Changes to the Intercondylar Ligaments of the Knee in Different Stages of Osteoarthritis—A Retrospective Cross-Sectional Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Image Evaluation
2.2. Statistics
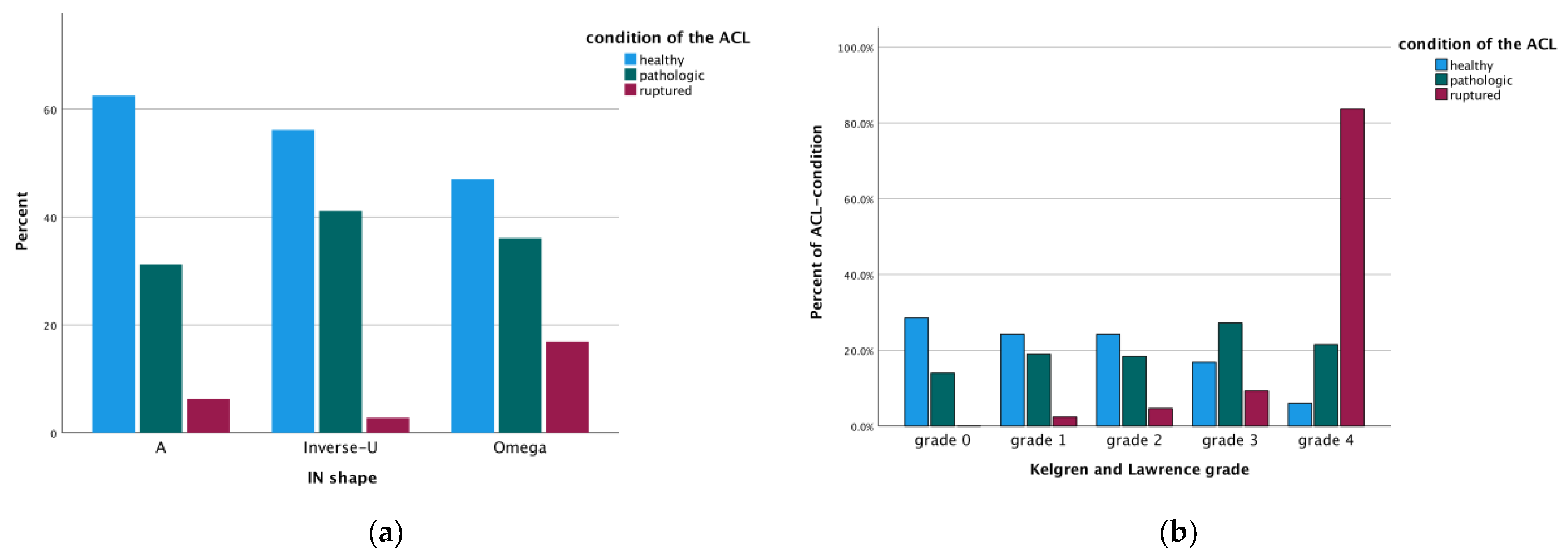
3. Results
Differences Between Coronal and Axial Plane and Intra- and Interobserver Reliability
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirtler, L.; Rohrich, S.; Kainberger, F. The Femoral Intercondylar Notch During Life: An Anatomic Redefinition With Patterns Predisposing to Cruciate Ligament Impingement. Am. J. Roentgenol. 2016, 207, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Ekdahl, M.; Shen, W.; Fu, F.H. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: An anatomic study. Arthrosc. J. Arthrosc. Relat. Surg. 2007, 23, 1218–1225. [Google Scholar] [CrossRef]
- Ferretti, M.; Levicoff, E.A.; Macpherson, T.A.; Moreland, M.S.; Cohen, M.; Fu, F.H. The fetal anterior cruciate ligament: An anatomic and histologic study. Arthrosc. J. Arthrosc. Relat. Surg. 2007, 23, 278–283. [Google Scholar] [CrossRef]
- Petersen, W.; Zantop, T. Anatomy of the anterior cruciate ligament with regard to its two bundles. Clin. Orthop. Relat. Res. 2007, 454, 35–47. [Google Scholar] [CrossRef]
- Siebold, R.; Ellert, T.; Metz, S.; Metz, J. Femoral insertions of the anteromedial and posterolateral bundles of the anterior cruciate ligament: Morphometry and arthroscopic orientation models for double-bundle bone tunnel placement—A cadaver study. Arthrosc. J. Arthrosc. Relat. Surg. 2008, 24, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Bicer, E.K.; Lustig, S.; Servien, E.; Selmi, T.A.; Neyret, P. Current knowledge in the anatomy of the human anterior cruciate ligament. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Tampere, T.; Van Hoof, T.; Cromheecke, M.; Van der Bracht, H.; Chahla, J.; Verdonk, P.; Victor, J. The anterior cruciate ligament: A study on its bony and soft tissue anatomy using novel 3D CT technology. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 236–244. [Google Scholar] [CrossRef]
- Skelley, N.W.; Castile, R.M.; York, T.E.; Gruev, V.; Lake, S.P.; Brophy, R.H. Differences in the microstructural properties of the anteromedial and posterolateral bundles of the anterior cruciate ligament. Am. J. Sports Med. 2015, 43, 928–936. [Google Scholar] [CrossRef]
- Girgis, F.G.; Marshall, J.L.; Monajem, A. The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clin. Orthop. Relat. Res. 1975, 106, 216–231. [Google Scholar] [CrossRef]
- Giron, F.; Cuomo, P.; Aglietti, P.; Bull, A.M.; Amis, A.A. Femoral attachment of the anterior cruciate ligament. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 250–256. [Google Scholar] [CrossRef]
- Mochizuki, T.; Muneta, T.; Nagase, T.; Shirasawa, S.; Akita, K.I.; Sekiya, I. Cadaveric knee observation study for describing anatomic femoral tunnel placement for two-bundle anterior cruciate ligament reconstruction. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 356–361. [Google Scholar] [CrossRef]
