Exploring the Effects of Manual Therapy on Somatosensory Tinnitus and Dizziness: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
- The patient can alter their tinnitus by voluntarily moving their head, neck, jaw, or eyes.
- The patient can change their tinnitus through specific somatic maneuvers.
- Tinnitus can be influenced by applying pressure to myofascial trigger points.

2.1. Measurements
2.2. Tinnitus Handicap Inventory (THI) Questionnaire
2.3. Dizziness Handicap Inventory (DHI)
2.4. Randomization Procedure
2.5. Statistical Methods
3. Results
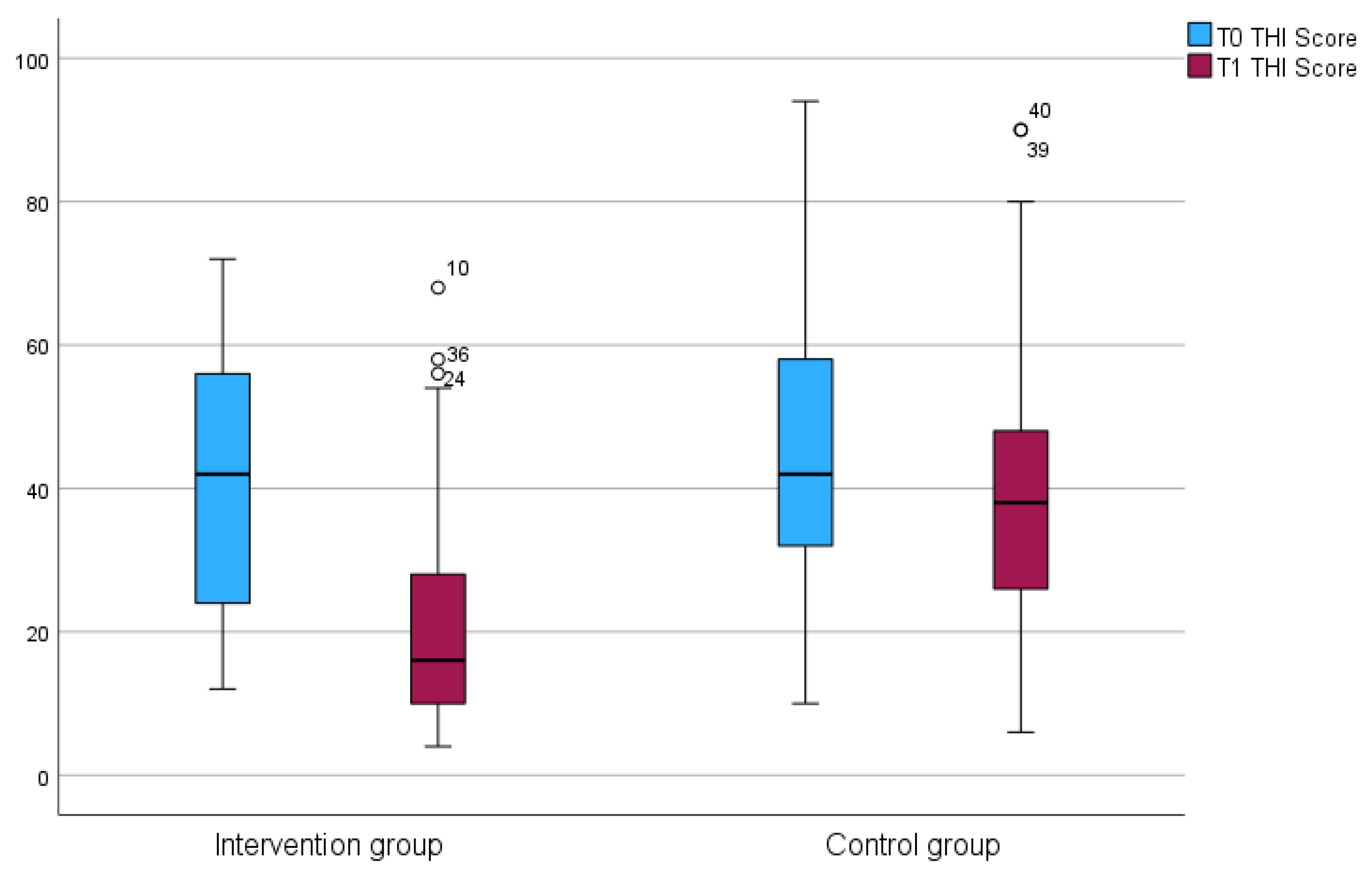
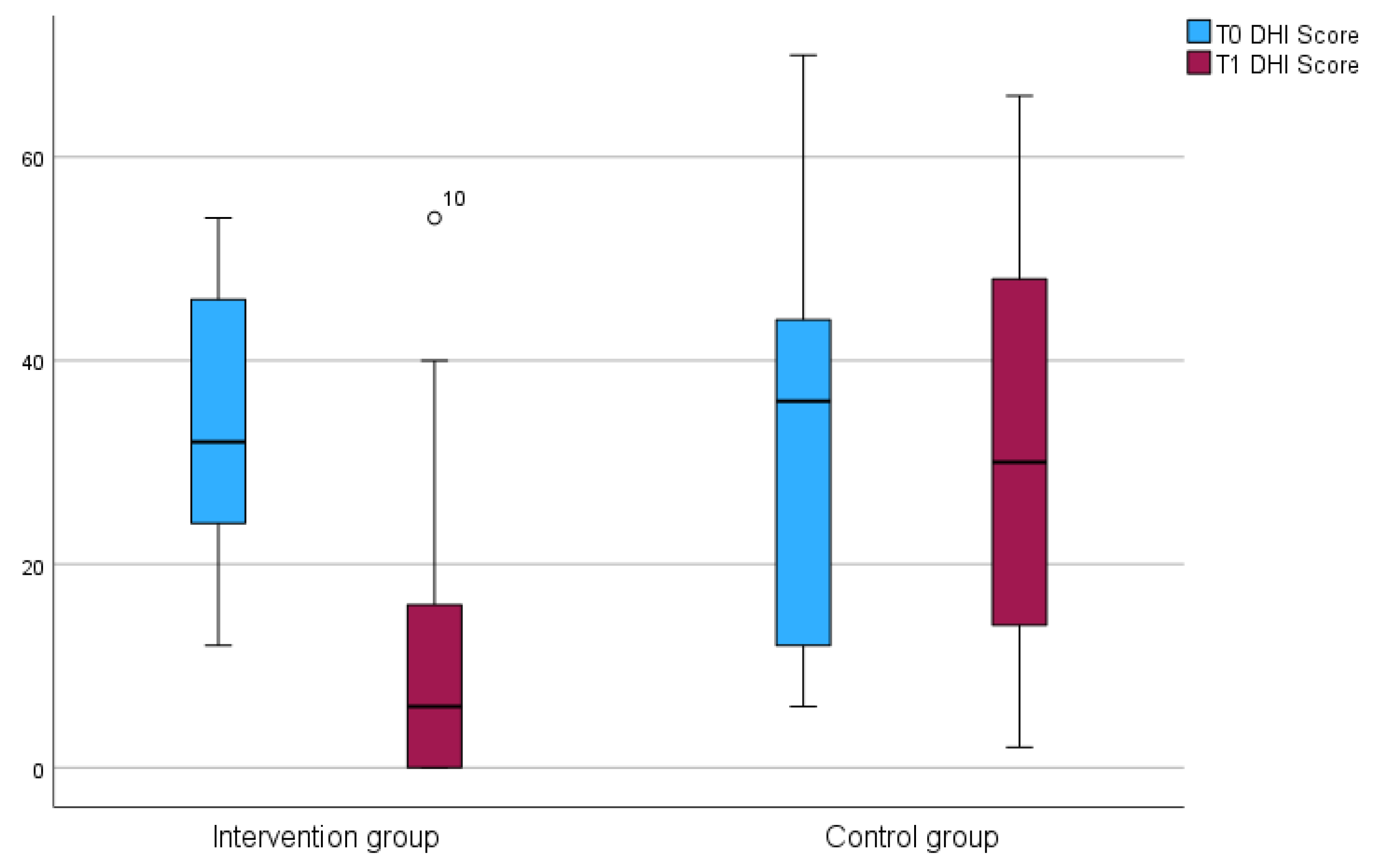
3.1. Modulation of Tinnitus and Dizziness
3.2. Pressure Pain of Head and Neck Muscles
3.3. Cervical Spine Mobility
3.4. Experiencing Tinnitus
3.5. Experiencing Dizziness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| THI | Tinnitus Handicap Inventory Questionnaire |
| DHI | Dizziness Handicap Inventory |
| CST | Cervicogenic somatosensory tinnitus |
References
- Jarach, C.M.; Lugo, A.; Scala, M.; van den Brandt, P.A.; Cederroth, C.R.; Odone, A.; Garavello, W.; Schlee, W.; Langguth, B.; Gallus, S. Global Prevalence and Incidence of Tinnitus: A Systematic Review and Meta-analysis. JAMA Neurol. 2022, 79, 888–900. [Google Scholar] [CrossRef]
- Landgrebe, M.; Azevedo, A.; Baguley, D.; Bauer, C.; Cacace, A.; Coelho, C.; Dornhoffer, J.; Figueiredo, R.; Flor, H.; Hajak, G.; et al. Methodological aspects of clinical trials in tinnitus: A proposal for an international standard. J. Psychosom. Res. 2012, 73, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, A.A.; Renton, J. A review of tinnitus. Aust. J. Gen. Pract. 2018, 47, 205–208. [Google Scholar] [CrossRef]
- Molnár, A.; Molnár, V.; Mavrogeni, P.; Maihoub, S. Fasting Glucose, Haemoglobin A1C (HbA1c), Blood Lipid, and Triglyceride–Glucose Index Parameters in Relation to Subjective Tinnitus. Biomedicines 2025, 13, 824. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, B.; Szczepek, A.J.; Brüggemann, P. Tinnitus–Klinik und Therapie. Laryngo-Rhino-Otol. 2017, 96, 47–59. [Google Scholar] [CrossRef]
- Biesinger, E.; Groth, A.; Höing, R.; Hölzl, M. Somatosensorischer Tinnitus. HNO 2015, 63, 266–271. [Google Scholar] [CrossRef]
