Does Sensory Integration Influence Gait Parameters in Healthy Older Adults? Insights from a Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection Criteria
2.3. Quality Assessment
2.4. Data Extraction
2.5. Data Synthesis and Analysis
3. Results
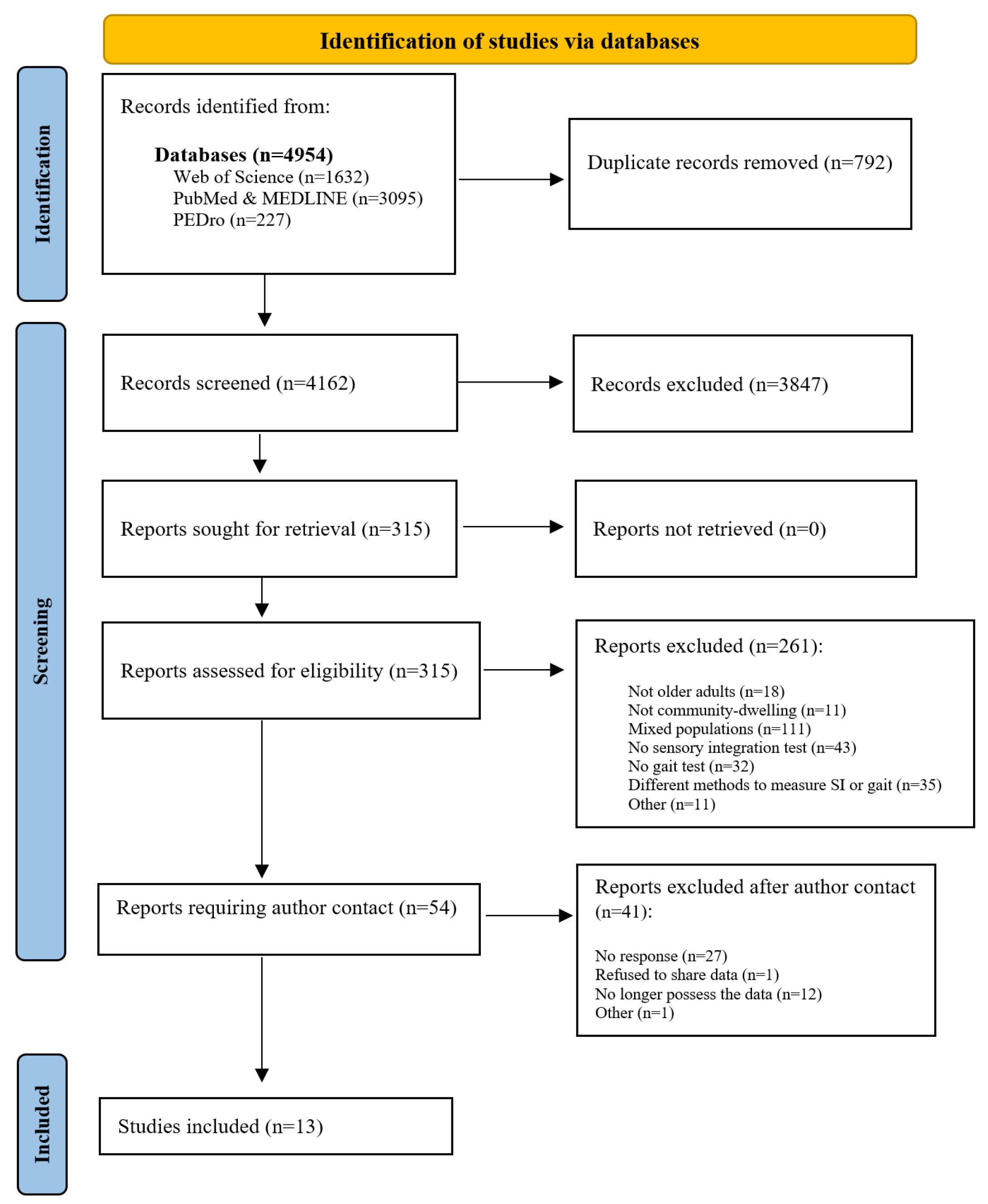
3.1. Study Selection
3.2. Characteristics of the Participants
3.3. Outcome Measurements
3.4. Risk of Bias
3.5. Qualitative Synthesis of the Findings
3.6. Quantitative Synthesis of the Findings
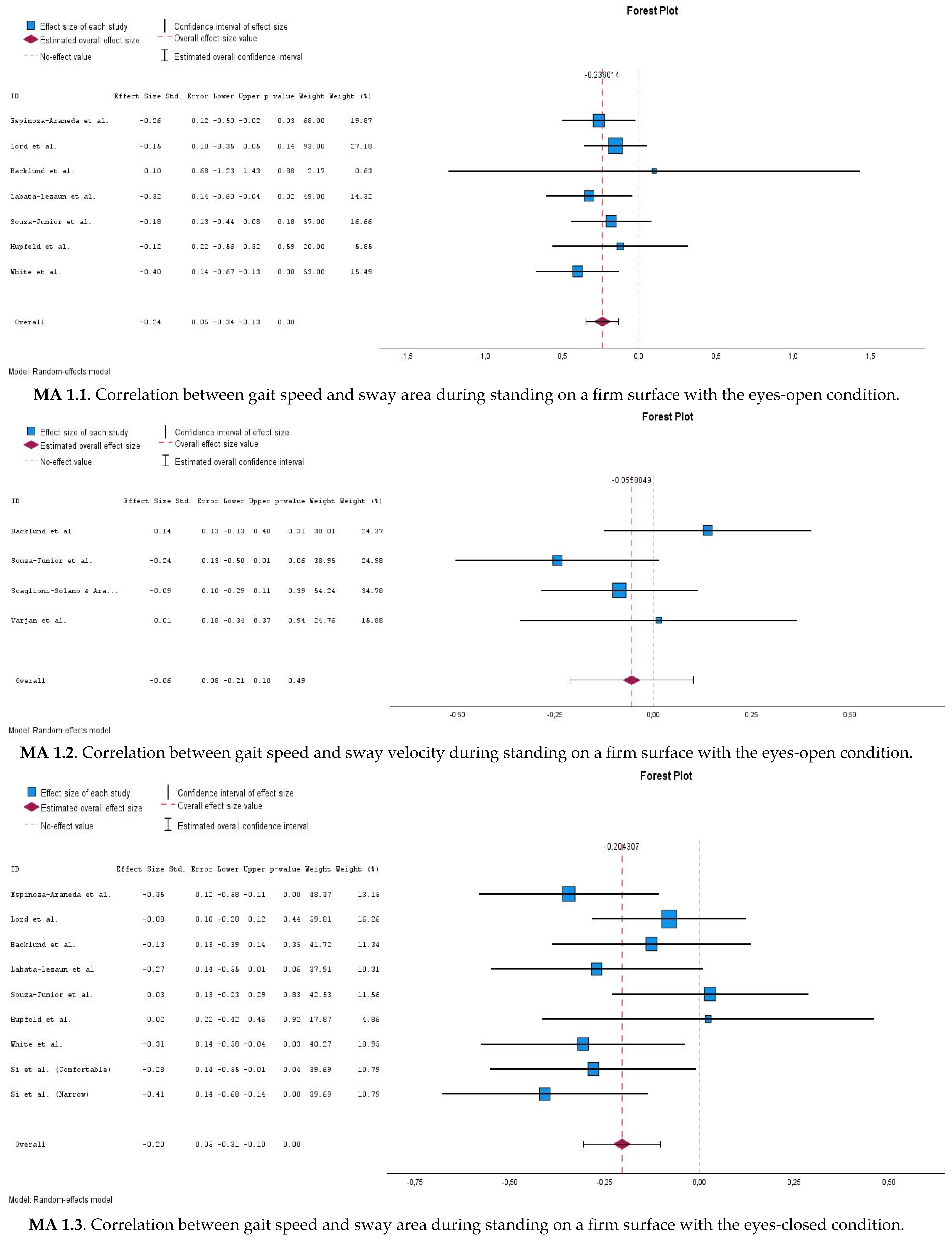
3.6.1. Correlations Between Gait Speed and Postural Sway Measures During Eyes-Open Stable Surface Condition (MA 1.1 and MA 1.2)
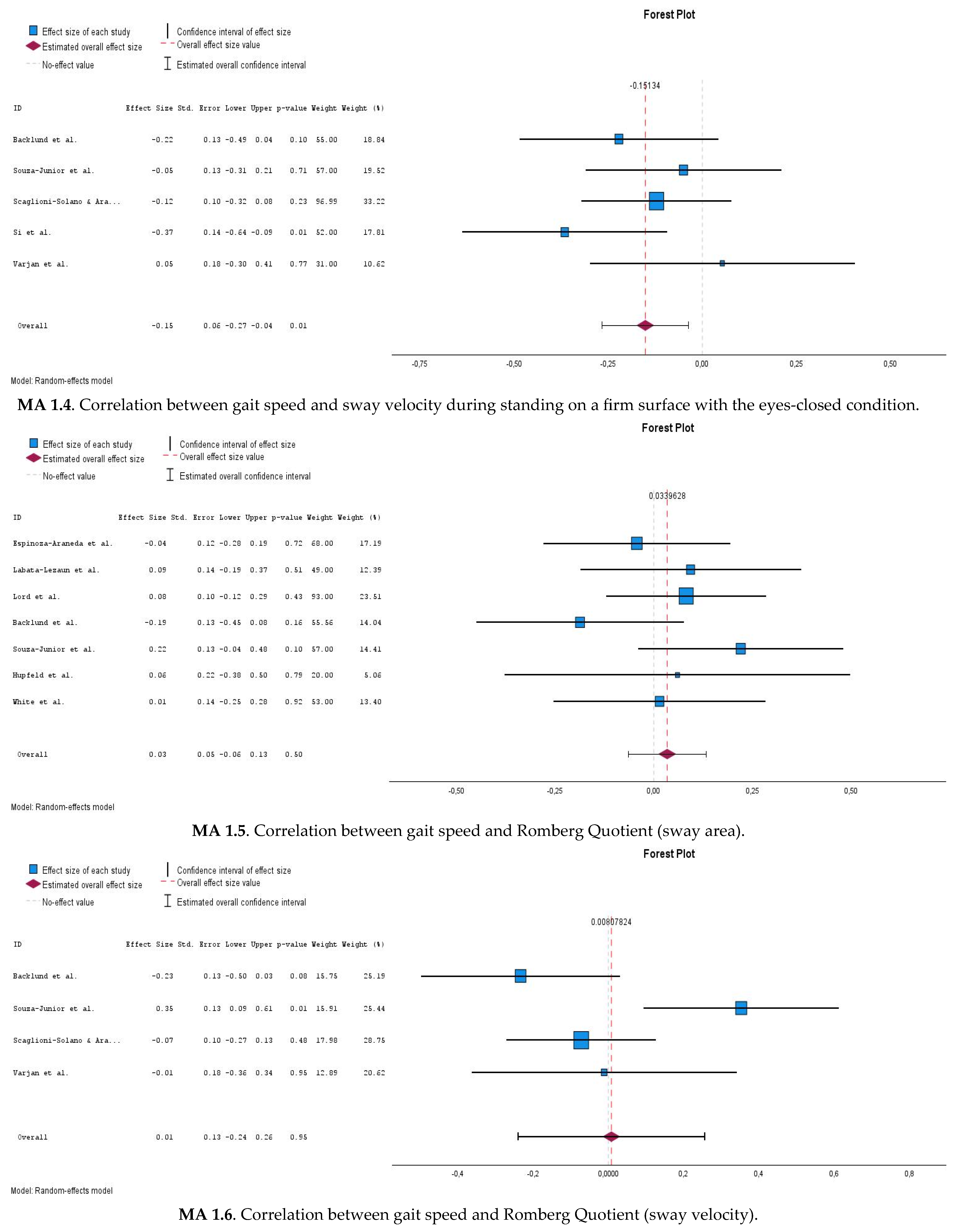
3.6.2. Correlations Between Gait Speed and Postural Sway Measures During Eyes-Closed Stable Surface Condition (MA 1.3 and MA 1.4)
3.6.3. Correlations Between Gait Speed and Romberg Quotient (MA 1.5 and MA 1.6)
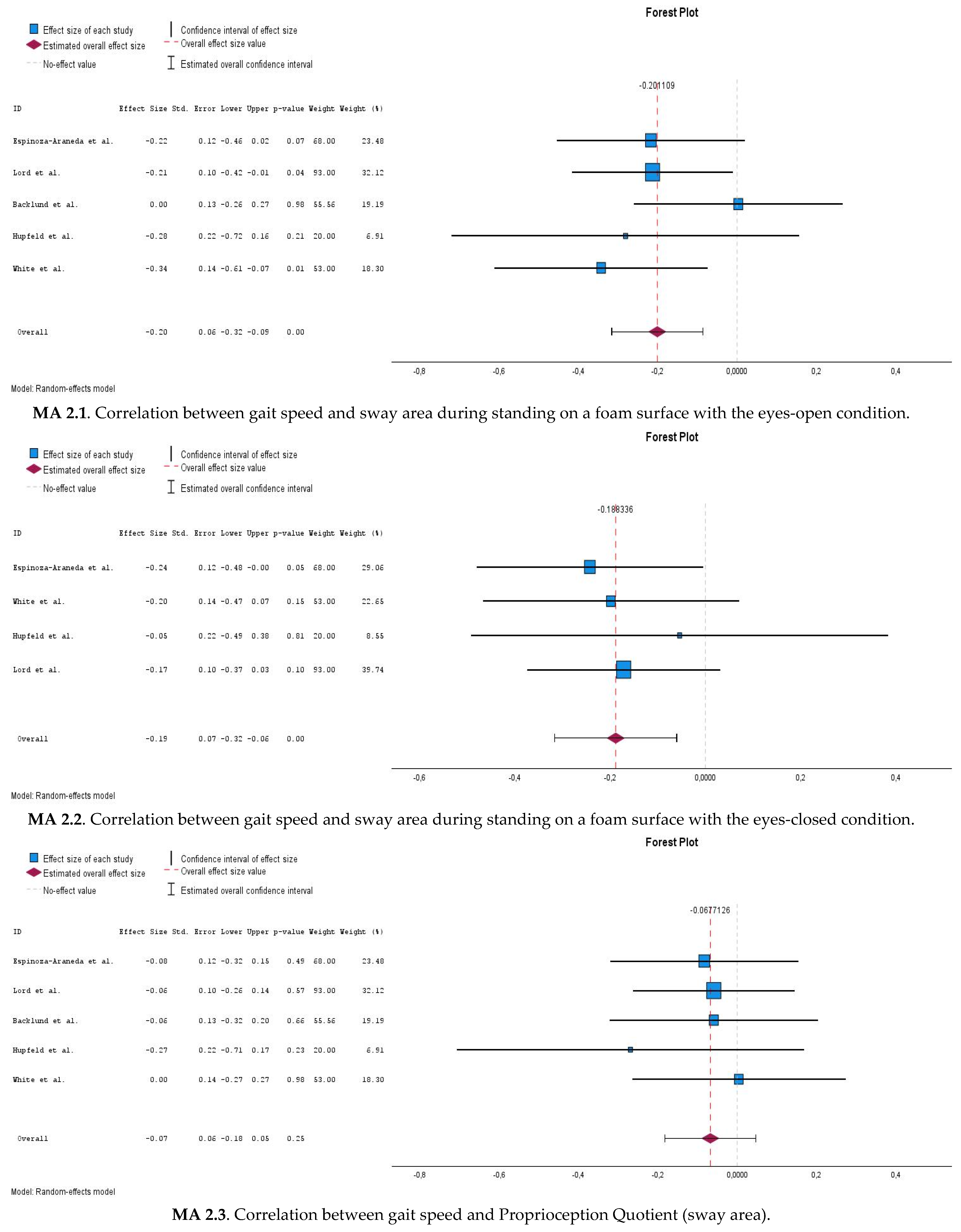
3.6.4. Correlations Between Gait Speed and Postural Sway Measures During Eyes-Open Compliant Surface Condition (MA 2.1)
3.6.5. Correlations Between Gait Speed and Postural Sway Measures During Eyes-Closed Compliant Surface Condition (MA 2.2)
3.6.6. Correlations Between Gait Speed and Proprioception Quotient (MA 2.3)
3.7. Moderation and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Horak, F.B.; Macpherson, J.M. Postural Orientation and Equilibrium. In Comprehensive Physiology; Prakash, Y.S., Ed.; Wiley: New York, NY, USA, 2011; pp. 255–292. ISBN 9780470650714. [Google Scholar]
- Fitzpatrick, R.; McCloskey, D.I. Proprioceptive, Visual and Vestibular Thresholds for the Perception of Sway during Standing in Humans. J. Physiol. 1994, 478, 173–186. [Google Scholar] [CrossRef]
- Osoba, M.Y.; Rao, A.K.; Agrawal, S.K.; Lalwani, A.K. Balance and Gait in the Elderly: A Contemporary Review. Laryngoscope Investig. Otolaryngol. 2019, 4, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.E.; Zobeiri, O.A. Proprioception and the Predictive Sensing of Active Self-Motion. Curr. Opin. Physiol. 2021, 20, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Translating Research into Clinical Practice, 5th ed.; Shumway-Cook, A., Woollacott, M.H., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2016; ISBN 9781496347725. [Google Scholar]
- Zhang, S.; Xu, W.; Zhu, Y.; Tian, E.; Kong, W. Impaired Multisensory Integration Predisposes the Elderly People to Fall: A Systematic Review. Front. Neurosci. 2020, 14, 411. [Google Scholar] [CrossRef]
