Transitional Lesions, One More Step Towards Understanding the Pathogenesis of Adenomyosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Tissue Collection
2.2. Immunohistochemistry
2.3. Immunofluorescence
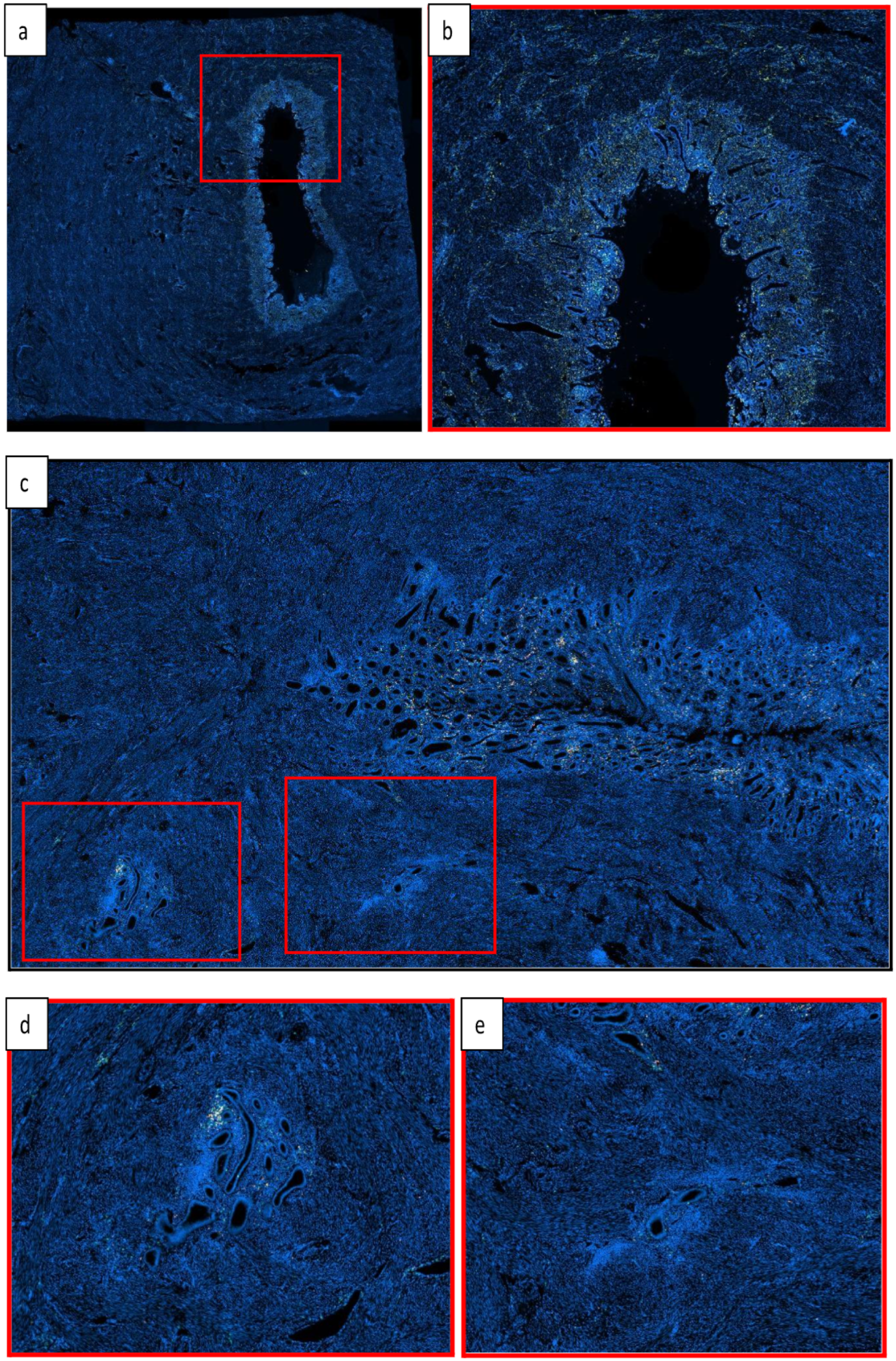
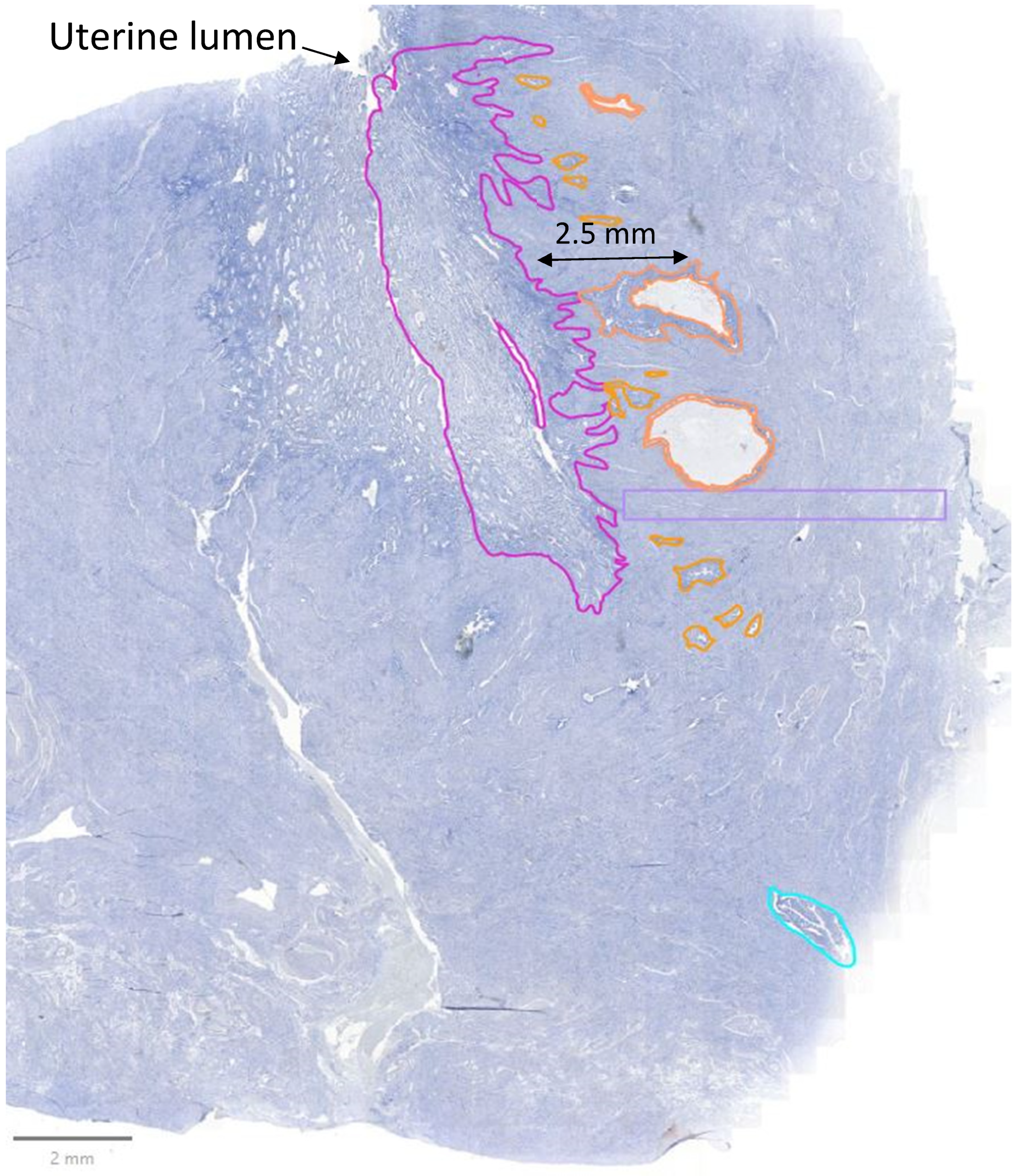
2.4. Analysis
2.5. Statistical Analysis
3. Results
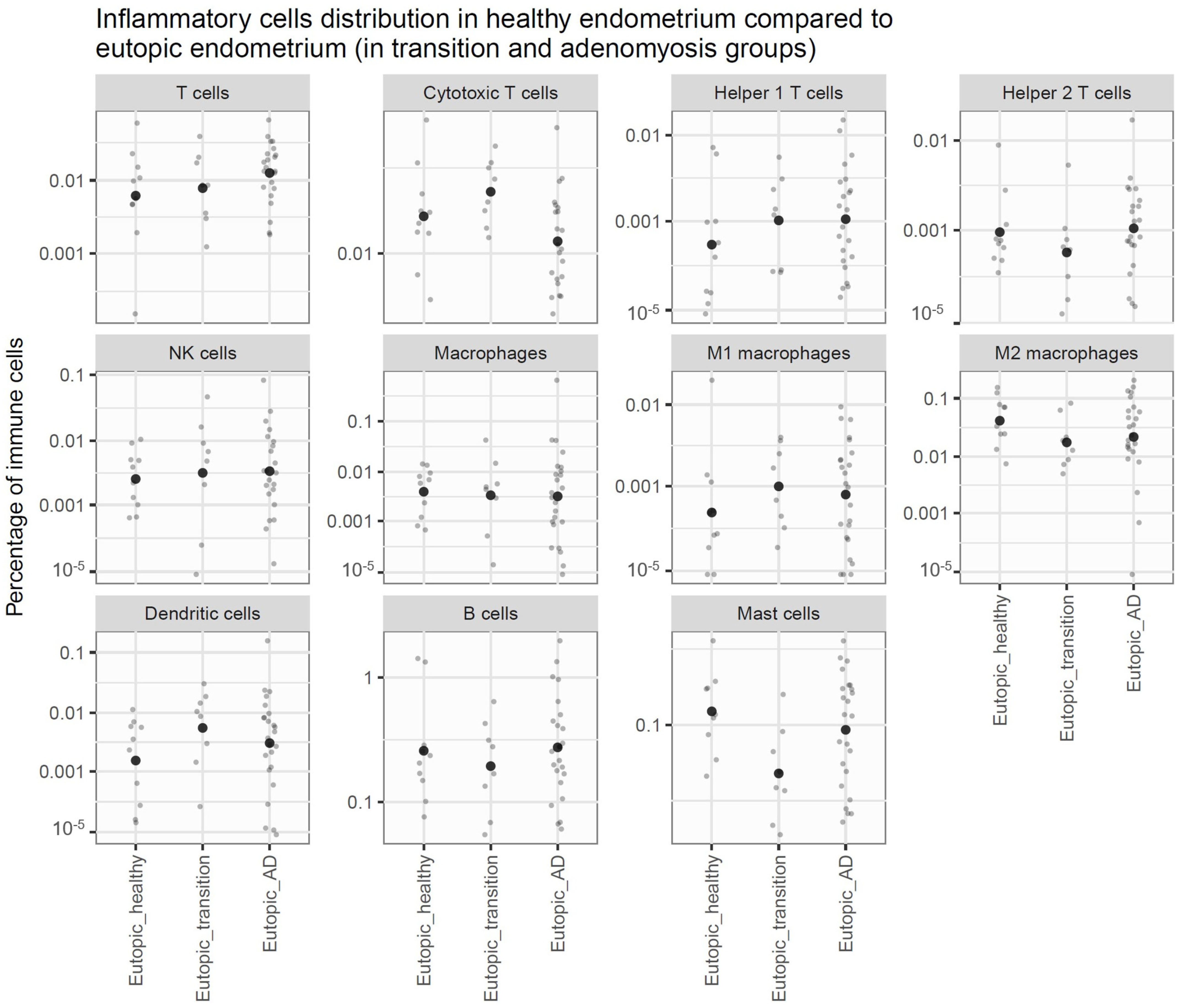
3.1. Comparison of Endometrium Between Controls and DISEASE GRoups
3.2. Comparison of Myometrium Between Controls and Disease Groups
3.3. Comparison of Lesions to Eutopic Endometrium Within Disease Groups
3.4. Phases of the Menstrual Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primary Antibody | Dilution | Antigen Retrieval | Secondary Antibody | Fluorochrome | Panel Position |
|---|---|---|---|---|---|
| GATA-3 (CST (Danvers, MA, USA) D13C9) | 1/800 | Citrate (pH 6) | Anti-rabbit | CF754 | 1 |
| NKp46 (Abcam (Cambridge, UK) EPR22403-57) | 1/100 | Citrate (pH 6) | Anti-rabbit | CF555 | 2 |
| T-bet (CST D6N8B) | 1/300 | EDTA (pH 9) | Anti-rabbit | AF594 | 3 |
| CD8 (Dako clone C8/144B) | 1/25 | EDTA (pH 9) | Anti-mouse | CF488 | 4 |
| CD3 (Invitrogen (Waltham, MA, USA) clone SP7) | 1/150 | Citrate (pH 6) | Anti-mouse | CF647 | 5 |
| Primary Antibody | Dilution | Antigen Retrieval | Secondary Antibody | Fluorochrome | Panel Position |
|---|---|---|---|---|---|
| CD86 (Abcam E2G8P) | 1/100 | EDTA (pH 9) | Anti-rabbit | CF488 | 1 |
| CD1a (Agilent clone 010) | 1/50 | EDTA (pH 9) | Anti-mouse | CF754 | 2 |
| CD163 (Sanbio Mob460-05) | 1/40 | Citrate (pH 6) | Anti-mouse | CF555 | 3 |
| CD68 (Abcam ab955) | 1/100 | Citrate (pH 6) | Anti-mouse | CF647 | 4 |
