Combining Self-Reported Information with Radiographic Bone Loss to Screen Periodontitis: A Performance Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Data and Participants
2.2. Outcome
2.3. Predictors
- Confirmation from self-reported and R-PBL data (Both group);
- Confirmation through self-report (SR group);
- Confirmation with R-PBL (R-PBL group);
- Either self-reported or R-PBL confirmation (Either group).
2.4. Sample Size
2.5. Statistical Analysis
- Model Either (or model 1)—Code 1 if positive result for self-reported and R-PBL data; Code 0 if at least one or both of them negative.
- Model SR (or model 2)—Code 1 if positive result for self-reported; Code 0 if negative result for self-reported.
- Model R-PBL (or model 3)—Code 1 if positive result for R-PBL; Code 0 if negative result for R-PBL.
- Model Both (or model 4)—Code 1 if positive result for self-reported OR R-PBL data; Code 0 if both of them showed negative results.
3. Results
3.1. Participants
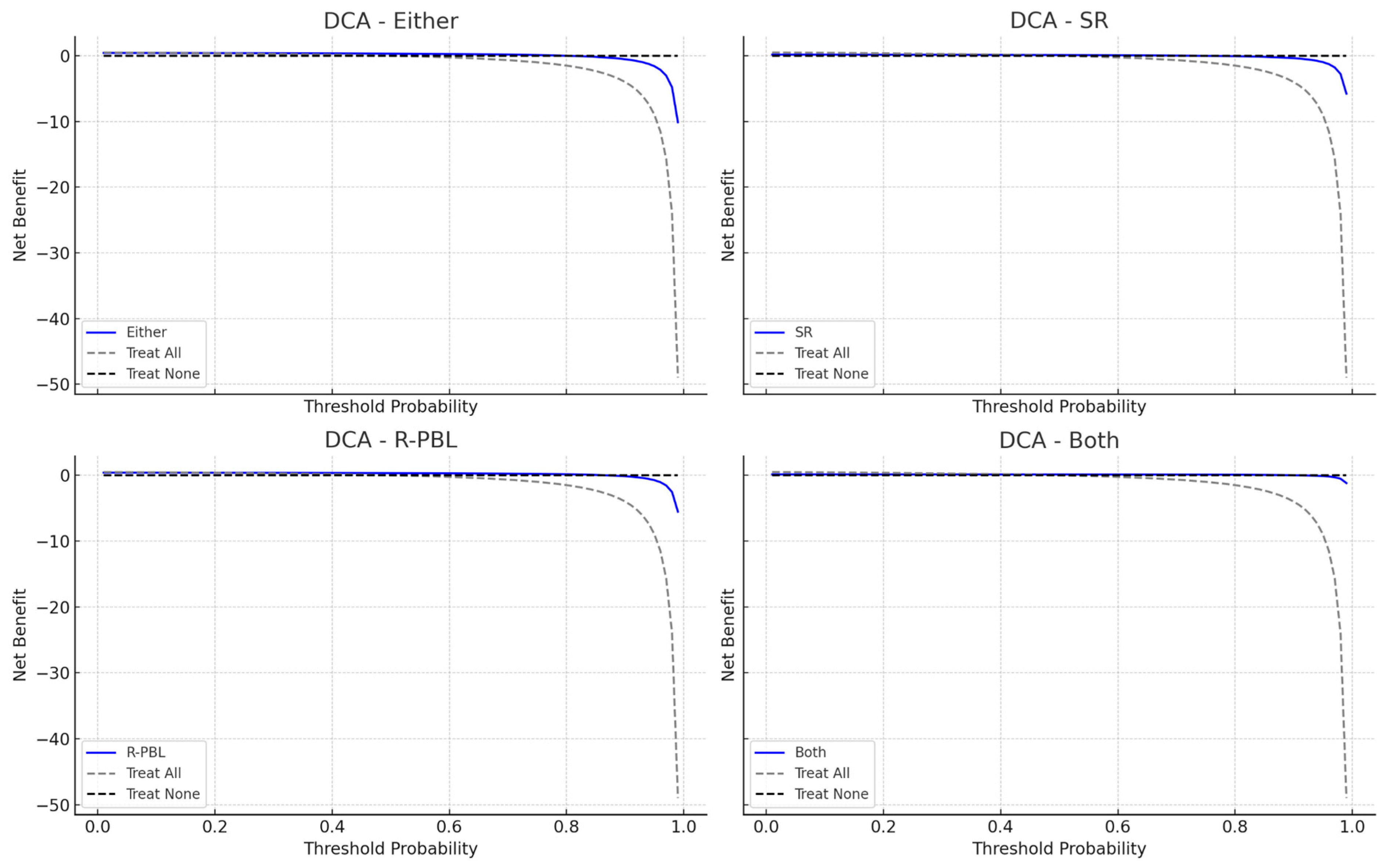
3.2. Models Performance for Periodontitis
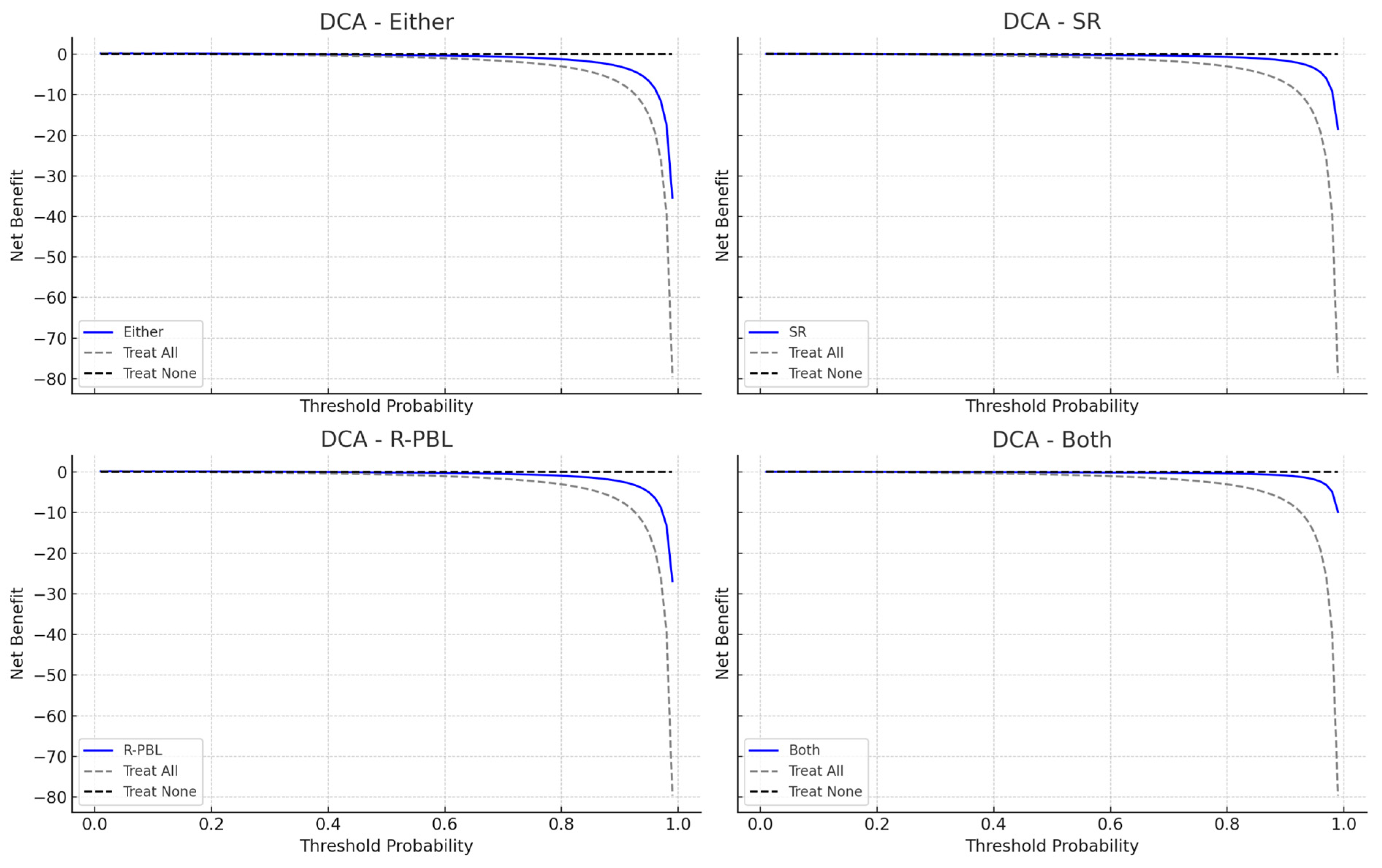
3.3. Models Performance for Severe Periodontitis
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nascimento, G.G.; Alves-Costa, S.; Romandini, M. Burden of Severe Periodontitis and Edentulism in 2021, with Projections up to 2050: The Global Burden of Disease 2021 Study. J. Periodontal Res. 2024, 59, 823–867. [Google Scholar] [CrossRef] [PubMed]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of Periodontitis in Dentate People between 2011 and 2020: A Systematic Review and Meta-Analysis of Epidemiological Studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef] [PubMed]
- Bernabe, E.; Marcenes, W.; Abdulkader, R.S.; Abreu, L.G.; Afzal, S.; Alhalaiqa, F.N.; Al-Maweri, S.; Alsharif, U.; Anyasodor, A.E.; Arora, A.; et al. Trends in the Global, Regional, and National Burden of Oral Conditions from 1990 to 2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2025, 405, 897–910. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, Fallacies and the Future. Periodontol 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Leira, Y.; Proença, L.; Chambrone, L.; Mendes, J.J. Economic Burden of Periodontitis in the United States and Europe—An Updated Estimation. J. Periodontol. 2021, 93, 373–379. [Google Scholar] [CrossRef]