- Pradels, A.; Pradon, D.; Hlavačková, P.; Diot, B.; Vuillerme, N. Sensory Re-Weighting in Human Bipedal Postural Control: The Effects of Experimentally-Induced Plantar Pain. PLoS ONE 2013, 8, e65510. [Google Scholar] [CrossRef] [PubMed]
- Merabet, L.B.; Pascual-Leone, A. Neural Reorganization Following Sensory Loss: The Opportunity of Change. Nat. Rev. Neurosci. 2010, 11, 44–52. [Google Scholar] [CrossRef]
- Overstall, P.W.; Exton-Smith, A.N.; Imms, F.J.; Johnson, A.L. Falls in the Elderly Related to Postural Imbalance. Br. Med. J. 1977, 1, 261–264. [Google Scholar] [CrossRef]
- Sturnieks, D.L.; St George, R.; Lord, S.R. Balance Disorders in the Elderly. Neurophysiol. Clin. Neurophysiol. 2008, 38, 467–478. [Google Scholar] [CrossRef]
- Zapparoli, L.; Mariano, M.; Paulesu, E. How the Motor System Copes with Aging: A Quantitative Meta-Analysis of the Effect of Aging on Motor Function Control. Commun. Biol. 2022, 5, 79. [Google Scholar] [CrossRef]
- Remaud, A.; Thuong-Cong, C.; Bilodeau, M. Age-Related Changes in Dynamic Postural Control and Attentional Demands Are Minimally Affected by Local Muscle Fatigue. Front. Aging Neurosci. 2016, 7, 257. [Google Scholar] [CrossRef]
- Freiberger, E.; Sieber, C.C.; Kob, R. Mobility in Older Community-Dwelling Persons: A Narrative Review. Front. Physiol. 2020, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jimenez, M. Normal Changes in Gait and Mobility Problems in the Elderly. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Maheu, M.; Houde, M.-S.; Landry, S.P.; Champoux, F. The Effects of Aging on Clinical Vestibular Evaluations. Front. Neurol. 2015, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Lopez, I.; Ishiyama, G.; Tang, Y.; Tokita, J.; Baloh, R.W.; Ishiyama, A. Regional Estimates of Hair Cells and Supporting Cells in the Human Crista Ampullaris. J. Neurosci. Res. 2005, 82, 421–431. [Google Scholar] [CrossRef]
- Alvarez, J.C.; Díaz, C.; Suárez, C.; Fernández, J.A.; González del Rey, C.; Navarro, A.; Tolivia, J. Aging and the Human Vestibular Nuclei: Morphometric Analysis. Mech. Ageing Dev. 2000, 114, 149–172. [Google Scholar] [CrossRef]
- Agrawal, Y.; Merfeld, D.M.; Horak, F.B.; Redfern, M.S.; Manor, B.; Westlake, K.P.; Holstein, G.R.; Smith, P.F.; Bhatt, T.; Bohnen, N.I.; et al. Aging, Vestibular Function, and Balance: Proceedings of a National Institute on Aging/National Institute on Deafness and Other Communication Disorders Workshop. J. Gerontol. Ser. A 2020, 75, 2471–2480. [Google Scholar] [CrossRef]