References
- Camboni, A.; Marbaix, E. Ectopic Endometrium: The Pathologist’s Perspective. Int. J. Mol. Sci. 2021, 22, 10974. [Google Scholar] [CrossRef] [PubMed]
- Stratopoulou, C.A.; Donnez, J.; Dolmans, M.-M. Origin and Pathogenic Mechanisms of Uterine Adenomyosis: What Is Known So Far. Reprod. Sci. 2021, 28, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- García-Solares, J.; Donnez, J.; Donnez, O.; Dolmans, M.-M. Pathogenesis of Uterine Adenomyosis: Invagination or Metaplasia? Fertil. Steril. 2018, 109, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Maclean, A.; Barzilova, V.; Patel, S.; Bates, F.; Hapangama, D.K. Characterising the Immune Cell Phenotype of Ectopic Adenomyosis Lesions Compared with Eutopic Endometrium: A Systematic Review. J. Reprod. Immunol. 2023, 157, 103925. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Santulli, P.; Jeljeli, M.; Vannuccini, S.; Marcellin, L.; Doridot, L.; Petraglia, F.; Batteux, F.; Chapron, C. Immunological Changes Associated with Adenomyosis: A Systematic Review. Human Reprod. Update 2021, 27, 108–129. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Daraï, E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil. Steril. 2018, 109, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, N.; Benidovskaya, E.; Beyaert, S.; Daumerie, A.; Maestre Osorio, F.; Aboubakar Nana, F.; Bouzin, C.; Van den Eynde, M. Multiplex Immunofluorescence Combined with Spatial Image Analysis for the Clinical and Biological Assessment of the Tumor Microenvironment. J. Vis. Exp. 2023, 196, e65220. [Google Scholar] [CrossRef] [PubMed]