- Kennedy, J.C.; Grainger, R.W. The posterior cruciate ligament. J. Trauma 1967, 7, 367–377. [Google Scholar] [CrossRef]
- Race, A.; Amis, A.A. Loading of the two bundles of the posterior cruciate ligament: An analysis of bundle function in a-P drawer. J. Biomech. 1996, 29, 873–879. [Google Scholar] [CrossRef]
- Sepulchre, P.; Blaimont, P. Biomechanics of the posterior cruciate ligament of the knee. Acta Orthop. Belg. 1986, 52, 437–448. [Google Scholar] [PubMed]
- Harner, C.D.; Xerogeanes, J.W.; Livesay, G.A.; Carlin, G.J.; Smith, B.A.; Kusayama, T.; Kashiwaguchi, S.; Woo, S.L. The human posterior cruciate ligament complex: An interdisciplinary study. Ligament morphology and biomechanical evaluation. Am. J. Sports Med. 1995, 23, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Gollehon, D.L.; Torzilli, P.A.; Warren, R.F. The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J. Bone Jt. Surg. Am. 1987, 69, 233–242. [Google Scholar] [CrossRef]
- Fox, R.J.; Harner, C.D.; Sakane, M.; Carlin, G.J.; Woo, S.L. Determination of the in situ forces in the human posterior cruciate ligament using robotic technology. A cadaveric study. Am. J. Sports Med. 1998, 26, 395–401. [Google Scholar] [CrossRef]
- Kaplan, E.B. The lateral meniscofemoral ligament of the knee joint. Bull. Hosp. Jt. Dis. 1956, 17, 176–182. [Google Scholar]
- Heller, L.; Langman, J. The Menisco-Femoral Ligaments of the Human Knee. J. Bone Jt. Surg. Br. Vol. 1964, 46, 307–313. [Google Scholar] [CrossRef]
- Poynton, A.R.; Javadpour, S.M.; Finegan, P.J.; O’Brien, M. The meniscofemoral ligaments of the knee. J. Bone Jt. Surg. Br. Vol. 1997, 79, 327–330. [Google Scholar] [CrossRef]
- Cho, J.M.; Suh, J.S.; Na, J.B.; Cho, J.H.; Kim, Y.; Yoo, W.K.; Lee, H.Y.; Chung, I.H. Variations in meniscofemoral ligaments at anatomical study and MR imaging. Skelet. Radiol. 1999, 28, 189–195. [Google Scholar] [CrossRef]
- Lee, B.Y.; Jee, W.H.; Kim, J.M.; Kim, B.S.; Choi, K.H. Incidence and significance of demonstrating the meniscofemoral ligament on MRI. Br. J. Radiol. 2000, 73, 271–274. [Google Scholar] [CrossRef]
- Erbagci, H.; Yildirim, H.; Kizilkan, N.; Gumusburun, E. An MRI study of the meniscofemoral and transverse ligaments of the knee. Surg. Radiol. Anat. 2002, 24, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Gupte, C.M.; Smith, A.; Jamieson, N.; Bull, A.M.; Thomas, R.D.; Amis, A.A. Meniscofemoral ligaments--structural and material properties. J. Biomech. 2002, 35, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Gupte, C.M.; Smith, A.; McDermott, I.D.; Bull, A.M.; Thomas, R.D.; Amis, A.A. Meniscofemoral ligaments revisited. Anatomical study, age correlation and clinical implications. J. Bone Jt. Surg. Br. Vol. 2002, 84, 846–851. [Google Scholar] [CrossRef]
- Amis, A.A.; Bull, A.M.; Gupte, C.M.; Hijazi, I.; Race, A.; Robinson, J.R. Biomechanics of the PCL and related structures: Posterolateral, posteromedial and meniscofemoral ligaments. Knee Surg. Sports Traumatol. Arthrosc. 2003, 11, 271–281. [Google Scholar] [CrossRef]