- Anson, E.; Pineault, K.; Bair, W.; Studenski, S.; Agrawal, Y. Reduced Vestibular Function Is Associated with Longer, Slower Steps in Healthy Adults during Normal Speed Walking. Gait Posture 2019, 68, 340–345. [Google Scholar] [CrossRef]
- Bárbara, R.C.S.; Freitas, S.M.S.F.; Bagesteiro, L.B.; Perracini, M.R.; Alouche, S.R. Gait Characteristics of Younger-Old and Older-Old Adults Walking Overground and on a Compliant Surface. Braz. J. Phys. Ther. 2012, 16, 375–380. [Google Scholar] [CrossRef]
- Duggan, E.; Donoghue, O.; Kenny, R.A.; Cronin, H.; Loughman, J.; Finucane, C. Time to Refocus Assessment of Vision in Older Adults? Contrast Sensitivity but Not Visual Acuity Is Associated with Gait in Older Adults. J. Gerontol. Ser. A 2017, 72, 1663–1668. [Google Scholar] [CrossRef]
- Jeka, J.J.; Allison, L.K.; Kiemel, T. The Dynamics of Visual Reweighting in Healthy and Fall-Prone Older Adults. J. Mot. Behav. 2010, 42, 197–208. [Google Scholar] [CrossRef]
- Reed, C.A.; Chaudhari, A.M.W.; Worthen-Chaudhari, L.C.; Bigelow, K.E.; Monfort, S.M. A New Perspective on Transient Characteristics of Quiet Stance Postural Control. PLoS ONE 2020, 15, e0237246. [Google Scholar] [CrossRef] [PubMed]
- McChesney, J.W.; Woollacott, M.H. The Effect of Age-Related Declines in Proprioception and Total Knee Replacement on Postural Control. J. Gerontol. Ser. A 2000, 55, M658–M666. [Google Scholar] [CrossRef]
- Behtani, L.; Paromov, D.; Moïn-Darbari, K.; Houde, M.-S.; Bacon, B.A.; Maheu, M.; Leroux, T.; Champoux, F. Sensory Reweighting for Postural Control in Older Adults with Age-Related Hearing Loss. Brain Sci. 2023, 13, 1623. [Google Scholar] [CrossRef] [PubMed]
- Kimijanová, J.; Svoboda, Z.; Han, J. Editorial: Sensory Control of Posture and Gait: Integration and Mechanisms to Maintain Balance during Different Sensory Conditions. Front. Hum. Neurosci. 2024, 18, 1378599. [Google Scholar] [CrossRef]
- Mahoney, J.R.; Verghese, J. Visual-Somatosensory Integration and Quantitative Gait Performance in Aging. Front. Aging Neurosci. 2018, 10, 377. [Google Scholar] [CrossRef]
- Mahoney, J.R.; Cotton, K.; Verghese, J. Multisensory Integration Predicts Balance and Falls in Older Adults. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2019, 74, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Lepers, R.; Bigard, A.X.; Diard, J.-P.; Gouteyron, J.-F.; Guezennec, C.Y. Posture Control after Prolonged Exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 55–61. [Google Scholar] [CrossRef]