- Binda, M.M.; Donnez, J.; Dolmans, M.-M. Targeting Mast Cells: A New Way to Treat Endometriosis. Expert Opin. Ther. Targets 2017, 21, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Anaf, V.; Chapron, C.; El Nakadi, I.; De Moor, V.; Simonart, T.; Noël, J.C. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil. Steril. 2006, 86, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Wang, J.; He, J.; Guo, X.; Li, T.; Zhang, X. The New Application of Mifepristone in the Relief of Adenomyosis-Caused Dysmenorrhea. Int. J. Med. Sci. 2020, 17, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Habiba, M.; Benagiano, G.; Guo, S.W. An Appraisal of the Tissue Injury and Repair (TIAR) Theory on the Pathogenesis of Endometriosis and Adenomyosis. Biomolecules 2023, 13, 975. [Google Scholar] [CrossRef] [PubMed]

| Pathology | Menstrual Phase | Age | BMI | Parity | Symptoms |
|---|---|---|---|---|---|
| Adenomyosis | Menstrual | 46 | 30.1 | G3P3 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Menstrual | 49 | 28.5 | G3P2 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Menstrual | 43 | 37.9 | G2P2 | Menorrhagia |
| Adenomyosis | Menstrual | 42 | Not found | G4P4 | Menorrhagia |
| Adenomyosis | Menstrual | 48 | Not found | G1P0 | Menorrhagia |
| Adenomyosis | Menstrual | 54 | 26.7 | Not found | Not found |
| Adenomyosis | Menstrual | 48 | 20.8 | G1P1 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Menstrual | 44 | 21.8 | G2P2 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Menstrual | 53 | 25.4 | G2P2 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Proliferative | 48 | 45.0 | G3P3 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Proliferative | 47 | 24.8 | G2P0 | Not found |