- Demoen, S.; Cardon, E.; Jacquemin, L.; Timmermans, A.; Van Rompaey, V.; Gilles, A.; Michiels, S. Health-Related Quality of Life in Subjective, Chronic Tinnitus Patients: A Scoping Review. J. Assoc. Res. Otolaryngol. 2024, 25, 103–129. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Jung, J.; Kim, J.; Lee, Y. Tinnitus and Its Association with Mental Health and Health-Related Quality of Life in an Older Population: A Nationwide Cross-Sectional Study. J. Appl. Gerontol. 2022, 41, 181–186. [Google Scholar] [CrossRef]
- Molnár, A.; Mavrogeni, P.; Tamás, L.; Maihoub, S. Correlation Between Tinnitus Handicap and Depression and Anxiety Scores. Ear Nose Throat J. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Michiels, S.; Ganz Sanchez, T.; Oron, Y.; Gilles, A.; Haider, H.F.; Erlandsson, S.; Bechter, K.; Vielsmeier, V.; Biesinger, E.; Nam, E.C.; et al. Diagnostic Criteria for Somatosensory Tinnitus: A Delphi Process and Face-to-Face Meeting to Establish Consensus. Trends Hear. 2018, 22, 2331216518796403. [Google Scholar] [CrossRef]
- Levine, R.A. Somatic modulation appears to be a fundamental attribute of tinnitus. In Proceedings of the Sixth International Tinnitus Seminar; The Tinnitus and Hyperacusis Center: London, UK, 1999. [Google Scholar]
- Biesinger, E. Funktionelle Störungen der Halswirbelsäule in ihrer Bedeutung für die Hals-Nasen-Ohrenheilkunde. In HNO Praxis Heute; Springer: Berlin/Heidelberg, Germany, 1989; pp. 129–147. [Google Scholar]
- Yacovino, D.A.; Hain, T.C. Clinical Characteristics of Cervicogenic-Related Dizziness and Vertigo. Semin. Neurol. 2013, 33, 244–255. [Google Scholar] [PubMed]
- Brandt, T. Cervical Vertigo–Reality or Fiction? Audiol. Neurotol. 1996, 1, 187–196. [Google Scholar] [CrossRef]
- Heymann, W.V.; Köneke, C. Tinnitus bei “Hirnstamm-Irritations-Syndrom”. Man. Med. 2009, 47, 239–246. [Google Scholar] [CrossRef]
- Shore, S.; Zhou, J.; Koehler, S. Neural mechanisms underlying somatic tinnitus. In Progress in Brain Research; Elsevier Science & Technology: Amsterdam, The Netherlands, 2007; pp. 107–123. [Google Scholar]