- Jevdjevic, M.; Listl, S. Global, Regional, and Country-Level Economic Impacts of Oral Conditions in 2019. J. Dent. Res. 2025, 104, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Mascarenhas, P.; Viana, J.; Proença, L.; Orlandi, M.; Leira, Y.; Chambrone, L.; Mendes, J.J.; Machado, V. An Umbrella Review of the Evidence Linking Oral Health and Systemic Noncommunicable Diseases. Nat. Commun. 2022, 13, 7614. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the Global Burden of Periodontal Diseases on Health, Nutrition and Wellbeing of Mankind: A Call for Global Action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef]
- Nijland, N.; Overtoom, F.; Gerdes, V.E.A.; Verhulst, M.J.L.; Su, N.; Loos, B.G. External Validation of a Rapid, Non-Invasive Tool for Periodontitis Screening in a Medical Care Setting. Clin. Oral. Investig. 2021, 25, 6661–6669. [Google Scholar] [CrossRef]
- World Health Organization. WHO Oral Health Resolution 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Tran, D.T.; Gay, I.; Du, X.L.; Fu, Y.; Bebermeyer, R.D.; Neumann, A.S.; Streckfus, C.; Chan, W.; Walji, M.F. Assessment of Partial-Mouth Periodontal Examination Protocols for Periodontitis Surveillance. J. Clin. Periodontol. 2014, 41, 846–852. [Google Scholar] [CrossRef]
- Feher, B.; Tussie, C.; Giannobile, W.V. Applied Artificial Intelligence in Dentistry: Emerging Data Modalities and Modeling Approaches. Front. Artif. Intell. 2024, 7, 1427517. [Google Scholar] [CrossRef]
- Revilla-León, M.; Gómez-Polo, M.; Barmak, A.B.; Inam, W.; Kan, J.Y.K.; Kois, J.C.; Akal, O. Artificial Intelligence Models for Diagnosing Gingivitis and Periodontal Disease: A Systematic Review. J. Prosthet. Dent. 2023, 130, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.; Proença, L.; Morgado, M.; Mendes, J.J.; Botelho, J. Accuracy of Panoramic Radiograph for Diagnosing Periodontitis Comparing to Clinical Examination. J. Clin. Med. 2020, 9, 2313. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, S.; Zou, T.; Jin, Z.; Jiang, S. Artificial Intelligence Models for Periodontitis Classification: A Systematic Review. J. Dent. 2025, 156, 105690. [Google Scholar] [CrossRef]