- Prieto, T.E.; Myklebust, J.B.; Hoffmann, R.G.; Lovett, E.G.; Myklebust, B.M. Measures of Postural Steadiness: Differences between Healthy Young and Elderly Adults. IEEE Trans. Biomed. Eng. 1996, 43, 956–966. [Google Scholar] [CrossRef]
- Yang, F.; Liu, X. Relative Importance of Vision and Proprioception in Maintaining Standing Balance in People with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2020, 39, 101901. [Google Scholar] [CrossRef]
- Howcroft, J.; Lemaire, E.D.; Kofman, J.; McIlroy, W.E. Elderly Fall Risk Prediction Using Static Posturography. PLoS ONE 2017, 12, e0172398. [Google Scholar] [CrossRef]
- Souza-Junior, E.L.S.; Schettino, L.; Araujo, C.M.; Pereira, R.; Oliveira, A.A.; Mascarenhas, C.H.M.; Fernandes, M.H. Factors Influencing Gait Speed in Community-Dwelling Older Women: A Bayesian Approach. Gait Posture 2022, 92, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Camicioli, R.; Panzer, V.P.; Kaye, J. Balance in the Healthy Elderly: Posturography and Clinical Assessment. Arch. Neurol. 1997, 54, 976–981. [Google Scholar] [CrossRef]
- Hughes, M.A.; Duncan, P.W.; Rose, D.K.; Chandler, J.M.; Studenski, S.A. The Relationship of Postural Sway to Sensorimotor Function, Functional Performance, and Disability in the Elderly. Arch. Phys. Med. Rehabil. 1996, 77, 567–572. [Google Scholar] [CrossRef]
- Aranda-García, S.; Busquets, A.; Planas, A.; Prat-Subirana, J.A.; Angulo-Barroso, R.M. Strength, Static Balance, Physical Activity, and Age Predict Maximal Gait Speed in Healthy Older Adults from a Rural Community: A Cross-Sectional Study. J. Aging Phys. Act. 2015, 23, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Si, B.; Zhu, H.; Wei, X.; Li, S.; Wu, X. The Mechanism of Static Postural Control in the Impact of Lower Limb Muscle Strength Asymmetry on Gait Performance in the Elderly. PeerJ 2024, 12, e17626. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing Bias in Studies of Prognostic Factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Van Wesemael, S.; Bogaerts, K.; De Baets, L.; Goossens, N.; Vlemincx, E.; Amerijckx, C.; Sohail, S.; Matheve, T.; Janssens, L. The Association between Pain-Related Psychological Variables and Postural Control in Low Back Pain: A Systematic Review and Meta-Analysis. Gait Posture 2024, 107, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; Cochrane: Oxford, UK, 2024; Available online: www.training.cochrane.org/handbook (accessed on 1 October 2024).