- Shore, S.E.; Roberts, L.E.; Langguth, B. Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nat. Rev. Neurol. 2016, 12, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Schiebler, T.H.; Korf, H. Anatomie; Steinkopff: Heidelberg, Germany, 2007. [Google Scholar]
- Yu, H.Z.; Gong, J.M.; Hong, G.W.; Zhou, R.Q.; Fu, X.P.; Fan, T.; Zheng, Y.Q.; Peng, Y.Q.; Li, J.; Wang, Y.F. The Effect of Physical Therapy on Somatosensory Tinnitus. J. Clin. Med. 2024, 13, 3496. [Google Scholar] [CrossRef] [PubMed]
- Cherian, K.; Cherian, N.; Cook, C.; Kaltenbach, J.A. Improving Tinnitus with Mechanical Treatment of the Cervical Spine and Jaw. J. Am. Acad. Audiol. 2013, 24, 544–555. [Google Scholar] [CrossRef]
- Delgado De La Serna, P.; Plaza-Manzano, G.; Cleland, J.; Fernández-De-Las-Peñas, C.; Martín-Casas, P.; Díaz-Arribas, M.J. Effects of cervico-mandibular manual therapy in patients with temporomandibular pain disorders and associated somatic tinnitus: A randomized clinical trial. Pain Med. 2020, 21, 613–624. [Google Scholar] [CrossRef]
- Fobbe, A.; Bökel, A.; Lesinski-Schiedat, A.; Gutenbrunner, C.; Sturm, C. Pilotstudie: Manualmedizinische Methodenevaluation zur Modulierbarkeit des Leitsymptoms Tinnitus. HNO 2022, 70, 675–684. [Google Scholar] [CrossRef]
- DGHNO-KHC. S3-Leitlinie Chronischer Tinnitus. 2021. Available online: https://register.awmf.org/de/leitlinien/detail/017-064 (accessed on 18 June 2025).
- Travell, J.; Simons, D.G. Handbuch der Muskel-Triggerpunkte; Urban & Fischer: Jena, Germany, 2002. [Google Scholar]
- Williams, M.A.; Williamson, E.; Gates, S.; Cooke, M.W. Reproducibility of the cervical range of motion (CROM) device for individuals with sub-acute whiplash associated disorders. Eur. Spine J. 2012, 21, 872–878. [Google Scholar] [CrossRef]
- Audette, I.; Dumas, J.; Côté, J.N.; De Serres, S.J. Validity and Between-Day Reliability of the Cervical Range of Motion (CROM) Device. J. Orthop. Sports Phys. Ther. 2010, 40, 318–323. [Google Scholar] [CrossRef]
- Tousignant, M.; Smeesters, C.; Breton, A.; Breton, É.; Corriveau, H. Criterion Validity Study of the Cervical Range of Motion (CROM) Device for Rotational Range of Motion on Healthy Adults. J. Orthop. Sports Phys. Ther. 2006, 36, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Zeman, F.; Koller, M.; Figueiredo, R.; Aazevedo, A.; Rates, M.; Coelho, C.; Kleinjung, T.; De Ridder, D.; Langguth, B.; Landgrebe, M. Tinnitus Handicap Inventory for Evaluating Treatment Effects: Which changes are clinically relevant? Otolaryngol.-Head Neck Surg. 2011, 145, 282–287. [Google Scholar] [CrossRef]