- Nijland, N.; Su, N.; Gerdes, V.E.A.; Loos, B.G. Attempts to Modify Periodontal Screening Models Based on a Self-Reported Oral Health Questionnaire in the Medical Care Setting. J. Clin. Periodontol. 2025, 52, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Blicher, B.; Joshipura, K.; Eke, P. Validation of Self-Reported Periodontal Disease: A Systematic Review. J. Dent. Res. 2005, 84, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B. Assessment of Self-Report Measures for Predicting Population Prevalence of Periodontitis. J. Periodontol. 2009, 80, 1371–1379. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis Or Diagnosis (TRIPOD): The TRIPOD Statement. Ann. Intern. Med. 2015, 162, 55–63. [Google Scholar] [CrossRef]
- Lindhe, J.; Nyman, S. The Role of Occlusion in Periodontal Disease and the Biological Rationale for Splinting in Treatment of Periodontitis. Oral Sci. Rev. 1977, 10, 11–43. [Google Scholar]
- Hamp, S.E.; Nyman, S.; Lindhe, J. Periodontal Treatment of Multirooted Teeth. Results after 5 Years. J. Clin. Periodontol. 1975, 2, 126–135. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.; Lyra, P.; Santos, C.; Proença, L.; Mendes, J.J.; Botelho, J. Self-Reported Measures of Periodontitis in a Portuguese Population: A Validation Study. J. Pers. Med. 2022, 12, 1315. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Holland, F. Decision Curve Analysis to Evaluate the Clinical Benefit of Prediction Models. Spine J. 2021, 21, 1643–1648. [Google Scholar] [CrossRef]
- Van Calster, B.; Wynants, L.; Verbeek, J.F.M.; Verbakel, J.Y.; Christodoulou, E.; Vickers, A.J.; Roobol, M.J.; Steyerberg, E.W. Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators. Eur. Urol. 2018, 74, 796–804. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Meyer, S.L. Toward Precision Public Health. J. Public. Health Dent. 2020, 80, S7–S13. [Google Scholar] [CrossRef] [PubMed]
- Gansky, S.A.; Shafik, S. At the Crossroads of Oral Health Inequities and Precision Public Health. J. Public. Health Dent. 2020, 80, S14–S22. [Google Scholar] [CrossRef]
- Swami, N.; Corti, C.; Curigliano, G.; Celi, L.A.; Dee, E.C. Exploring Biases in Predictive Modelling across Diverse Populations. Lancet Healthy Longev. 2022, 3, e88. [Google Scholar] [CrossRef]