- Amis, A.A.; Gupte, C.M.; Bull, A.M.; Edwards, A. Anatomy of the posterior cruciate ligament and the meniscofemoral ligaments. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 257–263; discussion 203. [Google Scholar] [CrossRef]
- Gupte, C.M.; Bull, A.M.; Thomas, R.D.; Amis, A.A. A review of the function and biomechanics of the meniscofemoral ligaments. Arthrosc. J. Arthrosc. Relat. Surg 2003, 19, 161–171. [Google Scholar] [CrossRef]
- Nagasaki, S.; Ohkoshi, Y.; Yamamoto, K.; Ebata, W.; Imabuchi, R.; Nishiike, J. The incidence and cross-sectional area of the meniscofemoral ligament. Am. J. Sports Med. 2006, 34, 1345–1350. [Google Scholar] [CrossRef]
- Domzalski, M.; Grzelak, P.; Gabos, P. Risk factors for Anterior Cruciate Ligament injury in skeletally immature patients: Analysis of intercondylar notch width using Magnetic Resonance Imaging. Int. Orthop. 2010, 34, 703–707. [Google Scholar] [CrossRef]
- Miljko, M.; Grle, M.; Kozul, S.; Kolobaric, M.; Djak, I. Intercondylar notch width and inner angle of lateral femoral condyle as the risk factors for anterior cruciate ligament injury in female handball players in Herzegovina. Coll. Antropol. 2012, 36, 195–200. [Google Scholar]
- Everhart, J.S.; Flanigan, D.C.; Simon, R.A.; Chaudhari, A.M. Association of noncontact anterior cruciate ligament injury with presence and thickness of a bony ridge on the anteromedial aspect of the femoral intercondylar notch. Am. J. Sports Med. 2010, 38, 1667–1673. [Google Scholar] [CrossRef]
- Anderson, A.F.; Dome, D.C.; Gautam, S.; Awh, M.H.; Rennirt, G.W. Correlation of anthropometric measurements, strength, anterior cruciate ligament size, and intercondylar notch characteristics to sex differences in anterior cruciate ligament tear rates. Am. J. Sports Med. 2001, 29, 58–66. [Google Scholar] [CrossRef]
- Stijak, L.; Malis, M.; Maksimovic, R.; Aksic, M.; Filipovic, B. The influence of the morphometric parameters of the intercondylar notch on rupture of the anterior cruciate ligament. Vojnosanit. Pregl. 2012, 69, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, D.H.; Lee, S.H.; Kim, J.M.; Kim, C.W.; Bin, S.I. Arthroscopic treatment of mucoid hypertrophy of the anterior cruciate ligament. Arthrosc. J. Arthrosc. Relat. Surg. 2008, 24, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Ahn, C.; Fung, D.T.; Ren, Y.; Zhang, L.Q. A knee-specific finite element analysis of the human anterior cruciate ligament impingement against the femoral intercondylar notch. J. Biomech. 2010, 43, 2039–2042. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Burnett, Q.M. Femoral intercondylar notch stenosis and correlation to anterior cruciate ligament injuries. A prospective study. Am. J. Sports Med. 1994, 22, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Fung, D.T.; Hendrix, R.W.; Koh, J.L.; Zhang, L.Q. ACL impingement prediction based on MRI scans of individual knees. Clin. Orthop. Relat. Res. 2007, 460, 210–218. [Google Scholar] [CrossRef]