- Kurre, A.; van Gool, C.J.; Bastiaenen, C.H.; Gloor-Juzi, T.; Straumann, D.; de Bruin, E.D. Translation, Cross-Cultural Adaptation and Reliability of the German Version of the Dizziness Handicap Inventory. Otol. Neurotol. 2009, 30, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.W.; Formby, C. Effect of Tinnitus Retraining Therapy vs Standard of Care on Tinnitus-Related Quality of Life: A Randomized Clinical Trial. JAMA Otolaryngol.–Head Neck Surg. 2019, 145, 597–608. [Google Scholar]
- Peroz, I. Funktionsstörungen des Kauorgans bei Tinnituspatienten im Vergleich zu einer Kontrollgruppe. HNO 2003, 51, 544–549. [Google Scholar] [CrossRef]
- Michiels, S.; Van de Heyning, P.; Truijen, S.; Hallemans, A.; De Hertogh, W. Does multi-modal cervical physical therapy improve tinnitus in patients with cervicogenic somatic tinnitus? Man. Ther. 2016, 26, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Kuo, C.H.; Hsieh, W.L.; Lee, S.D.; Lee, W.J.; Chen, L.K.; Kao, C.L. Anxiety, depression and quality of life (QoL) in patients with chronic dizziness. Arch. Gerontol. Geriatr. 2012, 54, 131–135. [Google Scholar] [CrossRef]
- Michiels, S.; De Hertogh, W.; Truijen, S.; Van de Heyning, P. Cervical Spine Dysfunctions in Patients with Chronic Subjective Tinnitus. Otol. Neurotol. 2015, 36, 741–745. [Google Scholar] [CrossRef]

| Category | Values | p | |
|---|---|---|---|
| Age, mean (SD) | 48 (13) | 0.239 | |
| Sex (female, n (%)) | 43 (61.4) | 0.091 | |
| Tinnitus duration, n (%) | 0.353 | ||
| <3 months | 7 (10) | ||
| <6 months | 8 (11.4) | ||
| <12 months | 9 (12.9) | ||
| >2 years | 14 (20) | ||
| >5 years | 11 (15.7) | ||
| >10 years | 21 (30) | ||
| Sound, n (%) | Whistling/beeping | 57 (81.4) | 0.131 |
| Humming | 18 (25.7) | 0.207 | |
| Knocking | 1 (1.4) | 0.162 | |
| Other | 11 (15.7) | 0.299 | |
| Localization, n (%) | 0.116 | ||
| Right ear | 8 (11.4) | ||
| Left ear | 19 (27.1) | ||
| On both sides | 43 (61.4) | ||
