CO2 Gap Alone Is Not a Prognostic Marker for 28-Day Survival of Patients Undergoing a Transcatheter Aortic Valve Replacement
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Measurement and Data Collection
2.3. Primary and Secondary Outcome
2.4. Statistical Analysis
3. Results
3.1. Baseline, Perioperative and Postoperative Clinical Characteristics
3.2. Primary and Secondary Outcomes
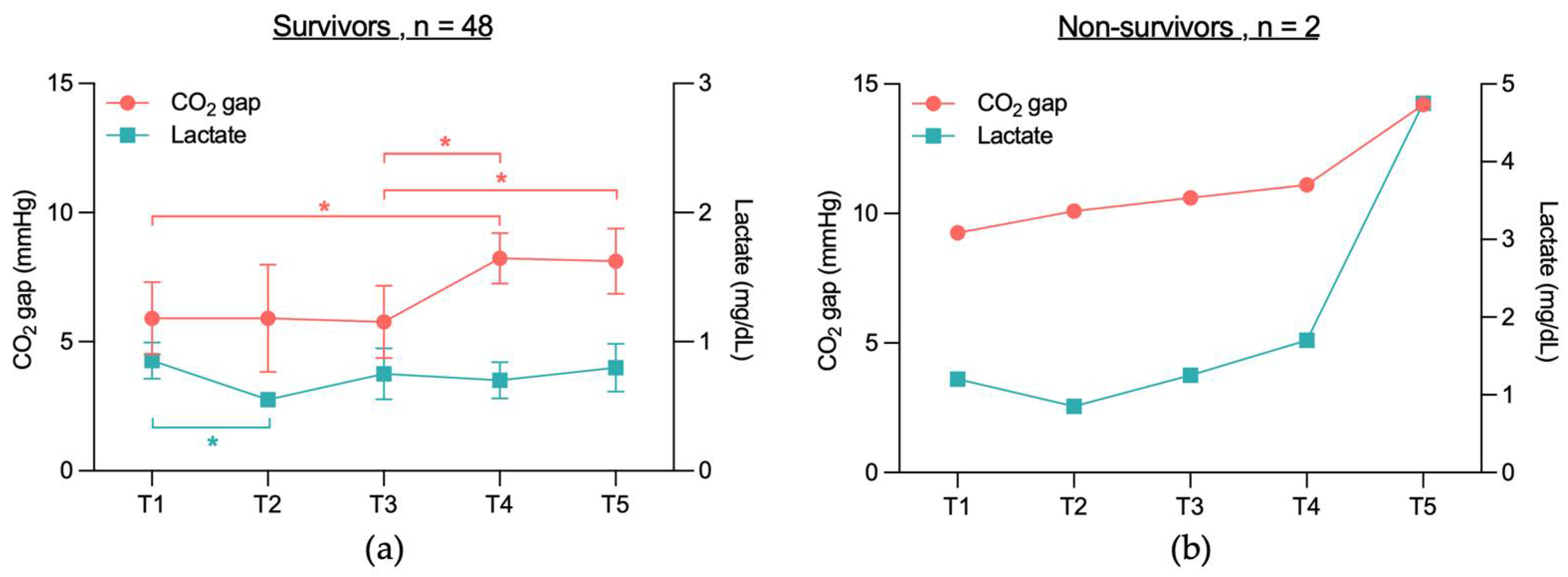
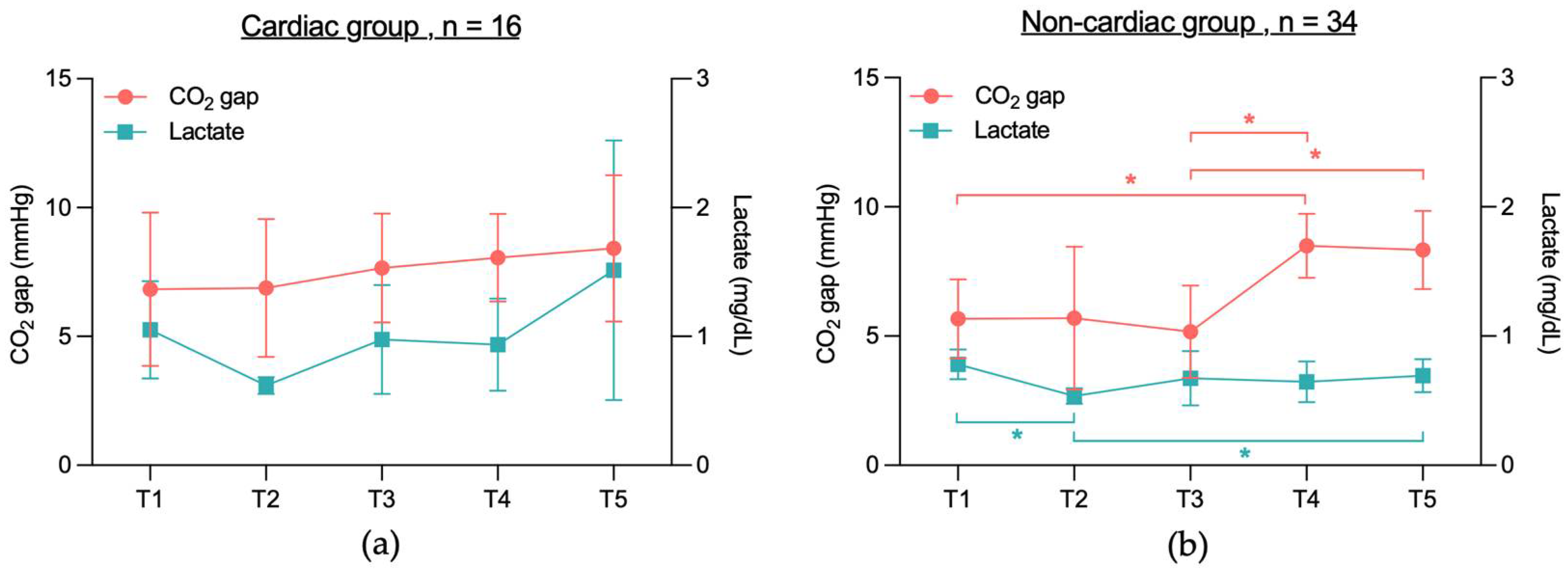
3.2.1. No Significant CO2 Gap Difference Between Cardiac and Non-Cardiac or Between Survivors and Non-Survivors
3.2.2. Limited Predictive Performance of CO2 Gap as a Prognostic Test for 28-Day Survival in Patients Undergoing a TAVR
3.2.3. No Significant Correlation Between CO2 Gap and Lactate Levels in 28-Day Survivors and Non-Survivors, Cardiac or Non-Cardiac Group
3.2.4. Difference in CO2 Content Calculated with the Douglas Equation Between Cardiac and Non-Cardiac Group and Between Survivors and Non-Survivors
3.2.5. Ratio of CO2 Gap to Arterial-to-Venous O2 Content Difference in Survivors and Non-Survivors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CO2 gap | venous-to-arterial difference in partial pressure of carbon dioxide |
| TAVR | transcatheter aortic valve replacement |
| LV-EF | left ventricular ejection fraction |
| ICU | intensive care unit |
| SOFA Score | Sequential organ failure assessment |
| EuroSCORE II | European system for cardiac operative risk evaluation II |
| MACE | major adverse cardiac events |
| NT-proBNP | N-terminal pro B-type natriuretic peptide |
| ROC | Receiver Operating Characteristics |
| AUC | Area Under the Curve |
Appendix A
| Characteristic | Survivor (n = 48) | Non-Survivor (n = 2) | p-Value |
|---|---|---|---|
| Baseline clinical characteristics | |||
| Age (years) | 80 ± 6.4 | 87 ± 4.2 | 0.132 |
| Male/Female | 29/19 (60.4%/39.6%) | 1/1 (50%/50%) | >0.999 |
| BMI (kg/m2) | 28.7 ± 4.4 | 30.2 ± 8.8 | 0.643 |
| Comorbidities | |||
| COPD | 7 (14.6%) | 0 (0%) | >0.999 |
| Pathological pulmonary function test 1 | 5 (10.9%) | 0 (0%) | >0.999 |
| Diabetes mellitus | 16 (33.3%) | 1 (50%) | >0.999 |
| Arterial hypertension | 38 (79.2%) | 2 (100%) | >0.999 |
| Liver cirrhosis | 0 (0%) | 0 (0%) | |
| Chronic kidney disease | 16 (33.3%) | 1 (50%) | >0.999 |
| Cardiac status at study entry | |||
| Chronic heart failure | 34 (70.8%) | 0 (0%) | 0.098 |
| Atrial fibrillation | 25 (52.1%) | 1 (50%) | >0.999 |
| History of myocardial infarction | 12 (25%) | 0 (0%) | >0.999 |
| LV-EF (%) | 52.5 ± 10.9 | 43 ± 24 | 0.589 |
| History of CABG surgery | 5 (10.4%) | 1 (50%) | 0.228 |
| Coronary artery disease (CAD) | 31 (64.6%) | 2 (100%) | 0.542 |
| Preoperative NT-proBNP level 2 (pg/mL) | 2272.3 ± 2893.6 | 3233 ± 1422.7 | 0.206 |
| Scores | |||
| SOFA | 1.4 ± 1 | 2.5 ± 0.7 | 0.147 |
| Euro II Score | 4 ± 3.8 | 9.5 ± 7.1 | 0.080 |
| Perioperative clinical characteristics | |||
| Length of TAVR (minutes) | 56.1 ± 18.7 | 101.5 ± 36.1 | 0.026 * |
| Patients with invasive ventilation during TAVR | 3 (6.3%) | 0 (0%) | >0.999 |
| All complications | 12 (25%) | 2 (100%) | 0.074 |
| CPR | 3 (6.3%) | 0 (0%) | >0.999 |
| Bleeding | 4 (8.3%) | 1 (50%) | 0.192 |
| AV block III° | 5 (10.4%) | 0 (0%) | >0.999 |
| Others 3 | 2 (4.2%) | 2 (100%) | 0.005 * |
| Postoperative clinical characteristics | |||
| Postoperative NT-proBNP level (pg/mL) | 2547.1 ± 3448.3 | 1856.5 ± 381.1 | 0.558 |
| Patients with invasive ventilation during ICU stay | 4 (8.3%) | 1 (50%) | 0.192 |