- Crocamo, C.; Bachi, B.; Calabrese, A.; Callovini, T.; Cavaleri, D.; Cioni, R.M.; Moretti, F.; Bartoli, F.; Carrà, G. Some of Us Are Most at Risk: Systematic Review and Meta-Analysis of Correlates of Depressive Symptoms among Healthcare Workers during the SARS-CoV-2 Outbreak. Neurosci. Biobehav. Rev. 2021, 131, 912–922. [Google Scholar] [CrossRef]
- Fujimoto, C.; Murofushi, T.; Chihara, Y.; Ushio, M.; Sugasawa, K.; Yamaguchi, T.; Yamasoba, T.; Iwasaki, S. Assessment of Diagnostic Accuracy of Foam Posturography for Peripheral Vestibular Disorders: Analysis of Parameters Related to Visual and Somatosensory Dependence. Clin. Neurophysiol. 2009, 120, 1408–1414. [Google Scholar] [CrossRef]
- Hunter, J.; Schmidt, F. Methods of Meta-Analysis Corrected Error and Bias in Research Findings; SAGE Publications: Newbury Park, CA, USA, 2004; Volume 20, ISBN 9781412904797. [Google Scholar]
- Christe, G.; Crombez, G.; Edd, S.; Opsommer, E.; Jolles, B.M.; Favre, J. Relationship between Psychological Factors and Spinal Motor Behaviour in Low Back Pain: A Systematic Review and Meta-Analysis. Pain 2021, 162, 672–686. [Google Scholar] [CrossRef]
- van Aert, R.C.M. Meta-Analyzing Partial Correlation Coefficients Using Fisher’s z Transformation. Res. Synth. Methods 2023, 14, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L. V Fitting Categorical Models to Effect Sizes from a Series of Experiments. J. Educ. Stat. 1982, 7, 119–137. [Google Scholar] [CrossRef]
- Sen, S.; Yildirim, I. A Tutorial on How to Conduct Meta-Analysis with IBM SPSS Statistics. Psych. 2022, 4, 640–667. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Cohen, J., Ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1988; ISBN 0-8058-0283-5. [Google Scholar]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley Online Library: Hoboken, NJ, USA, 2008; pp. 243–296. ISBN 9780470712184. [Google Scholar]
- Tanner-Smith, E.E.; Grant, S. Meta-Analysis of Complex Interventions. Annu. Rev. Public. Health 2018, 39, 135–151. [Google Scholar] [CrossRef]
- Bäcklund, T.; Frankel, J.; Israelsson, H.; Malm, J.; Sundström, N. Trunk Sway in Idiopathic Normal Pressure Hydrocephalus—Quantitative Assessment in Clinical Practice. Gait Posture 2017, 54, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.R.; Lloyd, D.G.; Li, S.K. Sensori-Motor Function, Gait Patterns and Falls in Community-Dwelling Women. Age Ageing 1996, 25, 292–299. [Google Scholar] [CrossRef]
- Scaglioni-Solano, P.; Aragón-Vargas, L.F. Gait Characteristics and Sensory Abilities of Older Adults Are Modulated by Gender. Gait Posture 2015, 42, 54–59. [Google Scholar] [CrossRef]
- Li, K.Z.H.; Roudaia, E.; Lussier, M.; Bherer, L.; Leroux, A.; McKinley, P.A. Benefits of Cognitive Dual-Task Training on Balance Performance in Healthy Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 1344–1352. [Google Scholar] [CrossRef]
- Hupfeld, K.E.; Hyatt, H.W.; Jerez, P.A.; Mikkelsen, M.; Hass, C.J.; Edden, R.A.E.; Seidler, R.D.; Porges, E.C. In Vivo Brain Glutathione Is Higher in Older Age and Correlates with Mobility. Cereb. Cortex 2021, 31, 4576–4594. [Google Scholar] [CrossRef]
- White, U.E.; Black, A.A.; Delbaere, K.; Wood, J.M. Determinants of Concern about Falling in Adults with Age-Related Macular Degeneration. Ophthalmic Physiol. Opt. 2021, 41, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Varjan, M.; Žiška Böhmerová, Ľ.; Oreská, Ľ.; Schickhofer, P.; Hamar, D. In Elderly Individuals, the Effectiveness of Sensorimotor Training on Postural Control and Muscular Strength Is Comparable to Resistance-Endurance Training. Front. Physiol. 2024, 15, 1386537. [Google Scholar] [CrossRef]
- Harro, C.C.; Garascia, C. Reliability and Validity of Computerized Force Platform Measures of Balance Function in Healthy Older Adults. J. Geriatr. Phys. Ther. 2019, 42, E57–E66. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Araneda, J.; Bravo-Carrasco, V.; Álvarez, C.; Marzuca-Nassr, G.N.; Muñoz-Mendoza, C.L.; Muñoz, J.; Caparrós-Manosalva, C. Postural Balance and Gait Parameters of Independent Older Adults: A Sex Difference Analysis. Int. J. Environ. Res. Public. Health 2022, 19, 4064. [Google Scholar] [CrossRef]
- Labata-Lezaun, N.; González-Rueda, V.; Rodríguez-Sanz, J.; López-de-Celis, C.; Llurda-Almuzara, L.; Rodríguez-Rubio, P.R.; Pérez-Bellmunt, A. Correlation between Physical Performance and Stabilometric Parameters in Older Adults. Medicina 2022, 58, 1211. [Google Scholar] [CrossRef]
- Kim, J.-W.; Eom, G.-M.; Kim, C.-S.; Kim, D.-H.; Lee, J.-H.; Park, B.K.; Hong, J. Sex Differences in the Postural Sway Characteristics of Young and Elderly Subjects during Quiet Natural Standing. Geriatr. Gerontol. Int. 2010, 10, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, E.; Zhang, Y. Association of Walking Pace and Fall-Related Injury among Chinese Older Adults: Data from the SAGE Survey. Complement. Ther. Clin. Pract. 2023, 50, 101710. [Google Scholar] [CrossRef]
- Adam, C.E.; Fitzpatrick, A.L.; Leary, C.S.; Hajat, A.; Ilango, S.D.; Park, C.; Phelan, E.A.; Semmens, E.O. Change in Gait Speed and Fall Risk among Community-Dwelling Older Adults with and without Mild Cognitive Impairment: A Retrospective Cohort Analysis. BMC Geriatr. 2023, 23, 328. [Google Scholar] [CrossRef]