| THI median (IQR) | 47 (35) | 0.145 | |
| DHI median (IQR) | 34 (25) | 0.363 |
| T0 | T1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Muscles, n (%) | CG Frequence n = 33 | IG Frequence n = 37 | Phi | p | CG Frequence n = 33 | IG Frequence n = 37 | Phi | p |
| Spleniuscapitis, right | 24 (72.7) | 25 (67.6) | 0.056 | 0.638 | 26 (78.8) | 11 (29.7) | 0.491 | <0.001 * |
| Spleniuscapitis, left | 31 (93.9) | 32 (86.5) | 0.124 | 0.299 | 27 (81.8) | 18 (48.7) | 0.346 | 0.004 * |
| Semispinaliscapitis, right | 21 (63.6) | 24 (64.9) | −0.013 | 0.915 | 23 (69.7) | 11 (29.7) | 0.399 | 0.001 * |
| Semispinaliscapitis, left | 29 (87.9) | 31 (83.8) | 0.058 | 0.625 | 27 (81.8) | 17 (45.9) | 0.371 | 0.002 * |
| Temporalis, right | 6 (18.2) | 7 (18.9) | −0.009 | 0.937 | 8 (24.2) | 4 (10.8) | 0.178 | 0.137 |
| Temporalis, left | 6 (18.2) | 4 (10.8) | 0.105 | 0.379 | 10 (30.3) | 3 (8.1) | 0.285 | 0.017 * |
| Masseter, right | 16 (48.5) | 10 (27.0) | 0.222 | 0.064 | 13 (39.4) | 10 (27.0) | 0.131 | 0.271 |
| Masseter, left | 21 (63.6) | 22 (59.5) | 0.043 | 0.720 | 17 (51.5) | 10 (27.0) | 0.251 | 0.036 * |
| Pterygoideus, right | 11 (33.4) | 10 (27.0) | 0.069 | 0.565 | 13 (39.4) | 6 (16.2) | 0.260 | 0.029 * |
| Pterygoideus, left | 14 (42.4) | 10 (27.0) | 0.162 | 0.175 | 17 (51.5) | 9 (24.3) | 0.281 | 0.019 * |
| Floor-of-mouth muscles, right | 9 (27.3) | 10 (27.0) | 0.003 | 0.982 | 17 (51.5) | 10 (27.0) | 0.251 | 0.036 * |
| Floor-of-mouth muscles, left | 11 (33.4) | 10 (27.0) | 0.069 | 0.565 | 20 (60.6) | 10 (27.0) | 0.339 | 0.005 * |
| Trapezius, right | 18 (54.6) | 14 (37.8) | 0.167 | 0.161 | 20 (60.6) | 8 (21.6) | 0.397 | 0.001 * |
| Trapezius, left | 14 (42.4) | 14 (37.8) | 0.047 | 0.696 | 24 (72.7) | 7 (18.9) | 0.541 | <0.001 * |
| Levator scapulae, right | 19 (57.6) | 12 (32.4) | 0.253 | 0.035 * | 20 (60.6) | 7 (18.9) | 0.428 | <0.001 * |
| Levator scapulae, left | 20 (60.6) | 10 (27.0) | 0.339 | 0.005 * | 20 (60.6) | 7 (18.9) | 0.428 | <0.001 * |
| Sternocleidomastoideus, right | 7 (21.2) | 6 (16.2) | 0.064 | 0.592 | 6 (18.2) | 3 (8.1) | 0.150 | 0.209 |
| Sternocleidomastoideus, left | 7 (21.2) | 8 (21.6) | −0.005 | 0.967 | 9 (27.3) | 6 (16.2) | 0.135 | 0.260 |