- Bhandari, A.; Wagner, T. Self-Reported Utilization of Health Care Services: Improving Measurement and Accuracy. Med. Care Res. Rev. 2006, 63, 217–235. [Google Scholar] [CrossRef]
- Riley, R.D.; Debray, T.P.A.; Collins, G.S.; Archer, L.; Ensor, J.; Smeden, M.; Snell, K.I.E. Minimum Sample Size for External Validation of a Clinical Prediction Model with a Binary Outcome. Stat. Med. 2021, 40, 4230–4251. [Google Scholar] [CrossRef]
- Tempelaar, D.; Rienties, B.; Nguyen, Q. Subjective Data, Objective Data and the Role of Bias in Predictive Modelling: Lessons from a Dispositional Learning Analytics Application. PLoS ONE 2020, 15, e0233977. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Nijman, S.; Denaxas, S.; Snell, K.I.E.; Uijl, A.; Nguyen, T.-L.; Asselbergs, F.W.; Debray, T.P.A. Internal-External Cross-Validation Helped to Evaluate the Generalizability of Prediction Models in Large Clustered Datasets. J. Clin. Epidemiol. 2021, 137, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Phua, K.; Wong, L.; Bin Goh, W.W. Extensions of the External Validation for Checking Learned Model Interpretability and Generalizability. Patterns 2020, 1, 100129. [Google Scholar] [CrossRef] [PubMed]


| Variable | Total |
|---|---|
| Age, mean (SD) [min–max] (years) | 46.5 (19.4) [18–83] |
| Sex, % (n) | |
| Female | 54.0 (81) |
| Male | 46.0 (69) |
| Periodontal diagnosis | |
| Healthy | 50.0 (75) |
| Stage I | 4.0 (6) |
| Stage II | 4.7 (7) |
| Stage III | 22.0 (33) |
| Stage IV | 19.3 (29) |
| Smoking habits | |
| Never | 54.7 (82) |
| Former | 18.0 (27) |
| Active | 27.3 (41) |
| Education | |
| Elementary | 9.3 (14) |
| Middle | 77.3 (116) |
| Higher | 13.3 (20) |
| Staging | Grading | Extent | |||
|---|---|---|---|---|---|
| A | B | C | Localized | Generalized | |
| I | 1.3 (1) | 2.7 (2) | 4.0 (3) | 1.3 (1) | 2.7 (2) |
| II | 1.3 (1) | 8.0 (6) | 0.0 (0) | 2.7 (2) | 6.7 (5) |
| III | 5.3 (4) | 25.3 (19) | 13.3 (10) | 6.7 (5) | 34.7 (26) |
| IV | 0.0 (0) | 16.0 (12) | 22.7 (17) | 0.0 (0) | 37.3 (28) |
| Model | Sensitivity | Specificity | AUC (95% CI) | E/O Ratio | Accuracy (%) | Youden’s Index (%) | Precision (%) |
|---|---|---|---|---|---|---|---|
| Either | 0.867 | 0.787 | 0.827 (0.767–0.882) | 1.08 | 82.7 | 65.3 | 80.2 |
| SR | 0.347 | 0.880 | 0.613 (0.545–0.680) | 0.467 | 61.3 | 22.7 | 74.3 |