- Adam, C.E.; Fitzpatrick, A.L.; Leary, C.S.; Hajat, A.; Phelan, E.A.; Park, C.; Semmens, E.O. The Association between Gait Speed and Falls in Community Dwelling Older Adults with and without Mild Cognitive Impairment. Int. J. Environ. Res. Public Health 2021, 18, 3712. [Google Scholar] [CrossRef]
- Fritz, S.; Lusardi, M. Walking Speed: The Sixth Vital Sign. J. Geriatr. Phys. Ther. 2009, 32, 46–49. [Google Scholar] [CrossRef]
- Kyrdalen, I.L.; Thingstad, P.; Sandvik, L.; Ormstad, H. Associations between Gait Speed and Well-Known Fall Risk Factors among Community-Dwelling Older Adults. Physiother. Res. Int. 2019, 24, e1743. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.; Nordström, A.; Gustafson, Y.; Westling, G.; Nordström, P. Increased Postural Sway during Quiet Stance as a Risk Factor for Prospective Falls in Community-Dwelling Elderly Individuals. Age Ageing 2017, 46, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, B.; Ye, H.; Duan, J.P. A Prospective Cohort Study on the Association between New Falls and Balancing Ability among Older Adults over 80 Years Who Are Independent. Exp. Gerontol. 2023, 180, 112259. [Google Scholar] [CrossRef]
- Chung, C.M.; Shin, S.; Lee, Y.G.; Lee, D.Y. Determination of the Predictors with the Greatest Influence on Walking in the Elderly. Medicina 2022, 58, 1640. [Google Scholar] [CrossRef]
- Stotz, A.; Hamacher, D.; Zech, A. Relationship between Muscle Strength and Gait Parameters in Healthy Older Women and Men. Int. J. Environ. Res. Public. Health 2023, 20, 5362. [Google Scholar] [CrossRef]
- Means, K.M.; Rodell, D.E.; O’Sullivan, P.S.; Winger, R.M. Comparison of a Functional Obstacle Course with an Index of Clinical Gait and Balance and Postural Sway. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1998, 53, M331–M335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steffen, T.M.; Mollinger, L.A. Age- and Gender-Related Test Performance in Community-Dwelling Adults. J. Neurol. Phys. Ther. 2005, 29, 181–188. [Google Scholar] [CrossRef]
- Takayanagi, N.; Sudo, M.; Yamashiro, Y.; Lee, S.; Kobayashi, Y.; Niki, Y.; Shimada, H. Relationship between Daily and In-Laboratory Gait Speed among Healthy Community-Dwelling Older Adults. Sci. Rep. 2019, 9, 3496. [Google Scholar] [CrossRef]
- Rojer, A.G.M.; Coni, A.; Mellone, S.; Van Ancum, J.M.; Vereijken, B.; Helbostad, J.L.; Taraldsen, K.; Mikolaizak, S.; Becker, C.; Aminian, K.; et al. Robustness of In-Laboratory and Daily-Life Gait Speed Measures over One Year in High Functioning 61- to 70-Year-Old Adults. Gerontology 2021, 67, 650–659. [Google Scholar] [CrossRef]
- Ganz, N.; Gazit, E.; Giladi, N.; Dawe, R.J.; Mirelman, A.; Buchman, A.S.; Hausdorff, J.M. Automatic Quantification of Tandem Walking Using a Wearable Device: New Insights into Dynamic Balance and Mobility in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 101–107. [Google Scholar] [CrossRef]
- Allison, L.K.; Kiemel, T.; Jeka, J.J. Multisensory Reweighting of Vision and Touch Is Intact in Healthy and Fall-Prone Older Adults. Exp. Brain Res. 2006, 175, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.M.; Wroblewski, K.E.; Huisingh-Scheetz, M.; Correia, C.; Lopez, K.J.; Chen, R.C.; Kern, D.W.; Schumm, P.L.; Dale, W.; McClintock, M.K. Global Sensory Impairment Predicts Morbidity and Mortality in Older U.S. Adults. J. Am. Geriatr. Soc. 2017, 65, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Diaconescu, A.O.; Hasher, L.; McIntosh, A.R. Visual Dominance and Multisensory Integration Changes with Age. Neuroimage 2013, 65, 152–166. [Google Scholar] [CrossRef]
- Pasma, J.H.; Engelhart, D.; Maier, A.B.; Schouten, A.C.; van der Kooij, H.; Meskers, C.G.M. Changes in Sensory Reweighting of Proprioceptive Information during Standing Balance with Age and Disease. J. Neurophysiol. 2015, 114, 3220–3233. [Google Scholar] [CrossRef]
- Yamagata, M.; Taniguchi, M.; Nakazato, K.; Wang, Z.; Yagi, M.; Fukumoto, Y.; Okada, S.; Okada, S.; Ichihashi, N. Fall Assessment in Healthy Older Adults: Approach Using Rambling-Trembling Decomposition Method. Clin. Biomech. 2024, 120, 106355. [Google Scholar] [CrossRef] [PubMed]
- Menant, J.C.; Schoene, D.; Sarofim, M.; Lord, S.R. Single and Dual Task Tests of Gait Speed Are Equivalent in the Prediction of Falls in Older People: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2014, 16, 83–104. [Google Scholar] [CrossRef]
- Chien, J.H.; Eikema, D.-J.A.; Mukherjee, M.; Stergiou, N. Locomotor Sensory Organization Test: A Novel Paradigm for the Assessment of Sensory Contributions in Gait. Ann. Biomed. Eng. 2014, 42, 2512–2523. [Google Scholar] [CrossRef]
| Number of the Meta-Analysis | Condition/Quotient | Outcome Measurement |
|---|---|---|
| Meta-analysis 1.1 | Eyes open/stable surface | Sway area |
| Meta-analysis 1.2 | Sway velocity | |
| Meta-analysis 1.3 | Eyes closed/stable surface | Sway area |
| Meta-analysis 1.4 | Sway velocity | |
| Meta-analysis 1.5 | Romberg Quotient | Sway area |