| Adenomyosis | Proliferative | 50 | 31.6 | Not found | Menorrhagia |
| Adenomyosis | Proliferative | 46 | 28.3 | G4P4 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Proliferative | 42 | 30.3 | G3P3 | Menorrhagia |
| Adenomyosis | Proliferative | 55 | Not found | G5P3 | Not found |
| Adenomyosis | Proliferative | 43 | Not found | Not found | Not found |
| Adenomyosis | Proliferative | 51 | 30.8 | G4P3 | Menorrhagia |
| Adenomyosis | Proliferative | 40 | Not found | G3P3 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Secretory | 47 | Not found | G2P2 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Secretory | 50 | 31.2 | G4P3 | Not found |
| Adenomyosis | Secretory | 46 | 30.5 | G5P2 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Secretory | 37 | 40.1 | G4P3 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Secretory | 45 | 36.3 | G1P1 | Menorrhagia |
| Adenomyosis | Secretory | 41 | 30.6 | G6P5 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Secretory | 45 | Not found | G2P2 | Menorrhagia |
| Adenomyosis | Secretory | 42 | 20.6 | G4P2 | Menorrhagia and dysmenorrhea |
| Adenomyosis | Secretory | 40 | Not found | G3P2 | Menorrhagia |
| Leiomyoma | Menstrual | 39 | 21.1 | G2P2 | Not found |
| Leiomyoma | Menstrual | 48 | 29.0 | G6P4 | Not found |
| Leiomyoma | Menstrual | 44 | 23.7 | G4P4 | Not found |
| Leiomyoma | Menstrual | 38 | 22.6 | G4P4 | Not found |
| Leiomyoma | Menstrual | 42 | Not found | Not found | Menorrhagia |
| Leiomyoma | Proliferative | 44 | 50.2 | Not found | Not found |
