; Hirata, Y.; Nagai, R. Diverse contribution of bone marrow-derived cells to vascular remodeling associated with pulmonary arterial hypertension and arterial neointimal formation. Circulation 2007, 115, 509–517. [Google Scholar] [CrossRef]
- Perros, F.; Dorfmuller, P.; Souza, R.; Durand-Gasselin, I.; Mussot, S.; Mazmanian, M.; Herve, P.; Emilie, D.; Simonneau, G.; Humbert, M. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur. Respir. J. 2007, 29, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Cracowski, J.L.; Chabot, F.; Labarere, J.; Faure, P.; Degano, B.; Schwebel, C.; Chaouat, A.; Reynaud-Gaubert, M.; Cracowski, C.; Sitbon, O.; et al. Proinflammatory cytokine levels are linked to death in pulmonary arterial hypertension. Eur. Respir. J. 2014, 43, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Huai, Q.; Mazar, A.P.; Kuo, A.; Parry, G.C.; Shaw, D.E.; Callahan, J.; Li, Y.; Yuan, C.; Bian, C.; Chen, L.; et al. Structure of human urokinase plasminogen activator in complex with its receptor. Science 2006, 311, 656–659. [Google Scholar] [CrossRef]
- Thuno, M.; Macho, B.; Eugen-Olsen, J. suPAR: The molecular crystal ball. Dis. Markers 2009, 27, 157–172. [Google Scholar] [CrossRef]
- Ni, W.; Han, Y.; Zhao, J.; Cui, J.; Wang, K.; Wang, R.; Liu, Y. Serum soluble urokinase-type plasminogen activator receptor as a biological marker of bacterial infection in adults: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 39481. [Google Scholar] [CrossRef]
- Lomholt, A.F.; Christensen, I.J.; Hoyer-Hansen, G.; Nielsen, H.J. Prognostic value of intact and cleaved forms of the urokinase plasminogen activator receptor in a retrospective study of 518 colorectal cancer patients. Acta Oncol. 2010, 49, 805–811. [Google Scholar] [CrossRef]
- Zimmermann, H.W.; Koch, A.; Seidler, S.; Trautwein, C.; Tacke, F. Circulating soluble urokinase plasminogen activator is elevated in patients with chronic liver disease, discriminates stage and aetiology of cirrhosis and predicts prognosis. Liver Int. 2012, 32, 500–509. [Google Scholar] [CrossRef]
- Borne, Y.; Persson, M.; Melander, O.; Smith, J.G.; Engstrom, G. Increased plasma level of soluble urokinase plasminogen activator receptor is associated with incidence of heart failure but not atrial fibrillation. Eur. J. Heart Fail. 2014, 16, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Eugen-Olsen, J.; Andersen, O.; Linneberg, A.; Ladelund, S.; Hansen, T.W.; Langkilde, A.; Petersen, J.; Pielak, T.; Moller, L.N.; Jeppesen, J.; et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J. Intern. Med. 2010, 268, 296–308. [Google Scholar] [CrossRef]
- Sidenius, N.; Nebuloni, M.; Sala, S.; Zerbi, P.; Price, R.W.; Gisslen, M.; Hagberg, L.; Vago, L.; Lazzarin, A.; Blasi, F.; et al. Expression of the urokinase plasminogen activator and its receptor in HIV-1-associated central nervous system disease. J. Neuroimmunol. 2004, 157, 133–139. [Google Scholar] [CrossRef]