| Meta-analysis 1.6 | Sway velocity | |
| Meta-analysis 2.1 | Eyes open/complaint surface | Sway area |
| Meta-analysis 2.2 | Eyes closed/complaint surface | Sway area |
| Meta-analysis 2.3 | Proprioception Quotient | Sway area |
| Author (Year) | Characteristics of Participants | Sensory Integration Conditions and Foot Position | Gait Test | Outcome Measurement(s) | Findings on Sensory Integration conditions | Findings on Quotients | Meta-Analysis |
|---|---|---|---|---|---|---|---|
| Espinoza-Araneda et al. (2022) [60] | Age: 69.93 ± 4.95 (61–80) F/M: 38/33 BMI: 29.04 ± 3.74 Fallers: NR | EOS ECS EOC ECC Foot position: NR | Unstandardized test (walking for 9 m) | 95% confidence ellipse area | Significant correlations: Gait speed x EOS: r: −0.253, p: 0.034 Gait speed x ECS: r: −0.332, p: 0.005 Gait speed x ECC: r: −0.238, p: 0.046 Foot clearance x EOS: r:0.237, p: 0.047 Foot clearance x ECS: r:0.328, p: 0.005 Foot clearance x ECC: r:0.251, p: 0.035 Cycle duration x EOS: r:0.238, p: 0.046 Cycle duration x ECS: r:0.309, p: 0.009 | No significant correlation between gait speed x RQ and gait speed x PQ No significant correlation between foot clearance x RQ and foot clearance x PQ No significant correlation between cycle duration x RQ and foot clearance x PQ No significant correlation between stride length x RQ and stride length x PQ | MA 1.1 MA 1.3 MA 1.5 MA 2.1 MA 2.2 MA 2.3 |
| Harro & Garascia (2019) [59] | Age: 67.8 ± 5.1 (60–80) F/M: 24/22 BMI: NR Fallers: No fallers | SOT (EOS, ECC) Foot position: hip-distance | 10 MWT | Equilibrium score | NR | No significant correlation between gait speed x VQ | |
| Backlund et al. (2017) [52] | Age: 71 ± 4.0 (NR) F/M: 29/29 BMI: NR Fallers: NR | EOS ECS EOC Foot position: self-selected (EOS, EOC) and feet together (EOS, ECS) | 10 MWT | Peak-to-peak Sway range Sway velocity of the trunk | No significant correlation between gait speed and conditions (for both outcome measurements) | No significant correlation between gait speed x RQ and gait speed x PQ (for both outcome measurements) | MA 1.1 MA 1.2 MA 1.3 MA 1.4 MA 1.5 MA 1.6 MA 2.1 MA 2.3 |
| Lord et al. (1996) [53] | Age: 72.8 ± 6.2 (NR) F/M: 96/0 BMI: NR Fallers: 29 (30.2%; Multiple fallers 11.5%) | EOS ECS EOC ECC Foot position: NR | Unstandardized test (Gait data collected for 20 steps) | Sway area of the trunk/CoM | Significant correlations: Gait speed x EOC: r: −0.21, p < 0.01 Gait speed x ECC: r: −0.17, p < 0.05 | No significant correlation between gait speed x RQ and gait speed x PQ | MA 1.1 MA 1.3 MA 1.5 MA 2.1 MA 2.2 MA 2.3 |
| Labata-Lezaun et al. (2022) [61] | Age: 73.7 ± 7.44 (62–93) F/M: 21/31 BMI: 28.3 ± 4.12 Fallers: NR | EOS ECS Foot position: at a 30° angle, heels 2 cm apart | 4 MWT | 95% confidence ellipse area | Significant correlations: Gait speed x EOS: r: −0.31, p: 0.025 | No significant correlation between gait speed x RQ | MA 1.1 MA 1.3 MA 1.5 |
| Camicioli et al. (1997) [34] | Age: 83.2 (66–102) F/M: 24/24 BMI: NR Fallers: 19 (21.6%) | SOT (EOC, ECC) Foot position: hip-distance | Unstandardized test (walking for 9 m) | Equilibrium score | No significant correlation between gait speed and conditions | Quotients are NR | |
| Souza-Junior et al. (2022) [33] | Age: 69.3 ± 5.9 (NR) F/M: 60/0 BMI: 26.6 ± 4.4 Fallers: NR | EOS ECS Foot position: at a 30° angle, heels 6 cm apart | 3 MWT | 95% confidence ellipse area Mean CoP velocity | No significant correlation between gait speed and conditions (for both outcome measurements) | Significant correlations: gait speed x RQ: r:0.339, p: 0.001 (mean velocity) | MA 1.1 MA 1.2 MA 1.3 MA 1.4 MA 1.5 MA 1.6 |
| Scaglioni-Solano & Aragon-Vargas (2015) ¥ [54] | Age: 70.6 ± 5.7 (NR) F/M: 74/26 BMI: 27.0 ± 4.2 Fallers: Included fallers | EOS ECS EOC ECC Foot position: NR | 10 MWT | Mean CoP velocity | No significant correlation between gait speed and conditions | No significant correlation between gait speed x RQ and gait speed x PQ | MA 1.2 MA 1.4 MA 1.6 |
| Li et al. (2010) [55] | Age: 76.2 (NR) F/M: 13/7 BMI: NR Fallers: NR | SOT (EOS, EOS with sway-referenced visual surroundings, EOC) Foot position: hip-distance | 6 min Walk Test | Equilibrium score | No significant correlation between gait speed and conditions | No significant correlation between gait speed x PQ | |
| Hupfeld et al. (2021) [56] | Age: 72.8 (NR) F/M: 11/12 BMI: 26.0 ± 3.9 Fallers: NR | mCTSIB (EOS, ECS, EOC, ECC) Foot position: NR | 4 min Walk Test | 95% confidence ellipse area (CoM) | No significant correlation between gait speed and conditions | No significant correlation between gait speed x RQ and gait speed x PQ | MA 1.1 MA 1.3 MA 1.5 MA 2.1 MA 2.2 MA 2.3 |
| White et al. (2021) [57] | Age: 75.4 ± 5.3 (NR) F/M: 31/25 BMI: NR Fallers: 20% single or multiple fallers | EOS ECS EOC ECC Foot position: NR | Unstandardized test (walking for 23 m) | Trace Length (mm) | Significant correlations: Gait speed x EOS: r: −0.377, p: 0.004 Gait speed x ECS: r: −0.298, p: 0.026 Gait speed x EOC: r: −0.330, p: 0.013 | No significant correlation between gait speed x RQ and gait speed x PQ | MA 1.1 MA 1.3 MA 1.5 MA 2.1 MA 2.2 MA 2.3 |