| Leiomyoma | Proliferative | 42 | 28.7 | G4P2 | Not found |
| Prolapsus | Proliferative | 56 | 20.8 | GXP3 | Not found |
| Leiomyoma | Proliferative | 35 | 21.1 | NA | Not found |
| Leiomyoma | Proliferative | 49 | 39.0 | G3P2 | Menorrhagia |
| Leiomyoma | Secretory | 41 | 24.3 | G2P2 | Menorrhagia |
| Leiomyoma | Secretory | 37 | Not found | G6P4 | Menorrhagia and dysmenorrhea |
| Leiomyoma | Secretory | 47 | NA | G4PX | Not found |
| Leiomyoma | Secretory | 40 | 28.3 | G3P2 | Menorrhagia and dysmenorrhea |
| Leiomyoma | Secretory | 43 | 24.7 | G3P2 | Menorrhagia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wacheul, E.; Dolmans, M.-M.; Ambroise, J.; Donnez, J.; Camboni, A. Transitional Lesions, One More Step Towards Understanding the Pathogenesis of Adenomyosis. J. Clin. Med. 2025, 14, 4578. https://doi.org/10.3390/jcm14134578
Wacheul E, Dolmans M-M, Ambroise J, Donnez J, Camboni A. Transitional Lesions, One More Step Towards Understanding the Pathogenesis of Adenomyosis. Journal of Clinical Medicine. 2025; 14(13):4578. https://doi.org/10.3390/jcm14134578
Chicago/Turabian StyleWacheul, Emilie, Marie-Madeleine Dolmans, Jérôme Ambroise, Jacques Donnez, and Alessandra Camboni. 2025. "Transitional Lesions, One More Step Towards Understanding the Pathogenesis of Adenomyosis" Journal of Clinical Medicine 14, no. 13: 4578. https://doi.org/10.3390/jcm14134578
APA StyleWacheul, E., Dolmans, M.-M., Ambroise, J., Donnez, J., & Camboni, A. (2025). Transitional Lesions, One More Step Towards Understanding the Pathogenesis of Adenomyosis. Journal of Clinical Medicine, 14(13), 4578. https://doi.org/10.3390/jcm14134578