- Hayek, S.S.; Sever, S.; Ko, Y.A.; Trachtman, H.; Awad, M.; Wadhwani, S.; Altintas, M.M.; Wei, C.; Hotton, A.L.; French, A.L.; et al. Soluble Urokinase Receptor and Chronic Kidney Disease. N. Engl. J. Med. 2015, 373, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Engstrom, G.; Bjorkbacka, H.; Hedblad, B. Soluble urokinase plasminogen activator receptor in plasma is associated with incidence of CVD. Results from the Malmo Diet and Cancer Study. Atherosclerosis 2012, 220, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Eapen, D.J.; Manocha, P.; Ghasemzadeh, N.; Patel, R.S.; Al Kassem, H.; Hammadah, M.; Veledar, E.; Le, N.A.; Pielak, T.; Thorball, C.W.; et al. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J. Am. Heart Assoc. 2014, 3, e001118. [Google Scholar] [CrossRef]
- Sorensen, M.H.; Gerke, O.; Eugen-Olsen, J.; Munkholm, H.; Lambrechtsen, J.; Sand, N.P.; Mickley, H.; Rasmussen, L.M.; Olsen, M.H.; Diederichsen, A. Soluble urokinase plasminogen activator receptor is in contrast to high-sensitive C-reactive-protein associated with coronary artery calcifications in healthy middle-aged subjects. Atherosclerosis 2014, 237, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, G.; Corban, M.T.; Hung, O.Y.; Eshtehardi, P.; Eapen, D.J.; Al-Kassem, H.; Rasoul-Arzrumly, E.; Gogas, B.D.; McDaniel, M.C.; Pielak, T.; et al. Plasma soluble urokinase-type plasminogen activator receptor level is independently associated with coronary microvascular function in patients with non-obstructive coronary artery disease. Atherosclerosis 2015, 239, 55–60. [Google Scholar] [CrossRef]
- Koller, L.; Stojkovic, S.; Richter, B.; Sulzgruber, P.; Potolidis, C.; Liebhart, F.; Mortl, D.; Berger, R.; Goliasch, G.; Wojta, J.; et al. Soluble Urokinase-Type Plasminogen Activator Receptor Improves Risk Prediction in Patients With Chronic Heart Failure. JACC Heart Fail. 2017, 5, 268–277. [Google Scholar] [CrossRef]
- Hodges, G.W.; Bang, C.N.; Eugen-Olsen, J.; Olsen, M.H.; Boman, K.; Ray, S.; Gohlke-Barwolf, C.; Kesaniemi, Y.A.; Jeppesen, J.L.; Wachtell, K. SuPAR Predicts Cardiovascular Events and Mortality in Patients With Asymptomatic Aortic Stenosis. Can. J. Cardiol. 2016, 32, 1462–1469. [Google Scholar] [CrossRef]
- Lyngbaek, S.; Marott, J.L.; Moller, D.V.; Christiansen, M.; Iversen, K.K.; Clemmensen, P.M.; Eugen-Olsen, J.; Jeppesen, J.L.; Hansen, P.R. Usefulness of soluble urokinase plasminogen activator receptor to predict repeat myocardial infarction and mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous intervention. Am. J. Cardiol. 2012, 110, 1756–1763. [Google Scholar] [CrossRef]
- Molkanen, T.; Ruotsalainen, E.; Thorball, C.W.; Jarvinen, A. Elevated soluble urokinase plasminogen activator receptor (suPAR) predicts mortality in Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1417–1424. [Google Scholar] [CrossRef]
- Smukowska-Gorynia, A.; Marcinkowska, J.; Chmara, E.; Malaczynska-Rajpold, K.; Slawek-Szmyt, S.; Cieslewicz, A.; Janus, M.; Araszkiewicz, A.; Jankiewicz, S.; Komosa, A.; et al. Neopterin as a Biomarker in Patients with Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension. Respiration 2018, 96, 222–230. [Google Scholar] [CrossRef]