| T0 | T1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Muscles, n (%) | CG Frequence n = 33 | IG Frequence n = 37 | Phi | p | CG Frequence n = 33 | IG Frequence n = 37 | Phi | p |
| Spleniuscapitis, right | 31 (93.9) | 37 (100) | −0.182 | 0.129 | 32 (96.9) | 27 (73.0) | 0.329 | 0.006 * |
| Spleniuscapitis, left | 31 (93.9) | 37 (100) | −0.182 | 0.129 | 31 (93.9) | 31 (83.8) | 0.159 | 0.182 |
| Semispinaliscapitis, right | 31 (93.9) | 33 (89.2) | 0.085 | 0.479 | 32 (96.9) | 25 (67.6) | 0.377 | 0.002 * |
| Semispinaliscapitis, left | 30 (90.9) | 37 (100) | −0.224 | 0.061 | 33 (100) | 26 (70.3) | 0.408 | 0.001 * |
| Temporalis, right | 11 (33.4) | 9 (24.3) | 0.100 | 0.405 | 19 (57.6) | 13 (35.1) | 0.225 | 0.060 |
| Temporalis, left | 9 (27.3) | 9 (24.3) | 0.034 | 0.778 | 16 (48.5) | 17 (46.0) | 0.025 | 0.832 |
| Masseter, right | 25 (75.8) | 19 (51.4) | 0.252 | 0.035 * | 28 (84.9) | 23 (62.2) | 0.255 | 0.033 * |
| Masseter, left | 25 (75.8) | 28 (75.7) | 0.001 | 0.994 | 29 (87.9) | 29 (78.4) | 0.126 | 0.292 |
| Pterygoideus, right | 18 (54.6) | 19 (51.4) | 0.032 | 0.789 | 26 (78.8) | 22 (59.5) | 0.208 | 0.082 |
| Pterygoideus, left | 23 (69.7) | 20 (54.1) | 0.160 | 0.180 | 23 (69.7) | 21 (56.8) | 0.134 | 0.263 |
| Floor-of-mouth muscles, right | 13 (39.4) | 14 (37.8) | 0.016 | 0.894 | 25 (75.8) | 12 (32.4) | 0.433 | <0.001 * |
| Floor-of-mouth muscles, left | 17 (51.5) | 12 (32.4) | 0.193 | 0.106 | 20 (60.6) | 9 (24.3) | 0.368 | 0.002 * |
| Trapezius, right | 29 (87.9) | 35 (94.6) | −0.120 | 0.316 | 32 (96.9) | 30 (81.1) | 0.249 | 0.037 * |
| Trapezius, left | 30 (90.9) | 34 (91.9) | −0.018 | 0.883 | 32 (96.9) | 26 (70.3) | 0.354 | 0.003 * |
| Levator scapulae, right | 28 (84.9) | 31 (83.8) | 0.015 | 0.903 | 31 (93.9) | 26 (70.3) | 0.304 | 0.011 * |
| Levator scapulae, left | 31 (93.9) | 32 (86.5) | 0.124 | 0.299 | 29 (87.9) | 21 (56.8) | 0.344 | 0.004 * |
| Sternocleidomastoideus, right | 9 (27.3) | 7 (18.9) | 0.099 | 0.406 | 8 (24.2) | 8 (21,6) | 0.031 | 0.794 |
| Sternocleidomastoideus, left | 4 (12.1) | 5 (13.5) | −0.021 | 0.862 | 8 (24.2) | 8 (21.6) | 0.031 | 0.794 |
| T0, n (%) | IG Median (IQR) n = 37 | CG Median (IQR) n = 33 | U | p |
|---|---|---|---|---|