| Si et al. (2024) θ [37] | Age: 67.4 (NR) F/M: 32/23 BMI: 23.7 Fallers: NR | EOS ECS Foot position: self-selected and feet together in both conditions | Unstandardized test (walking for 5.2 m) | Sway Velocity Index 95% confidence ellipse area | Sway Velocity Index No significant correlations between gait speed and conditions in self-selected stance Significant correlations in narrow stance: Gait speed x ECS: r: −0.350, p: 0.009 95% confidence ellipse area Significant correlations in self-selected stance: Gait speed x ECS: r: −0.273, p: 0.044 Significant correlations in narrow stance: Gait speed x ECS: r: −0.387, p: 0.004 Sway Velocity Index No significant correlations between cadence and conditions in self-selected stance Significant correlations in narrow stance: Cadence x ECS: r: −0.281, p: 0.038 95% confidence ellipse area No significant correlations between cadence and conditions in self-selected stance Significant correlations in narrow stance: Cadence x ECS: r: −0.279, p: 0.039 | Quotients are NR | MA 1.3 MA 1.4 |
| Varjan et al. (2024) [58] | Age: 72.7 ± 4.4 (65–75) F/M: 34/0 BMI: NR Fallers: NR | EOS ECS Foot position: hip-width apart, toes pointing outwards | 10 MWT | Mean CoP velocity | No significant correlations between gait speed and conditions | No significant correlation between gait speed x RQ | MA 1.2 MA 1.4 MA 1.6 |
| Author (Year) | Study Participation | Study Attrition | Prognostic Factor Measurement | Outcome Measurement | Study Confounding | Statistical Analysis and Reporting | Overall |
|---|---|---|---|---|---|---|---|
| Espinoza-Araneda et al. (2022) [60] | High | Low | Moderate | Moderate | High | High | High |
| Harro & Garascia (2019) [59] | High | Low | Low | Low | High | High | High |
| Backlund et al. (2017) [52] | High | Low | Moderate | Low | High | High | High |
| Lord et al. (1996) [53] | High | High | High | Moderate | High | High | High |
| Labata-Lezaun et al. (2022) [61] | High | Low | Moderate | Low | High | High | High |
| Camicioli et al. (1997) [34] | High | Low | Low | Moderate | Moderate | Moderate | High |
| Souza-Junior et al. (2022) [33] | High | High | Low | Low | Moderate | Low | High |
| Scaglioni-Solano & Aragon-Vargas (2015) [54] | High | High | Low | Low | High | High | High |
| Li et al. (2010) [55] | High | High | Moderate | Low | High | High | High |
| Hupfeld et al. (2021) [56] | High | High | Moderate | Low | High | High | High |
| White et al. (2021) [57] | High | High | High | Moderate | High | High | High |
| Si et al. (2024) [37] | High | Low | Low | Low | High | Low | High |
| Varjan et al. (2024) [58] | High | Low | Low | Low | High | High | High |
| Meta-Analysis | Condition/Quotient and Outcome Measurement | n | n | Pooled Effect Size | 95% CI | p-Value | I2 |
|---|---|---|---|---|---|---|---|
| Meta-analysis 1.1 | Gait speed x EOS (sway area)  | 7 | 416 | −0.235 | −0.340–(−0.13) | <0.001 | 0% |
| Meta-analysis 1.2 | Gait speed x EOS (sway velocity)  | 4 | 252 | −0.056 | −0.213–0.101 | 0.486 | 31.6% |
| Meta-analysis 1.3 | Gait speed x ECS (sway area)  | 9 | 526 | −0.201 | −0.306–(−0.102) | <0.001 | 24.6% |
| Meta-analysis 1.4 | Gait speed x ECS (sway velocity)  | 5 | 307 | −0.149 | −0.266–(−0.37) | 0.01 | 0% |
| Meta-analysis 1.5 | Gait speed x RQ (sway area) 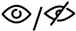 | 7 | 416 | 0.034 | −0.065–0.133 | 0.499 | 0% |
| Meta-analysis 1.6 | Gait speed x RQ (sway velocity)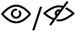 | 4 | 252 | 0.008 | −0.240–0.256 | 0.949 | 72.0% |
| Meta-analysis 2.1 | Gait speed x EOC (sway area) | 5 | 304 | −0.198 | −0.316–(−0.086) | <0.001 | 0% |
| Meta-analysis 2.2 | Gait speed x ECC (sway area)  | 4 | 246 | −0.186 | −0.316–(−0.060) | 0.004 | 0% |
| Meta-analysis 2.3 | Gait speed x PQ (sway area) 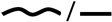 | 5 | 304 | −0.068 | −0.183–0.047 | 0.249 | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolbaşı, E.N.; van der Hulst, E.G.; Spildooren, J.; Janssens, L.; Meyns, P. Does Sensory Integration Influence Gait Parameters in Healthy Older Adults? Insights from a Systematic Review with Meta-Analysis. J. Clin. Med. 2025, 14, 4545. https://doi.org/10.3390/jcm14134545
Kolbaşı EN, van der Hulst EG, Spildooren J, Janssens L, Meyns P. Does Sensory Integration Influence Gait Parameters in Healthy Older Adults? Insights from a Systematic Review with Meta-Analysis. Journal of Clinical Medicine. 2025; 14(13):4545. https://doi.org/10.3390/jcm14134545
Chicago/Turabian StyleKolbaşı, Esma Nur, Elisabeth G. van der Hulst, Joke Spildooren, Lotte Janssens, and Pieter Meyns. 2025. "Does Sensory Integration Influence Gait Parameters in Healthy Older Adults? Insights from a Systematic Review with Meta-Analysis" Journal of Clinical Medicine 14, no. 13: 4545. https://doi.org/10.3390/jcm14134545
APA StyleKolbaşı, E. N., van der Hulst, E. G., Spildooren, J., Janssens, L., & Meyns, P. (2025). Does Sensory Integration Influence Gait Parameters in Healthy Older Adults? Insights from a Systematic Review with Meta-Analysis. Journal of Clinical Medicine, 14(13), 4545. https://doi.org/10.3390/jcm14134545