- Karakurt, C.; Baspinar, O.; Celik, F.S.; Taskapan, C.; Sahin, A.D.; Yologlu, S. Serum Pentraxin 3 and hs-CRP Levels in Children with Severe Pulmonary Hypertension. Balk. Med. J. 2014, 31, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, J.A.; Horne, B.D.; Saeed, W.; Sardar, M.R.; Zolty, R. Galectin-3 Levels Are Elevated and Predictive of Mortality in Pulmonary Hypertension. Heart Lung Circ. 2017, 26, 1208–1215. [Google Scholar] [CrossRef]
- Kim, N.H.; Delcroix, M.; Jais, X.; Madani, M.M.; Matsubara, H.; Mayer, E.; Ogo, T.; Tapson, V.F.; Ghofrani, H.A.; Jenkins, D.P. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801915. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Chan, S.Y.; Weir, E.K. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1322–H1331. [Google Scholar] [CrossRef]
- Farber, H.W.; Loscalzo, J. Pulmonary arterial hypertension. N. Engl. J. Med. 2004, 351, 1655–1665. [Google Scholar] [CrossRef]
- Aldabbous, L.; Abdul-Salam, V.; McKinnon, T.; Duluc, L.; Pepke-Zaba, J.; Southwood, M.; Ainscough, A.J.; Hadinnapola, C.; Wilkins, M.R.; Toshner, M.; et al. Neutrophil Extracellular Traps Promote Angiogenesis: Evidence From Vascular Pathology in Pulmonary Hypertension. Arter. Thromb. Vasc. Biol. 2016, 36, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Vonk Noordegraaf, A.; Chin, K.M.; Haddad, F.; Hassoun, P.M.; Hemnes, A.R.; Hopkins, S.R.; Kawut, S.M.; Langleben, D.; Lumens, J.; Naeije, R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019, 53, 1801900. [Google Scholar] [CrossRef]
- Humbert, M.; Sitbon, O.; Chaouat, A.; Bertocchi, M.; Habib, G.; Gressin, V.; Yaici, A.; Weitzenblum, E.; Cordier, J.F.; Chabot, F.; et al. Pulmonary arterial hypertension in France: Results from a national registry. Am. J. Respir. Crit. Care Med. 2006, 173, 1023–1030. [Google Scholar] [CrossRef]
- Badesch, D.B.; Raskob, G.E.; Elliott, C.G.; Krichman, A.M.; Farber, H.W.; Frost, A.E.; Barst, R.J.; Benza, R.L.; Liou, T.G.; Turner, M.; et al. Pulmonary arterial hypertension: Baseline characteristics from the REVEAL Registry. Chest 2010, 137, 376–387. [Google Scholar] [CrossRef]
- Ogawa, A.; Satoh, T.; Tamura, Y.; Fukuda, K.; Matsubara, H. Survival of Japanese Patients With Idiopathic/Heritable Pulmonary Arterial Hypertension. Am. J. Cardiol. 2017, 119, 1479–1484. [Google Scholar] [CrossRef]
- Lim, Y.; Low, T.T.; Chan, S.P.; Teo, T.W.; Jang, J.J.; Yip, N.; Kuntjoro, I.; Tay, E.L.; Yip, J.W. Pulmonary arterial hypertension in a multi-ethnic Asian population: Characteristics, survival and mortality predictors from a 14-year follow-up study. Respirology 2019, 24, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Kramer, T.; Pan, Z.; Eichstaedt, C.A.; Spiesshoefer, J.; Benjamin, N.; Olsson, K.M.; Meyer, K.; Vizza, C.D.; Vonk-Noordegraaf, A.; et al. Mortality in pulmonary arterial hypertension: Prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur. Respir. J. 2017, 50, 1700740. [Google Scholar] [CrossRef]