| R-PBL | 0.787 | 0.880 | 0.833 (0.771–0.892) | 0.907 | 83.3 | 66.7 | 86.7 |
| Both | 0.267 | 0.973 | 0.620 (0.565–0.675) | 0.293 | 62.0 | 24.0 | 90.9 |
| Model | Sensitivity | Specificity | AUC (95% CI) | E/O Ratio | Accuracy (%) | Youden’s Index (%) | Precision (%) |
|---|---|---|---|---|---|---|---|
| Either | 0.931 | 0.553 | 0.742 (0.672–0.802) | 2.79 | 62.7 | 48.4 | 33.3 |
| SR | 0.241 | 0.769 | 0.505 (0.420–0.599) | 1.21 | 66.7 | 1.0 | 20.0 |
| R-PBL | 0.931 | 0.661 | 0.796 (0.724–0.853) | 2.34 | 71.3 | 59.2 | 39.7 |
| Both | 0.241 | 0.876 | 0.559 (0.473–0.645) | 0.76 | 75.3 | 11.7 | 31.8 |
| Model | Either | SR | R-PBL | Both | Either | SR | R-PBL | Both |
|---|---|---|---|---|---|---|---|---|
| Either | - | <0.001 | 0.773 | <0.001 | - | <0.001 | <0.001 | <0.001 |
| SR | - | - | <0.001 | 0.773 | - | - | <0.001 | <0.001 |
| R-PBL | - | - | - | <0.001 | - | - | - | <0.001 |
| Both | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, J.J.; Neves, M.; Supiot, C.; Pinto, L.; Tenda, D.; Silva, N.; Proença, L.; Leira, Y.; Machado, V.; Botelho, J. Combining Self-Reported Information with Radiographic Bone Loss to Screen Periodontitis: A Performance Study. J. Clin. Med. 2025, 14, 4531. https://doi.org/10.3390/jcm14134531
Mendes JJ, Neves M, Supiot C, Pinto L, Tenda D, Silva N, Proença L, Leira Y, Machado V, Botelho J. Combining Self-Reported Information with Radiographic Bone Loss to Screen Periodontitis: A Performance Study. Journal of Clinical Medicine. 2025; 14(13):4531. https://doi.org/10.3390/jcm14134531
Chicago/Turabian StyleMendes, José João, Margarida Neves, Clara Supiot, Leonor Pinto, Diogo Tenda, Nuno Silva, Luís Proença, Yago Leira, Vanessa Machado, and João Botelho. 2025. "Combining Self-Reported Information with Radiographic Bone Loss to Screen Periodontitis: A Performance Study" Journal of Clinical Medicine 14, no. 13: 4531. https://doi.org/10.3390/jcm14134531
APA StyleMendes, J. J., Neves, M., Supiot, C., Pinto, L., Tenda, D., Silva, N., Proença, L., Leira, Y., Machado, V., & Botelho, J. (2025). Combining Self-Reported Information with Radiographic Bone Loss to Screen Periodontitis: A Performance Study. Journal of Clinical Medicine, 14(13), 4531. https://doi.org/10.3390/jcm14134531