| Number of patients requiring red blood cell transfusion 4 | 8 (16.7%) | 2 (100%) | 0.037 * |
| All complications | 40 (83%) | 2 (100%) | >0.999 |
| Bleeding at the vascular access site or pseudoaneurysm | 15 (31.3%) | 1 (50%) | 0.542 |
| Mild paravalvular leakage | 16 (33.3%) | 0 (0%) | >0.999 |
| AV block III° | 8 (16.7%) | 0 (0%) | >0.999 |
| Atrial fibrillation requiring therapy 5 | 4 (8.3%) | 0 (0%) | >0.999 |
| New permanent pacemaker implantation | 18 (37.5%) | 0 (0%) | 0.530 |
| Other cardiac arrhythmias 6 | 19 (39.6%) | 0 (0%) | 0.519 |
| Acute kidney injury | 6 (12.5%) | 0 (0%) | >0.999 |
| Infection | 12 (25%) | 0 (0%) | >0.999 |
| Stroke | 1 (2.1%) | 0 (0%) | >0.999 |
| Delirium | 1 (2.1%) | 0 (0%) | >0.999 |
| Others 7 | 16 (33.3%) | 2 (100%) | 0.125 |
| Major adverse cardiac events (MACE) 8 | 0 (0%) | 2 (100%) | <0.001 * |
Appendix B
| T1 | T2 | T3 | T4 | T5 | p-Value | |
|---|---|---|---|---|---|---|
| Arterial CO2 pressure (mmHg) | 42 (36.6–46.2) | 48.5 (41.2–52.3) | 48.2 (43.2–52.1) | 44.7 (5.7) | 41.9 (38–47.2) | <0.001 * |
| Central venous CO2 pressure (mmHg) | 47.8 (43.9–51.5) | 54.3 (49.4–57.8) | 54.2 (49.6–57.1) | 52.1 (49.6–55.4) | 49.7 (46.1–53.2) | <0.001 * |
| SaO2 (%) | 99.4 (98.1–100.1) | 99.9 (99.6–100.2) | 99.8 (99.1–100.1) | 99.8 (98.9–100.2) | 96.6 (94.3–98.3) | <0.001 * |
| SvO2 (%) | 71.5 (8.7) | 74 (7.5) | 76.9 (9.3) | 75.7 (69.1–80.4) | 64.2 (8.7) | <0.001 * |
| aHb (g/dL) | 12.3 (1.7) | 11.3 (1.6) | 10.7 (1.5) | 10.6 (1.6) | 11.5 (1.8) | <0.001 * |
| vHb (g/dL | 12.3 (1.7) | 11.2 (1.7) | 10.6 (1.5) | 10.6 (1.7) | 11.7 (1.7) | <0.001 * |
| Lactate (mg/dL) | 0.8 (0.6–1) | 0.6 (0.5–0.6) | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) | 0.6 (0.5–0.8) | <0.001 * |
| SpO2 (%) | 100 (98.8–100) | 100 (99–100) | 100 (99–100) | 99.5 (98–100) | 96 (93.8–98) | <0.001 * |
| HR (bpm) | 64.5 (61–74.3) | 73 (16) | 74 (63.8–89.5) | 69.5 (60–78) | 60.5 (55–69.3) | <0.001 * |
| MAP (mmHg) | 87 (76–95) | 75 (13) | 79 (14) | 73 (11) | 72 (11) | <0.001 * |
| CVP 1 (mmHg) | 12 (6) | 15 (3) | 17 (4) | 12 (8–18) | 10 (4) | <0.001 * |
| O2 gas flow rate (L/min) | 6 (5.4–6) | 6 (6–6) | 6 (6–6) | 6 (6–6) | 2 (0.8–2) | <0.001 * |
| Infusion rate of norepinephrine (μg/kg/min) | 0.000 (0.0000–0.0000) | 0.0000 (0.0000–0.0324) | 0.0000 (0.0000–0.0000) | 0.0000 (0.0000–0.0235) | 0.0000 (0.0000–0.0000) | <0.001 * |
| Infusion rate of epinephrine (μg/kg/min) | 0.0000 (0.0000–0.0000) | 0.0000 (0.0000–0.0000) | 0.0000 (0.0000–0.0000) | 0.0000 (0.0000–0.0000) | 0.0000 (0.0000–0.0000) | 0.713 |
References
- Van Hemelrijck, M.; Taramasso, M.; De Carlo, C.; Kuwata, S.; Regar, E.; Nietlispach, F.; Ferrero, A.; Weber, A.; Maisano, F. Recent advances in understanding and managing aortic stenosis. F1000Research 2018, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Sharma, R.P.; Cubeddu, R.J.; Aaron, L.; Abdelfattah, O.M.; Koulogiannis, K.P.; Marcoff, L.; Naguib, M.; Kapadia, S.R.; Makkar, R.R.; et al. The Mortality Burden of Untreated Aortic Stenosis. J. Am. Coll. Cardiol. 2023, 82, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Virtanen, M.P.O.; Eskola, M.; Jalava, M.P.; Husso, A.; Laakso, T.; Niemelä, M.; Ahvenvaara, T.; Tauriainen, T.; Maaranen, P.; Kinnunen, E.-M.; et al. Comparison of Outcomes After Transcatheter Aortic Valve Replacement vs Surgical Aortic Valve Replacement Among Patients with Aortic Stenosis at Low Operative Risk. JAMA Netw. Open 2019, 2, e195742. [Google Scholar] [CrossRef]