- Kylhammar, D.; Kjellstrom, B.; Hjalmarsson, C.; Jansson, K.; Nisell, M.; Soderberg, S.; Wikstrom, G.; Radegran, G. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur. Heart J. 2018, 39, 4175–4181. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Miller, D.P.; Gomberg-Maitland, M.; Frantz, R.P.; Foreman, A.J.; Coffey, C.S.; Frost, A.; Barst, R.J.; Badesch, D.B.; Elliott, C.G.; et al. Predicting survival in pulmonary arterial hypertension: Insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010, 122, 164–172. [Google Scholar] [CrossRef]
- Arvanitaki, A.; Boutsikou, M.; Anthi, A.; Apostolopoulou, S.; Avgeropoulou, A.; Demerouti, E.; Farmakis, D.; Feloukidis, C.; Giannakoulas, G.; Karvounis, H.; et al. Epidemiology and initial management of pulmonary arterial hypertension: Real-world data from the Hellenic pulmOnary hyPertension rEgistry (HOPE). Pulm. Circ. 2019, 9, 2045894019877157. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, M.Z.; Diederichsen, S.Z.; Mickley, H.; Steffensen, F.H.; Lambrechtsen, J.; Sand, N.P.R.; Christensen, K.L.; Olsen, M.H.; Diederichsen, A.; Gronhoj, M.H. Prognostic value of suPAR and hs-CRP on cardiovascular disease. Atherosclerosis 2018, 271, 245–251. [Google Scholar] [CrossRef]
- Backes, Y.; van der Sluijs, K.F.; Mackie, D.P.; Tacke, F.; Koch, A.; Tenhunen, J.J.; Schultz, M.J. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: A systematic review. Intensive Care Med. 2012, 38, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Moons, L.; Bouche, A.; Shapiro, S.D.; Collen, D.; Carmeliet, P. Deficiency of urokinase-type plasminogen activator-mediated plasmin generation impairs vascular remodeling during hypoxia-induced pulmonary hypertension in mice. Circulation 2001, 103, 2014–2020. [Google Scholar] [CrossRef]
- Fuhrman, B. The urokinase system in the pathogenesis of atherosclerosis. Atherosclerosis 2012, 222, 8–14. [Google Scholar] [CrossRef]
- Waltz, D.A.; Fujita, R.M.; Yang, X.; Natkin, L.; Zhuo, S.; Gerard, C.J.; Rosenberg, S.; Chapman, H.A. Nonproteolytic role for the urokinase receptor in cellular migration in vivo. Am. J. Respir. Cell Mol. Biol. 2000, 22, 316–322. [Google Scholar] [CrossRef]
- Kruger, R.; Rasmussen, L.M.; Argraves, W.S.; Eugen-Olsen, J.; Nielsen, O.W.; Blyme, A.; Willenheimer, R.; Wachtell, K.; Olsen, M.H. Extracellular matrix biomarker, fibulin-1, is closely related to NT-proBNP and soluble urokinase plasminogen activator receptor in patients with aortic valve stenosis (the SEAS study). PLoS ONE 2014, 9, e101522. [Google Scholar] [CrossRef] [PubMed]
- du Plooy, C.S.; Kruger, R.; Huisman, H.W.; Rasmussen, L.M.; Eugen-Olsen, J.; Schutte, A.E. Extracellular matrix biomarker, fibulin-1 and its association with soluble uPAR in a bi-ethnic South African population: The SAfrEIC study. Heart Lung Circ. 2015, 24, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Lyu, L.; Yu, T.; Zuo, C.; Fu, J.; He, Y.; Wan, Q.; Wan, N.; Jia, D.; Lyu, A. Macrophage-Derived Legumain Promotes Pulmonary Hypertension by Activating the MMP (Matrix Metalloproteinase)-2/TGF (Transforming Growth Factor)-beta1 Signaling. Arter. Thromb. Vasc. Biol. 2019, 39, e130–e145. [Google Scholar] [CrossRef] [PubMed]