| Inclination | 55 (15) | 55 (13) | 569.00 | 0.622 |
| Reclination | 60 (20) | 60 (20) | 610.00 | 0.995 |
| Lateral inclination, right | 35 (15) | 35 (10) | 583.00 | 0.742 |
| Lateral inclination, left | 35 (15) | 35 (13) | 556.50 | 0.518 |
| Rotation, right | 60 (8) | 60 (10) | 565.50 | 0.577 |
| Rotation, left | 60 (10) | 60 (13) | 534.00 | 0.351 |
| T1, n (%) | ||||
| Inclination | 50 (25) | 55 (18) | 556.00 | 0.518 |
| Reclination | 65 (20) | 55 (18) | 516.50 | 0.265 |
| Lateral inclination, right | 40 (15) | 35 (10) | 467.50 | 0.087 |
| Lateral inclination, left | 40 (15) | 35 (10) | 466.00 | 0.084 |
| Rotation, right | 60 (10) | 60 (10) | 343.00 | 0.001 * |
| Rotation, left | 60 (10) | 60 (10) | 395.50 | 0.008 * |
| THI | DHI | |||
|---|---|---|---|---|
| Mann–Whitney U = 227.000; z = −4.520; p < 0.001 *; n = 70 | Mann–Whitney U = 29.500; z = −2.826; p = 0.003 *; n = 26 | |||
| Intervention Group (n = 37) | Control Group (n = 33) | Intervention Group (n = 13) | Control Group (n = 13) | |
| Relevant improvement, n (%) | 12 (32.4) | 7 (21.2) (7) | 1 (7.7) | 3 (23.1) |
| Strong improvement, n (%) | 18 (48.7) | 2 (6.1) | 8 (61.5) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bökel, A.; Fobbe, A.; Lesinski-Schiedat, A.; Sturm, C. Exploring the Effects of Manual Therapy on Somatosensory Tinnitus and Dizziness: A Randomized Controlled Trial. J. Clin. Med. 2025, 14, 4579. https://doi.org/10.3390/jcm14134579
Bökel A, Fobbe A, Lesinski-Schiedat A, Sturm C. Exploring the Effects of Manual Therapy on Somatosensory Tinnitus and Dizziness: A Randomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(13):4579. https://doi.org/10.3390/jcm14134579
Chicago/Turabian StyleBökel, Andrea, Andreas Fobbe, Anke Lesinski-Schiedat, and Christian Sturm. 2025. "Exploring the Effects of Manual Therapy on Somatosensory Tinnitus and Dizziness: A Randomized Controlled Trial" Journal of Clinical Medicine 14, no. 13: 4579. https://doi.org/10.3390/jcm14134579
APA StyleBökel, A., Fobbe, A., Lesinski-Schiedat, A., & Sturm, C. (2025). Exploring the Effects of Manual Therapy on Somatosensory Tinnitus and Dizziness: A Randomized Controlled Trial. Journal of Clinical Medicine, 14(13), 4579. https://doi.org/10.3390/jcm14134579