- Tam, D.Y.; Vo, T.X.; Wijeysundera, H.C.; Ko, D.T.; Rocha, R.V.; Friedrich, J.; Fremes, S.E. Transcatheter vs Surgical Aortic Valve Replacement for Aortic Stenosis in Low-Intermediate Risk Patients: A Meta-analysis. Can. J. Cardiol. 2017, 33, 1171–1179. [Google Scholar] [CrossRef]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Teirstein, P.S.; DeFrain, M.; Muppala, M.; et al. 3-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2023, 81, 1663–1674. [Google Scholar] [CrossRef]
- Blankenberg, S.; Seiffert, M.; Vonthein, R.; Baumgartner, H.; Bleiziffer, S.; Borger, M.A.; Choi, Y.-H.; Clemmensen, P.; Cremer, J.; Czerny, M.; et al. Transcatheter or Surgical Treatment of Aortic-Valve Stenosis. N. Engl. J. Med. 2024, 390, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef] [PubMed]
- Wundram, S.; Seoudy, H.; Dümmler, J.C.; Ritter, L.; Frank, J.; Puehler, T.; Lutter, G.; Lutz, M.; Saad, M.; Bramlage, P.; et al. Is the outcome of elective vs non-elective patients undergoing transcatheter aortic valve implantation different? Results of a single-centre, observational assessment of outcomes at a large university clinic. BMC Cardiovasc. Disord. 2023, 23, 295. [Google Scholar] [CrossRef] [PubMed]
- Dres, M.; Monnet, X.; Teboul, J.-L. Hemodynamic management of cardiovascular failure by using PCO2 venous-arterial difference. J. Clin. Monit. Comput. 2012, 26, 367–374. [Google Scholar] [CrossRef]
- Lamia, B.; Monnet, X.; Teboul, J.L. Meaning of arterio-venous PCO2 difference in circulatory shock. Minerva Anestesiol. 2006, 72, 597–604. [Google Scholar]
- Mekontso-Dessap, A.; Castelain, V.; Anguel, N.; Bahloul, M.; Schauvliege, F.; Richard, C.; Teboul, J.-L. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med. 2002, 28, 272–277. [Google Scholar] [CrossRef]
- Monnet, X.; Julien, F.; Ait-Hamou, N.; Lequoy, M.; Gosset, C.; Jozwiak, M.; Persichini, R.; Anguel, N.; Richard, C.; Teboul, J.-L. Lactate and Venoarterial Carbon Dioxide Difference/Arterial-Venous Oxygen Difference Ratio, but Not Central Venous Oxygen Saturation, Predict Increase in Oxygen Consumption in Fluid Responders*. Crit. Care Med. 2013, 41, 1412–1420. [Google Scholar] [CrossRef]
- Muller, G.; Mercier, E.; Vignon, P.; Henry-Lagarrigue, M.; Kamel, T.; Desachy, A.; Botoc, V.; Plantefève, G.; Frat, J.; Bellec, F.; et al. Prognostic significance of central venous-to-arterial carbon dioxide difference during the first 24 hours of septic shock in patients with and without impaired cardiac function. Br. J. Anaesth. 2017, 119, 239–248. [Google Scholar] [CrossRef]
- Cuschieri, J.; Rivers, E.P.; Donnino, M.W.; Katilius, M.; Jacobsen, G.; Nguyen, H.B.; Pamukov, N.; Horst, H.M. Central venous-arterial carbon dioxide difference as an indicator of cardiac index. Intensive Care Med. 2005, 31, 818–822. [Google Scholar] [CrossRef]
- Robin, E.; Futier, E.; Pires, O.; Fleyfel, M.; Tavernier, B.; Lebuffe, G.; Vallet, B. Central venous-to-arterial carbon dioxide difference as a prognostic tool in high-risk surgical patients. Crit. Care 2015, 19, 227. [Google Scholar] [CrossRef]
- Futier, E.; Robin, E.; Jabaudon, M.; Guerin, R.; Petit, A.; Bazin, J.-E.; Constantin, J.-M.; Vallet, B. Central venous O2 saturation and venous-to-arterial CO2 difference as complementary tools for goal-directed therapy during high-risk surgery. Crit. Care 2010, 14, R193. [Google Scholar] [CrossRef] [PubMed]
- EuroScore Website—Calculator, n.d. Available online: https://www.euroscore.org/index.php?id=17&lang=en (accessed on 9 November 2024).