- Legany, N.; Toldi, G.; Distler, J.H.; Beyer, C.; Szalay, B.; Kovacs, L.; Vasarhelyi, B.; Balog, A. Increased plasma soluble urokinase plasminogen activator receptor levels in systemic sclerosis: Possible association with microvascular abnormalities and extent of fibrosis. Clin. Chem. Lab. Med. 2015, 53, 1799–1805. [Google Scholar] [CrossRef]
- Butt, S.; Jeppesen, J.L.; Iversen, L.V.; Fenger, M.; Eugen-Olsen, J.; Andersson, C.; Jacobsen, S. Association of soluble urokinase plasminogen activator receptor levels with fibrotic and vascular manifestations in systemic sclerosis. PLoS ONE 2021, 16, e0247256. [Google Scholar] [CrossRef]
- Yokokawa, T.; Boucherat, O.; Martineau, S.; Lemay, S.E.; Breuils-Bonnet, S.; Krishna, V.; Kalyana-Sundaram, S.; Jeyaseelan, J.; Potus, F.; Bonnet, S.; et al. Prognostic Significance of Proteomics-Discovered Circulating Inflammatory Biomarkers in Patients With Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2024, 13, e032888. [Google Scholar] [CrossRef]
- Mirna, M.; Rohm, I.; Jirak, P.; Wernly, B.; Baz, L.; Paar, V.; Kretzschmar, D.; Hoppe, U.C.; Schulze, P.C.; Lichtenauer, M.; et al. Analysis of Novel Cardiovascular Biomarkers in Patients With Pulmonary Hypertension (PH). Heart Lung Circ. 2019, 29, 337–344. [Google Scholar] [CrossRef]
| Control Group (n = 45) | PH Group (n = 44) | p | |
|---|---|---|---|
| Group 1 PH, n (%) | - | 36 (82) | |
| Group 4 PH, n (%) | - | 8 (18) | |
| Clinical parameters | |||
| Gender, male, n (%) | 12 (26.7) | 12 (27.3) | 0.949 |
| Age, years | 51.24 ± 12.48 | 51.05 ± 18.18 | 0.952 |
| BMI, kg/m2 | 27.38 ± 3.76 | 27.05 ± 3.73 | 0.674 |
| Smoking, n (%) | 3 (6.7) | 2 (4.5) | 1 |
| Diabetes mellitus, n (%) | 7 (15.6) | 9 (20.5) | 0.547 |
| Hypertension, n (%) | 11 (24.4) | 11 (25) | 0.952 |
| Systolic blood pressure, mmHg | 120 (100–130) | 110 (100–130) | 0.041 * |
| Diastolic blood pressure, mmHg | 70 (70–70) | 70 (62.50–80) | 0.903 |
| Heart Rate, beats per minute | 70 (70–80) | 82.50 (70–95) | 0.005 * |
| 6 min walking test, m | 650 (622.50–680) | 372.50 (312.50–407.50) | <0.001 * |
| Laboratory parameters | |||
| WBC count, ×109/L | 7.72 ± 1.82 | 7.60 ± 2.92 | 0.802 |
| Hemoglobin, g/dL | 14.54 ± 1.41 | 12.65 ± 2.33 | <0.001 * |
| Fasting blood glucose, mg/dL | 94 (102–87.50) | 93 (82.25–100.75) | 0.290 |
| GFR, mL/per minute | 111.63 ± 19.48 | 103.71 ± 30.62 | 0.148 |
| ESR, mm/h | 13 (10.50–20.0) | 18 (7.25–33.25) | 0.160 |
| CRP, mg/L | 2.01 (1.05–3.03) | 1.39 (0.60–3.56) | 0.536 |
| NT-proBNP, pg/mL | 37.60 (24.40–80.50) | 442.00 (120.75–1516.75) | <0.001 * |
| suPAR, pg/mL | 65.52 (53.06–80.91) | 73.14 (62.77–167.13) | 0.012 * |
| Echocardiographic parameters | |||
| LV end-diastolic diameter, mm | 48 (46–49) | 46(42–48.75) | 0.024 * |
| LVEF, % | 60 (60–60) | 55.50 (55–60) | <0.001 * |
| Systolic PAB, mmHg | 28 (26.50–29.50) | 69 (53.50–80) | <0.001 * |
| TAPSE, mm | 23 (21–24) | 16 (14–18) | <0.001 * |
| RAA, cm2 | 15.00 (13.85–16.30) | 27.55 (24.90–30.60) | <0.001 * |
| Medications | |||
| RAS blockers, n (%) | 11 (24.4) | 17 (38.6) | 0.149 |
| Beta blockers, n (%) | 13 (28.9) | 13 (29.5) | 0.946 |
| Calcium channel blockers, n (%) | 4 (8.9) | 4 (9.1) | 1 |
| Statins, n (%) | 4 (8.9) | 8 (18.2) | 0.230 |