- Souryal, T.O.; Freeman, T.R. Intercondylar notch size and anterior cruciate ligament injuries in athletes. A prospective study. Am. J. Sports Med. 1993, 21, 535–539. [Google Scholar] [CrossRef]
- Feagin, J.A., Jr.; Lambert, K.L. Mechanism of injury and pathology of anterior cruciate ligament injuries. Orthop. Clin. N. Am. 1985, 16, 41–45. [Google Scholar] [CrossRef]
- Fleming, B.C.; Renstrom, P.A.; Beynnon, B.D.; Engstrom, B.; Peura, G.D.; Badger, G.J.; Johnson, R.J. The effect of weightbearing and external loading on anterior cruciate ligament strain. J. Biomech. 2001, 34, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, G.; Benoit, D.L.; Lamontagne, M.; Caraffa, A.; Liti, A. In vivo anterior cruciate ligament strain behaviour during a rapid deceleration movement: Case report. Knee Surg. Sports Traumatol. Arthrosc. 2003, 11, 307–311. [Google Scholar] [CrossRef]
- Lahlaidi, A.; Vaclavek, J. The posterior menisco-femoral ligaments and their significance in organogenesis. Bull. Assoc. Anat. 1975, 59, 177–183. [Google Scholar]
- Hassine, D.; Feron, J.M.; Henry-Feugeas, M.C.; Schouman-Claeys, E.; Guerin Surville, H.; Frija, G. The meniscofemoral ligaments: Magnetic resonance imaging and anatomic correlations. Surg. Radiol. Anat. 1992, 14, 59–63. [Google Scholar] [CrossRef]
- Coulier, B. Signification of the unusual delineation of the anterior meniscofemoral ligament of Humphrey during knee arthro-CT. Surg. Radiol. Anat. 2009, 31, 121–128. [Google Scholar] [CrossRef]
- Müller, W. The Knee: Form, Function and Ligamentous Reconstruction Surgery; Springer: Berlin, Germany, 1982. [Google Scholar]
- Brantigan, O.C.; Voshell, A.F. The Mechanics of the Ligaments and Menisci of the Knee Joint. J. Bone Jt. Surg. Am. 1941, 23, 44–66. [Google Scholar]
- Last, R.J. Some anatomical details of the knee joint. J. Bone Jt. Surg. Br. Vol. 1948, 30, 683–688. [Google Scholar] [CrossRef]
- Amadi, H.O.; Gupte, C.M.; Lie, D.T.; McDermott, I.D.; Amis, A.A.; Bull, A.M. A biomechanical study of the meniscofemoral ligaments and their contribution to contact pressure reduction in the knee. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 1004–1008. [Google Scholar] [CrossRef]
- Lertwanich, P.; Martins, C.A.; Kato, Y.; Ingham, S.J.; Kramer, S.; Linde-Rosen, M.; Smolinski, P.; Fu, F.H. Contribution of the meniscofemoral ligament as a restraint to the posterior tibial translation in a porcine knee. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1277–1281. [Google Scholar] [CrossRef]
- Forkel, P.; Herbort, M.; Schulze, M.; Rosenbaum, D.; Kirstein, L.; Raschke, M.; Petersen, W. Biomechanical consequences of a posterior root tear of the lateral meniscus: Stabilizing effect of the meniscofemoral ligament. Arch. Orthop. Trauma Surg. 2013, 133, 621–626. [Google Scholar] [CrossRef]