- Sequential Organ Failure Assessment (SOFA) Score n.d. Available online: https://www.mdcalc.com/calc/691/sequential-organ-failure-assessment-sofa-score (accessed on 14 February 2025).
- Mallat, J.; Vallet, B. Ratio of venous-to-arterial PCO2 to arteriovenous oxygen content difference during regional ischemic or hypoxic hypoxia. Sci. Rep. 2021, 11, 10172. [Google Scholar] [CrossRef]
- Created in BioRender: Scientific Image and Illustration Software. Available online: https://BioRender.com (accessed on 21 June 2025).
- Ospina-Tascón, G.A.; Bautista-Rincón, D.F.; Umaña, M.; Tafur, J.D.; Gutiérrez, A.; García, A.F.; Bermúdez, W.; Granados, M.; Arango-Dávila, C.; Hernández, G. Persistently high venous-to-arterial carbon dioxide differences during early resuscitation are associated with poor outcomes in septic shock. Crit. Care 2013, 17, R294. [Google Scholar] [CrossRef]
- Vallée, F.; Vallet, B.; Mathe, O.; Parraguette, J.; Mari, A.; Silva, S.; Samii, K.; Fourcade, O.; Genestal, M. Central venous-to-arterial carbon dioxide difference: An additional target for goal-directed therapy in septic shock? Intensive Care Med. 2008, 34, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Köhler, W.M.; Freitag-Wolf, S.; Lambers, M.; Lutz, M.; Niemann, P.M.; Petzina, R.; Lutter, G.; Bramlage, P.; Frey, N.; Frank, D. Preprocedural but not periprocedural high-sensitive Troponin T levels predict outcome in patients undergoing transcatheter aortic valve implantation. Cardiovasc. Ther. 2016, 34, 385–396. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Stortecky, S.; Franzone, A.; O’Sullivan, C.J.; Praz, F.; Zuk, K.; Räber, L.; Pilgrim, T.; Moschovitis, A.; Fiedler, G.M.; et al. Post-Procedural Troponin Elevation and Clinical Outcomes Following Transcatheter Aortic Valve Implantation. J. Am. Heart Assoc. 2016, 5, e002430. [Google Scholar] [CrossRef] [PubMed]
- Stähli, B.E.; Gebhard, C.; Saleh, L.; Falk, V.; Landmesser, U.; Nietlispach, F.; Maisano, F.; Lüscher, T.F.; Maier, W.; Binder, R.K. N-terminal pro-B-type natriuretic peptide-ratio predicts mortality after transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2015, 85, 1240–1247. [Google Scholar] [CrossRef]
- Mecher, C.E.; Rackow, E.C.; Astiz, M.E.; Weil, M.H. Venous hypercarbia associated with severe sepsis and systemic hypoperfusion. Crit. Care Med. 1990, 18, 585–589. [Google Scholar] [CrossRef]