| Anticoagulant therapy, n (%) | 1 (2.2) | 16 (36.4) | <0.001 * |
| Oral anti-diabetics, n (%) | 7 (15.9) | 6 (13.6) | 0.764 |
| Variables | Univariate | Multiple | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Systolic blood pressure, mmHg | 0.968 (0.940–0.998) | 0.034 | 0.875 (0.780–0.981 | 0.022 |
| Heart Rate, beats per minute | 1.073 (1.027–1.121) | 0.002 | 1.117 (0.951–1.314) | 0.178 |
| Hemoglobin, g/dL | 0.583 (0.441–0.772) | <0.001 | 0.701 (0.266–1.848) | 0.472 |
| GFR | 0.988 (0.971–1.005) | 0.150 | 0.970 (0.899–1.047) | 0.438 |
| ESR, mm/h | 1.043 (1.008–1.080) | 0.016 | 1.177 (1.038–1.335) | 0.011 * |
| NT- ProBNP, pg/mL | 1.015 (1.007–1.024) | <0.001 | 1.025 (1.009–1.042) | 0.003 * |
| suPAR, pg/mL | 1.007 (1.016–1.032) | 0.047 | 1.047 (1.004–1.093) | 0.032 * |
| LV end-diastolic diameter, mm | 0.896 (0.805–0.997) | 0.044 | 0.877 (0.630–1.220) | 0.435 |
| LVEF, % | 0.701 (0.581–0.846) | <0.001 | 0.901 (0.464–1.747) | 0.757 |
| Group 1 PH (n = 36) | Group 4 PH (n = 8) | p | |
|---|---|---|---|
| Clinical parameters | |||
| Gender, male, n (%) | 9 (25) | 3 (37.5) | 0.663 |
| Age, years | 51.17 ± 19.19 | 50.50 ± 13.70 | 0.927 |
| BMI, kg/m2 | 27.10 ± 3.89 | 26.85 ± 3.16 | |
| Diabetes mellitus, n (%) | 8 (22.2) | 1 (12.5) | 1.000 |
| Hypertension, n (%) | 11 (30.6) | 0 (0) | 0.170 |
| 6 min walking test, m | 345.14 ± 71.07 | 437.50 ± 68.19 | 0.002 * |
| Laboratory parameters | |||
| Hemoglobin, g/dL | 12.72 ± 2.49 | 12.29 ± 1.50 | 0.637 |
| NT-proBNP, pg/mL | 520.50 (182.50–2516.75) | 162.50 (69.72–585.75) | 0.092 |
| suPAR, pg/mL | 70.57 (63.90–235.76) | 75.48 (62.31–80.08) | 0.800 |
| Echocardiographic parameters | |||
| LVEF, % | 57 (55–60) | 58 (55–60) | 0.731 |
| Systolic PAB, mmHg | 74.11 ± 22.53 | 63.25 ± 25.26 | 0.234 |
| TAPSE, mm | 15.89 ± 2.20 | 16.87 ± 3.64 | 0.319 |
| RAA, cm2 | 27.80 ± 3.77 | 27.95 ± 4.94 | 0.923 |
| Heart catheterization parameters | |||
| Systolic PAB, mmHg | 77.42 ± 16.98 | 69.37 ± 24.37 | 0.270 |
| Mean PAB, mmHg | 46.97 ± 9.83 | 42 ± 14.21 | 0.241 |
| Cardiac index, L/min/m2 | 2.48 ± 0.38 | 2.62 ± 0.49 | 0.383 |
| PCWP, mmHg | 10.75 ± 2.01 | 9.87 ± 0.35 | 0.230 |
| Right atrial pressure, mmHg | 9.44 ± 2.12 | 8.12 ± 2.23 | 0.122 |
| PVR, Woods | 7.62 ± 2.57 | 5.43 ± 4.29 | 0.063 |
| Medications | |||
| RAS blockers, n (%) | 16 (44.4) | 1 (12.5) | 0.125 |
| Calcium channel blockers, n (%) | 4 (11.1) | 0 (0) | 1.000 |
| Anticoagulant therapy, n (%) | 11 (30.6) | 8(100) | <0.001 |
| Pulmonary artery hypertension medications | |||
| Number of PH drugs | 0.008 * | ||
| 1, n (%) | 14 (38.9) | 8 (100) | |
| 2, n (%) | 16 (44.4) | 0 (0) | |
| 3, n (%) | 6 (16.7) | 0 (0) | |
| -Endothelin receptor antagonists | |||
| Bosentan, n (%) | 21 (58.3) | 1 (12.5) | 0.046 * |
| Macitentan, n (%) | 12 (33.3) | 0(0) | 0.084 * |
| Ambricentan, n (%) | 0 (0) | 0 (0) | - |
| -Phosphodiesterase-5 inhibitors | |||
| Sildenafil, n (%) | 4 (11.1) | 0(0) | 0.434 |
| Tadalafil, n (%) | 18 (50) | 0(0) | 0.014 * |
| -Prostacyclin analogs | |||
| Ilioprost (inhalated), n (%) | 5 (13.99) | 0 (0) | 0.566 |
| Epoprostenol (IV), n (%) | 1 (2.8) | 0 (0) | 1 |