- Raicea, V.; Kovacs, J.; Moraru, L.; Suciu, H. Coronary Sinus Lactate as Marker of Myocardial Ischemia in Cardiac Surgery: Correlation with Morbidity and Mortality after Cardiac Surgery/Lactatul din sinusul coronarian-marker al ischemiei miocardice în chirurgia cardiacă: Corelaţii cu morbiditatea şi mortalitatea postoperatorie. Rom. Rev. Lab. Med. 2015, 23, 189–197. [Google Scholar] [CrossRef]
- Nguyen, H.B.; Rivers, E.P.; Knoblich, B.P.; Jacobsen, G.; Muzzin, A.; Ressler, J.A.; Tomlanovich, M.C. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock*. Crit. Care Med. 2004, 32, 1637–1642. [Google Scholar] [CrossRef]
- Meregalli, A.; Oliveira, R.P.; Friedman, G. Occult hypoperfusion is associated with increased mortality in hemodynamically stable, high-risk, surgical patients. Crit. Care 2004, 8, R60. [Google Scholar] [CrossRef]
- Zymliński, R.; Biegus, J.; Sokolski, M.; Siwołowski, P.; Nawrocka-Millward, S.; Todd, J.; Jankowska, E.A.; Banasiak, W.; Cotter, G.; Cleland, J.G.; et al. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur. J. Heart Fail. 2018, 20, 1011–1018. [Google Scholar] [CrossRef]
- Contenti, J.; Occelli, C.; Corraze, H.; Lemoël, F.; Levraut, J. Long-Term β-Blocker Therapy Decreases Blood Lactate Concentration in Severely Septic Patients*. Crit. Care Med. 2015, 43, 2616–2622. [Google Scholar] [CrossRef] [PubMed]
- Contenti, J.; Occelli, C.; Lemoel, F.; Ferrari, P.; Levraut, J. Blood lactate measurement within the emergency department: A two-year retrospective analysis. Am. J. Emerg. Med. 2019, 37, 401–406. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Quintairos e Silva, A.; Couto, L.; Taccone, F.S. The value of blood lactate kinetics in critically ill patients: A systematic review. Crit. Care 2016, 20, 257. [Google Scholar] [CrossRef]
- Sun, X.-G.; Hansen, J.E.; Stringer, W.W.; Ting, H.; Wasserman, K. Carbon dioxide pressure-concentration relationship in arterial and mixed venous blood during exercise. J. Appl. Physiol. 2001, 90, 1798–1810. [Google Scholar] [CrossRef] [PubMed]
- Teboul, J.-L.; Scheeren, T. Understanding the Haldane effect. Intensive Care Med. 2017, 43, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Jakob, S.M.; Groeneveld, A.B.J.; Teboul, J.-L. Venous–arterial CO2 to arterial–venous O2 difference ratio as a resuscitation target in shock states? Intensive Care Med. 2015, 41, 936–938. [Google Scholar] [CrossRef]
- Ospina-Tascón, G.A.; Umaña, M.; Bermúdez, W.; Bautista-Rincón, D.F.; Hernandez, G.; Bruhn, A.; Granados, M.; Salazar, B.; Arango-Dávila, C.; De Backer, D. Combination of arterial lactate levels and venous-arterial CO2 to arterial-venous O2 content difference ratio as markers of resuscitation in patients with septic shock. Intensive Care Med. 2015, 41, 796–805. [Google Scholar] [CrossRef]
- Mesquida, J.; Saludes, P.; Gruartmoner, G.; Espinal, C.; Torrents, E.; Baigorri, F.; Artigas, A. Central venous-to-arterial carbon dioxide difference combined with arterial-to-venous oxygen content difference is associated with lactate evolution in the hemodynamic resuscitation process in early septic shock. Crit. Care 2015, 19, 126. [Google Scholar] [CrossRef]
- Heuer, J.F.; Sauter, P.; Pelosi, P.; Herrmann, P.; Brück, W.; Perske, C.; Schöndube, F.; A Crozier, T.; Bleckmann, A.; Beißbarth, T.; et al. Effects of pulmonary acid aspiration on the lungs and extra-pulmonary organs: A randomized study in pigs. Crit. Care 2012, 16, R35. [Google Scholar] [CrossRef] [PubMed]
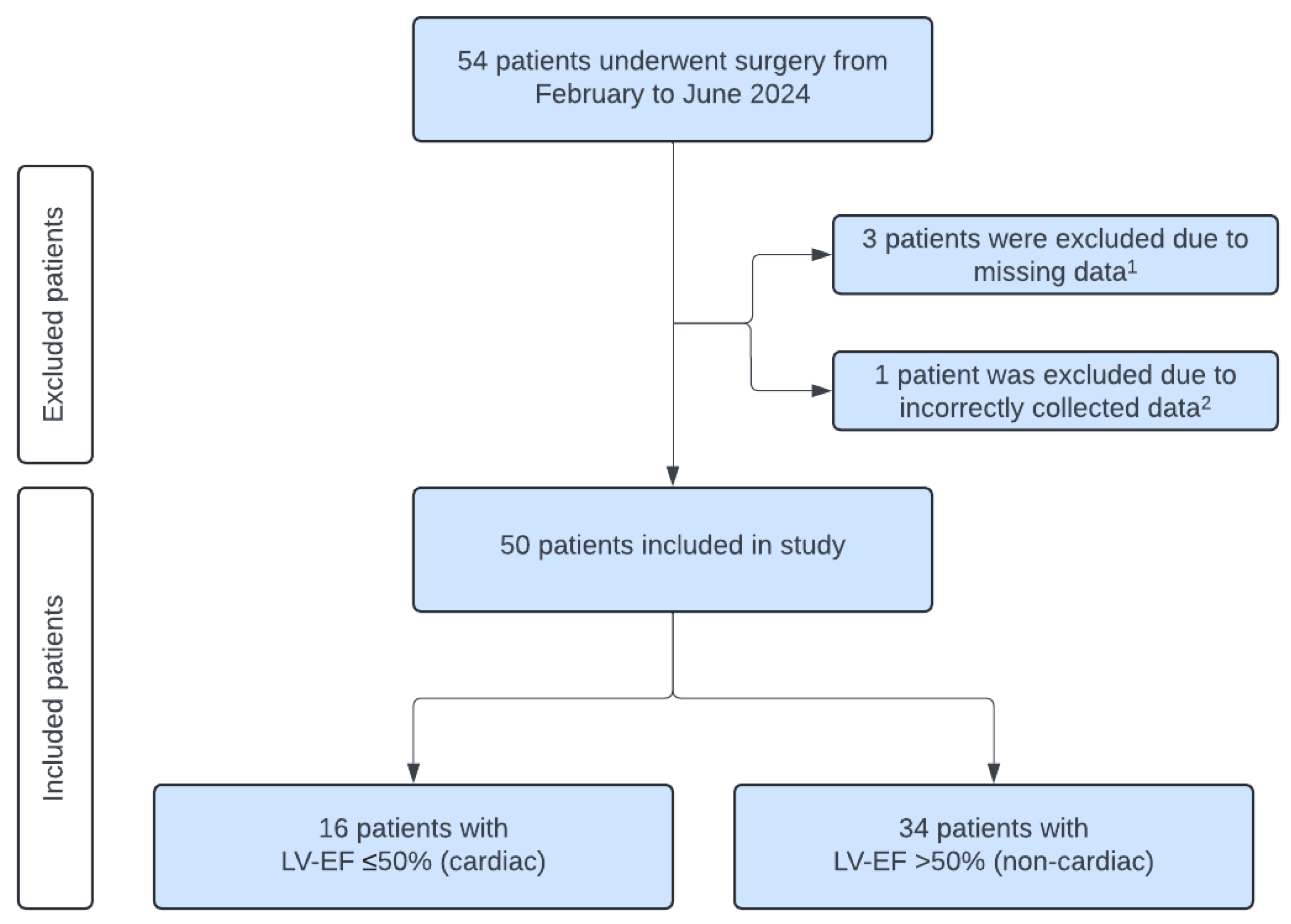
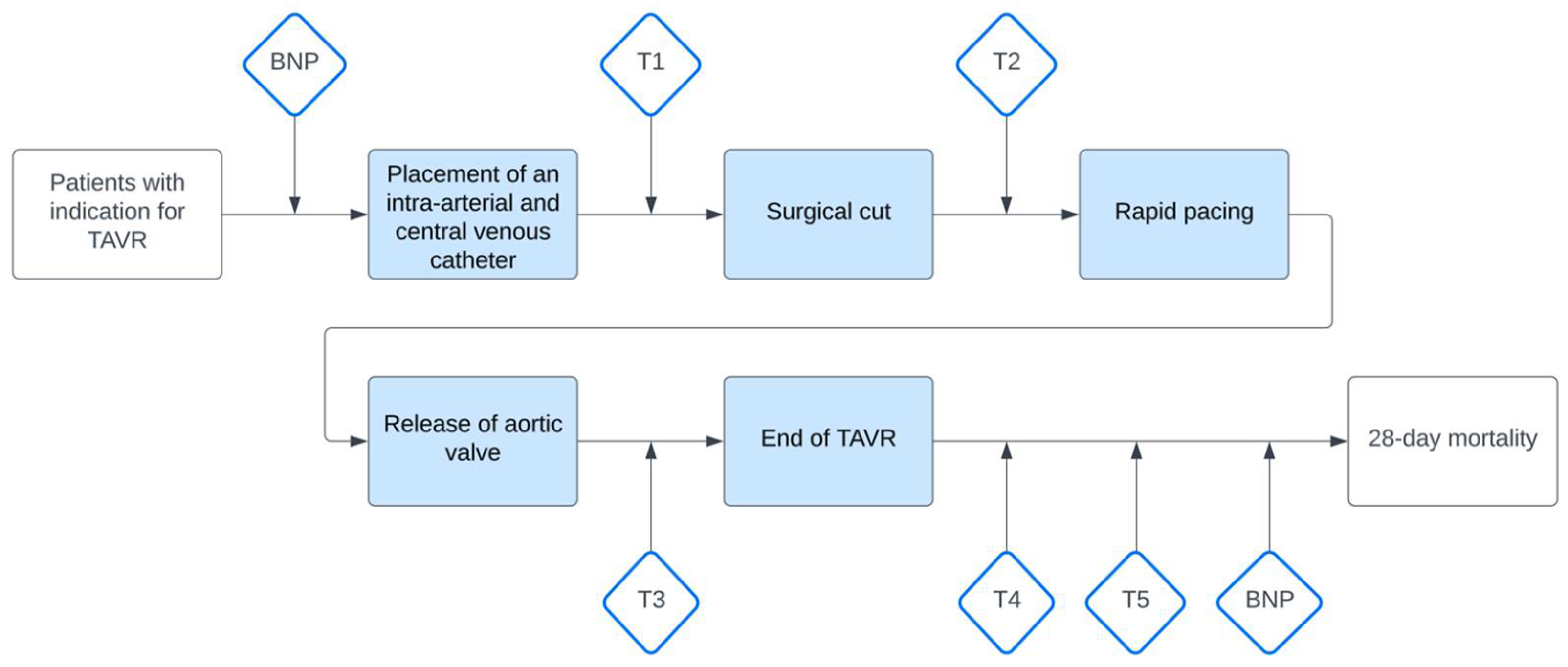
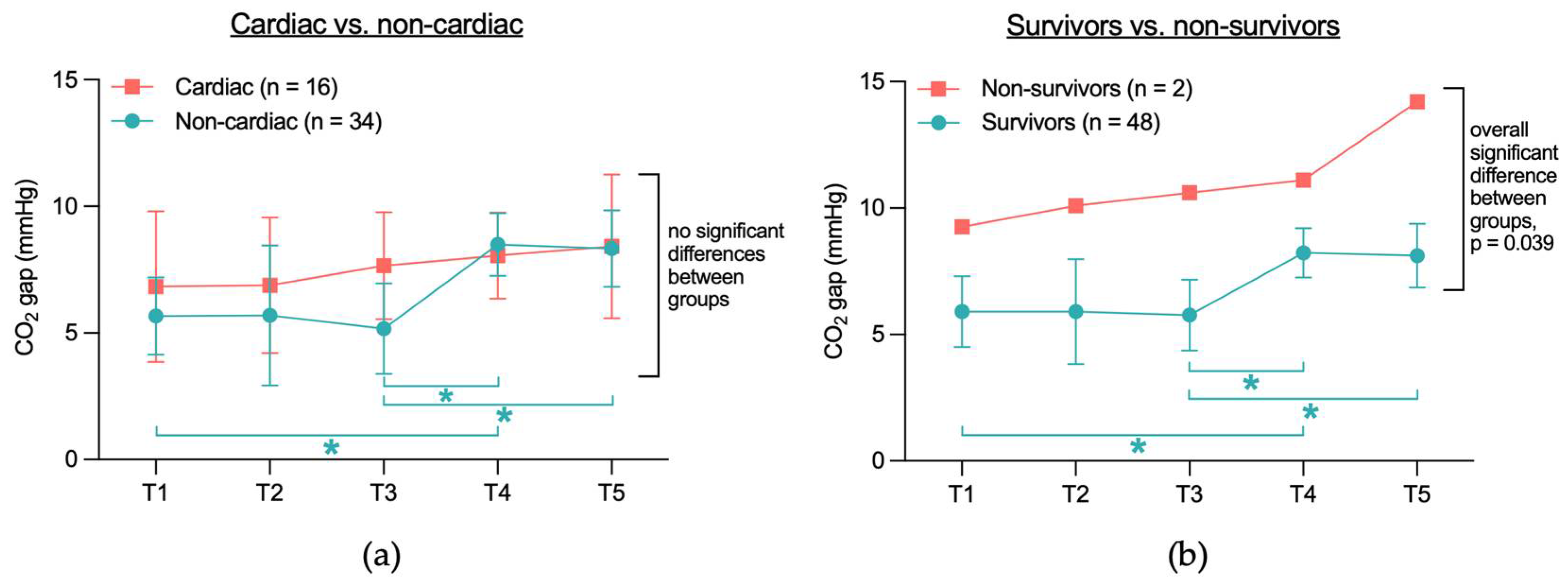
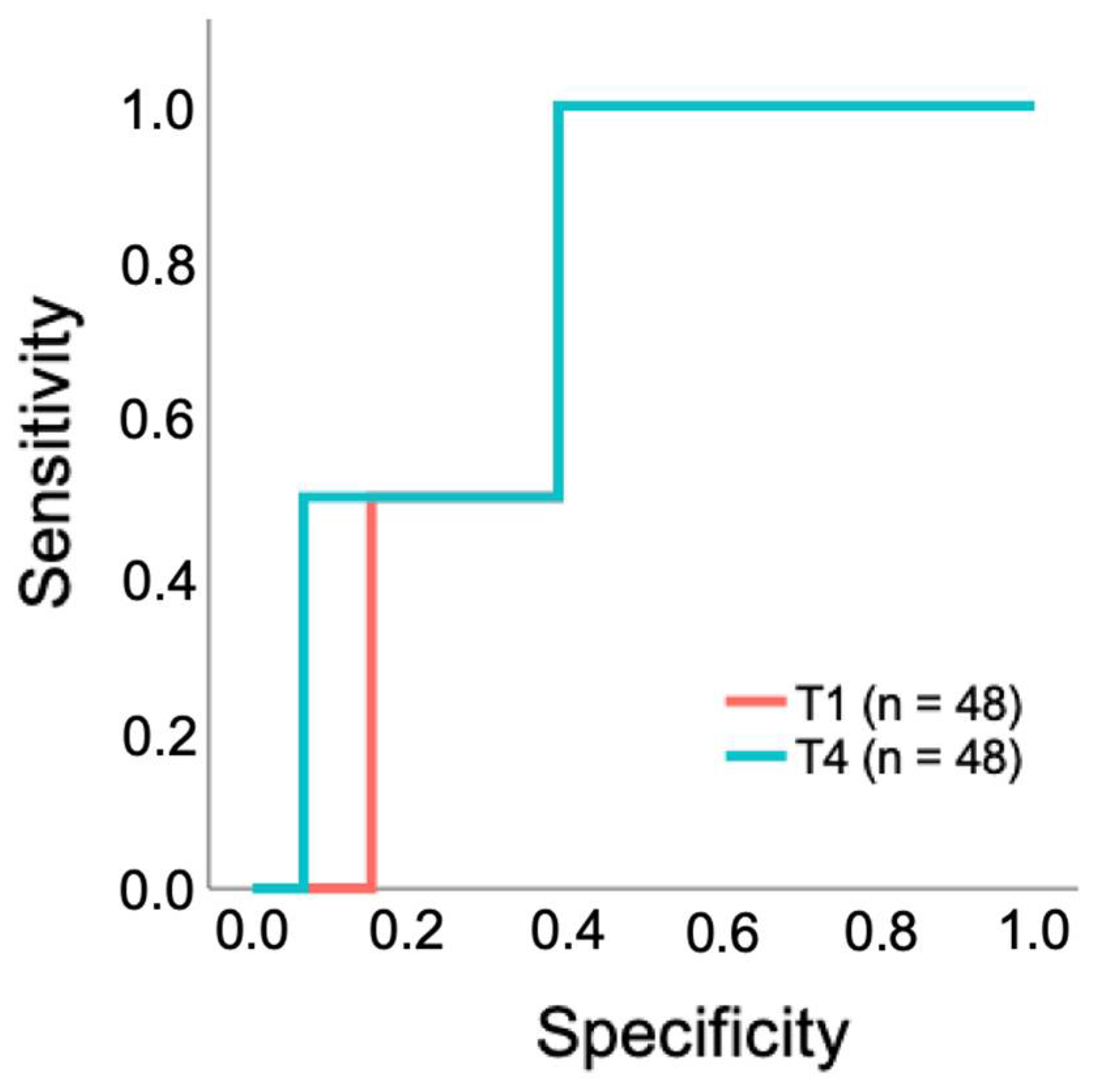


| Characteristic | All Patients n = 50 | Non-Cardiac n = 34 | Cardiac n = 16 | p-Value |
|---|---|---|---|---|
| Baseline clinical characteristics | ||||
| Age (years) | 82 (76–86) | 82 (75–86) | 83 (79–85) | 0.851 |
| Male/Female | 30/20 (60%/40%) | 17/17 (50%/50%) | 13/3 (81.3%/18.8%) | 0.062 |
| BMI (kg/m2) | 28.6 (25.7–31) | 28.7 (24.8–31.7) | 27.6 (25.8–30) | 0.835 |
| Comorbidities | ||||
| COPD | 7 (14%) | 5 (14.7%) | 2 (12.5%) | >0.999 |
| Pathological pulmonary function test 1 | 5 (10.4%) | 3 (8.8%) | 2 (14.3%) | 0.621 |
| Diabetes mellitus | 17 (34%) | 12 (35.3%) | 5 (31.3%) | >0.999 |
| Arterial hypertension | 40 (80%) | 27 (79.4%) | 13 (81.3%) | >0.999 |
| Liver cirrhosis | 0 (0%) | 0 (0%) | 0 (0%) | >0.999 |
| Chronic kidney disease | 17 (34%) | 10 (29.4%) | 7 (43.8%) | 0.353 |
| Cardiac status at study entry | ||||
| Chronic heart failure | 34 (68%) | 21 (61.8%) | 13 (81.3%) | 0.208 |
| Atrial fibrillation | 26 (52%) | 14 (41.2%) | 12 (75%) | 0.035 * |
| History of myocardial infarction | 12 (24%) | 6 (17.6%) | 6 (37.5%) | 0.163 |
| LV-EF (%) | 57.5 (48.8–60) | 60 (57–60) | 37.5 (29.3–48.8) | < 0.001 * |
| History of CABG surgery | 6 (12%) | 5 (14.7%) | 1 (6.3%) | 0.650 |
| Coronary artery disease (CAD) | 33 (66%) | 18 (52.9%) | 15 (93.8%) | 0.004 * |