| -Riociguat, n (%) | 3 (8.3) | 8 (100) | <0.001 * |
| Study Population (n = 89) r Value | p Value | |
|---|---|---|
| Clinical parameters | ||
| Age, years | −0.055 | 0.644 |
| BMI, kg/m2 | 0.124 | 0.247 |
| Systolic blood pressure, mmHg | −0.030 | 0.777 |
| Diastolic blood pressure, mmHg | 0.030 | 0.783 |
| Heart Rate, beats per minute | 0.144 | 0.178 |
| 6 min walking test, m | −0.310 | 0.003 * |
| Laboratory parameters | ||
| WBC count, ×109/L | 0.041 | 0.705 |
| Hemoglobin, g/dL | −0.040 | 0.707 |
| Fasting blood glucose, mg/dL | −0.065 | 0.545 |
| GFR, mL/per minute | −0.047 | 0.659 |
| ESR, mm/h | −0.010 | 0.990 |
| C-reactive protein, mg/L | 0.043 | 0.686 |
| NT-proBNP, pg/mL | 0.287 | 0.006 |
| Echocardiographic parameters | ||
| LV end-diastolic diameter, mm | 0.034 | 0.752 |
| LVEF, % | −0.016 | 0.885 |
| Systolic PAB, mmHg | 0.241 | 0.023 * |
| TAPSE, mm | −0.295 | 0.005 * |
| RAA, cm2 | 0.194 | 0.068 |
| Cardiac Index, lt/m2 | −0.047 | 0.764 |
| PVR, woods | 0.004 | 0.979 |
| RAP, mmHg | 0.133 | 0.390 |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| B ± SE | 95% CI | p Value | B ± SE | 95% CI | p Value | |
| Heart Rate, beats per minute | 3.012 ± 1.475 | 0.079–5.944 | 0.044 | 1.473 ± 1.591 | −1.690–4.637 | 0.357 |
| 6-MWD, m | −0.363 ± 0.123 | −0.607–(-)0.118 | 0.004 | −0.363 ± 0.123 | −0.607–(-)0.118 | 0.004 * |
| NT-proBNP, pg/mL | 0.012 ± 0.016 | −0.020–0.044 | 0.472 | - | - | - |
| Systolic PAB, mmHg | 1.316 ± 0.714 | −0.103–2.735 | 0.069 | −0.795 ± 1.104 | −2.950–1.441 | 0.496 |
| TAPSE, mm | −12.303 ± 4.873 | −21.988–(-)2.619 | 0.013 | −8.007 ± 9.217 | −26.336–10.323 | 0.388 |
| RAA, cm2 | 6.957 ± 2.751 | 1.489–12.424 | 0.013 | 0.515 ± 7.132 | −13.669–14.700 | 0.943 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tunçez, A.; Yalçın, M.U.; Tezcan, H.; Altunkeser, B.B.; Öztürk, B.; Aydoğan, C.; Toprak, A.; Polat, O.C.; Aygül, N.; Demir, K.; et al. Assessment of Serum suPAR Levels in Patients with Group 1 and Group 4 Pulmonary Hypertension. J. Clin. Med. 2025, 14, 4671. https://doi.org/10.3390/jcm14134671
Tunçez A, Yalçın MU, Tezcan H, Altunkeser BB, Öztürk B, Aydoğan C, Toprak A, Polat OC, Aygül N, Demir K, et al. Assessment of Serum suPAR Levels in Patients with Group 1 and Group 4 Pulmonary Hypertension. Journal of Clinical Medicine. 2025; 14(13):4671. https://doi.org/10.3390/jcm14134671
Chicago/Turabian StyleTunçez, Abdullah, Muhammed Ulvi Yalçın, Hüseyin Tezcan, Bülent Behlül Altunkeser, Bahadır Öztürk, Canan Aydoğan, Aslıhan Toprak, Onur Can Polat, Nazif Aygül, Kenan Demir, and et al. 2025. "Assessment of Serum suPAR Levels in Patients with Group 1 and Group 4 Pulmonary Hypertension" Journal of Clinical Medicine 14, no. 13: 4671. https://doi.org/10.3390/jcm14134671
APA StyleTunçez, A., Yalçın, M. U., Tezcan, H., Altunkeser, B. B., Öztürk, B., Aydoğan, C., Toprak, A., Polat, O. C., Aygül, N., Demir, K., Gürses, K. M., Özen, Y., Akyürek, F., & Tunçez, H. B. (2025). Assessment of Serum suPAR Levels in Patients with Group 1 and Group 4 Pulmonary Hypertension. Journal of Clinical Medicine, 14(13), 4671. https://doi.org/10.3390/jcm14134671