| Preoperative NT-proBNP level 2 (pg/mL) | 1434 (678.5–2659) | 1089 (479–1914) | 2599 (1915–6154) | 0.001 * |
| Scores | ||||
| SOFA | 2 (0.8–2) | 2 (0–2) | 2 (1–2) | 0.539 |
| EuroSCORE II | 3.1 (1.8–4.3) | 2.8 (1.8–3.7) | 3.4 (2.2–5.7) | 0.114 |
| Perioperative clinical characteristics | ||||
| Length of TAVR (min) | 53.5 (42.3–67) | 53 (42–63) | 58 (45–72) | 0.355 |
| Patients with invasive ventilation during TAVR | 3 (6%) | 2 (5.9%) | 1 (6.3%) | >0.999 |
| All complications | 14 (28%) | 9 (26.5%) | 5 (31.3%) | 0.746 |
| CPR | 3 (6%) | 1 (2.9%) | 2 (12.5%) | 0.237 |
| Bleeding | 5 (10%) | 3 (8.8%) | 2 (12.5%) | 0.650 |
| AV block III° | 5 (10%) | 5 (14.7%) | 0 (0%) | 0.163 |
| Others 3 | 4 (8%) | 2 (5.9%) | 2 (12.5%) | 0.584 |
| Postoperative clinical characteristics | ||||
| Postoperative NT-proBNP level (pg/mL) | 1493 (715–2498) | 1180 (529–1944) | 2547 (957–7202) | 0.007 * |
| Patients with invasive ventilation during ICU stay | 5 (10%) | 3 (8.8%) | 2 (12.5%) | 0.650 |
| Number of patients requiring red blood cell transfusion 4 | 10 (20%) | 5 (14.7%) | 5 (31.3%) | 0.256 |
| All complications | 42 (84%) | 27 (79.4%) | 15 (93.8%) | 0.409 |
| Bleeding at the vascular access site or pseudoaneurysm | 16 (32%) | 10 (29.4%) | 6 (37.5%) | 0.746 |
| Mild paravalvular leakage | 16 (32%) | 12 (35.3%) | 4 (25%) | 0.533 |
| AV block III° | 18 (36%) | 13 (38.2%) | 5 (31.3%) | 0.757 |
| Atrial fibrillation requiring therapy 5 | 4 (8%) | 2 (5.9%) | 2 (12.5%) | 0.584 |
| New permanent pacemaker implantation | 18 (36%) | 13 (38.2%) | 5 (31.3%) | 0.757 |
| Other cardiac arrhythmias 6 | 19 (38%) | 12 (35.3%) | 7 (43.8%) | 0.756 |
| Acute kidney injury | 6 (12%) | 4 (11.8%) | 2 (12.5%) | >0.999 |
| Infection 7 | 12 (24%) | 5 (14.7%) | 7 (43.8%) | 0.036 * |
| Stroke | 1 (2%) | 1 (2.9%) | 0 (0%) | >0.999 |
| Delirium | 1 (2%) | 1 (2.9%) | 0 (0%) | >0.999 |
| Others 8 | 18 (36%) | 12 (35.3%) | 6 (37.5%) | >0.999 |
| Major adverse cardiac events (MACE) 9 | 2 (4%) | 1 (2.9%) | 1 (6.3%) | 0.542 |
| Characteristic | All Patients n = 50 | Non-Cardiac n = 34 | Cardiac n = 16 | p-Value | ANOVA Adjusted p-Value 1 |
|---|---|---|---|---|---|
| 28-day mortality 2 | 2 (4.2%) | 1 (3.1%) | 1 (6.3%) | 0.560 | 0.796 |
| ICU mortality | 2 (4%) | 1 (2.9%) | 1 (6.3%) | 0.542 | 0.876 |
| ICU readmission | 2 (4%) | 2 (5.9%) | 0 (0%) | 0.458 | 0.297 |
| ICU LOS (days) | 1 (1–2) | 1 (1–2) | 1 (1–1.8) | 0.928 | 0.687 |
| Hospital LOS (days) | 10.5 (7.5–2) | 9 (6.8–21) | 14 (9.3–21) | 0.172 | 0.309 |
| Clinical Variable | Overall Model Quality | Optimal Cutoff | Youden Index | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|
| T1 CO2 gap | 0.53 | 7.3 mmHg | 0.609 | 100 | 61 | 0.728 |
| T4 CO2 gap | 0.52 | 8.7 mmHg | 0.609 | 100 | 61 | 0.772 |
| Survivors Group | p-Value | Spearman r |
|---|---|---|
| CO2 gap T1 vs. lactate T1 | 0.103 | 0.238 |
| CO2 gap T2 vs. lactate T2 | 0.778 | 0.042 |
| CO2 gap T3 vs. lactate T3 | 0.329 | −0.144 |
| CO2 gap T4 vs. lactate T4 | 0.957 | −0.008 |
| CO2 gap T5 vs. lactate T5 | 0.746 | −0.048 |
| a: Cardiac group | p-Value | Spearman r |
| CO2 gap T1 vs. lactate T1 | 0.121 | 0.404 |
| CO2 gap T2 vs. lactate T2 | 0.039 * | 0.525 |
| CO2 gap T3 vs. lactate T3 | 0.720 | −0.096 |
| CO2 gap T4 vs. lactate T4 | 0.705 | 0.102 |
| CO2 gap T5 vs. lactate T5 | 0.422 | 0.214 |
| b: Non-cardiac group | p-Value | Spearman r |
| CO2 gap T1 vs. lactate T1 | 0.367 | 0.160 |
| CO2 gap T2 vs. lactate T2 | 0.679 | −0.074 |
| CO2 gap T3 vs. lactate T3 | 0.205 | −0.223 |
| CO2 gap T4 vs. lactate T4 | 0.714 | −0.065 |
| CO2 gap T5 vs. lactate T5 | 0.859 | −0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiehoff, L.; Simons, J.A.; Wiegand, S.B.; Schälte, G.; Schröder, J.W.; Fischbach, A. CO2 Gap Alone Is Not a Prognostic Marker for 28-Day Survival of Patients Undergoing a Transcatheter Aortic Valve Replacement. J. Clin. Med. 2025, 14, 4612. https://doi.org/10.3390/jcm14134612
Thiehoff L, Simons JA, Wiegand SB, Schälte G, Schröder JW, Fischbach A. CO2 Gap Alone Is Not a Prognostic Marker for 28-Day Survival of Patients Undergoing a Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine. 2025; 14(13):4612. https://doi.org/10.3390/jcm14134612
Chicago/Turabian StyleThiehoff, Lisa, Julia Alexandra Simons, Steffen B. Wiegand, Gereon Schälte, Jörg W. Schröder, and Anna Fischbach. 2025. "CO2 Gap Alone Is Not a Prognostic Marker for 28-Day Survival of Patients Undergoing a Transcatheter Aortic Valve Replacement" Journal of Clinical Medicine 14, no. 13: 4612. https://doi.org/10.3390/jcm14134612
APA StyleThiehoff, L., Simons, J. A., Wiegand, S. B., Schälte, G., Schröder, J. W., & Fischbach, A. (2025). CO2 Gap Alone Is Not a Prognostic Marker for 28-Day Survival of Patients Undergoing a Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine, 14(13), 4612. https://doi.org/10.3390/jcm14134612