- Gupte, C.M.; Bull, A.M.; Thomas, R.D.; Amis, A.A. The meniscofemoral ligaments: Secondary restraints to the posterior drawer. Analysis of anteroposterior and rotary laxity in the intact and posterior-cruciate-deficient knee. J. Bone Jt. Surg. Br. Vol. 2003, 85, 765–773. [Google Scholar] [CrossRef]
- Rohrich, S.; Kainberger, F.; Hirtler, L. Evaluation of age-dependent morphometrics of the meniscofemoral ligaments in reference to the posterior cruciate ligament in routine MRI. Eur. Radiol. 2018, 28, 2369–2379. [Google Scholar] [CrossRef]
- Kennedy, J.C.; Hawkins, R.J.; Willis, R.B.; Danylchuck, K.D. Tension studies of human knee ligaments. Yield point, ultimate failure, and disruption of the cruciate and tibial collateral ligaments. J. Bone Jt. Surg. Am. 1976, 58, 350–355. [Google Scholar] [CrossRef]
- Race, A.; Amis, A.A. The mechanical properties of the two bundles of the human posterior cruciate ligament. J. Biomech. 1994, 27, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bintoudi, A.; Natsis, K.; Tsitouridis, I. Anterior and posterior meniscofemoral ligaments: MRI evaluation. Anat. Res. Int. 2012, 2012, 839724. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Rheumatism in miners. II. X-ray study. Br. J. Ind. Med. 1952, 9, 197–207. [Google Scholar] [CrossRef][Green Version]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of rheumatoid arthritis. Ann. Rheum. Dis. 1957, 16, 485–493. [Google Scholar] [CrossRef]
- Hirtler, L.; Schreiner, M.; Röhrich, S.; Kandathil, S.A.; Kainberger, F. Morphological changes to the intercondylar space in different stages of osteoarthritis—A retrospective cross-sectional study. Ann. Anat.-Anat. Anz. 2025, 259, 152388. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Everhart, J.S.; Flanigan, D.C.; Chaudhari, A.M. Anteromedial ridging of the femoral intercondylar notch: An anatomic study of 170 archival skeletal specimens. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 80–87. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Y.; Geng, B.; Tan, X.; Zhang, B.; Jayswal, C.K.; Khan, M.S.; Meng, H.; Ding, N.; Jiang, J.; et al. Intercondylar Notch Stenosis of Knee Osteoarthritis and Relationship between Stenosis and Osteoarthritis Complicated with Anterior Cruciate Ligament Injury: A Study in MRI. Medicine 2016, 95, e3439. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T.; Bowes, M.A.; Niu, J.; De Souza, K.M.; Vincent, G.R.; Goggins, J.; Zhang, Y.; Felson, D.T. Magnetic resonance imaging-based three-dimensional bone shape of the knee predicts onset of knee osteoarthritis: Data from the osteoarthritis initiative. Arthritis Rheum. 2013, 65, 2048–2058. [Google Scholar] [CrossRef]
- Marshall, J.L. Periarticular osteophytes. Initiation and formation in the knee of the dog. Clin. Orthop. Relat. Res. 1969, 62, 37–47. [Google Scholar]
- Leon, H.O.; Blanco, C.E.; Guthrie, T.B.; Martinez, O.J. Intercondylar notch stenosis in degenerative arthritis of the knee. Arthrosc. J. Arthrosc. Relat. Surg. 2005, 21, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Staeubli, H.U.; Adam, O.; Becker, W.; Burgkart, R. Anterior cruciate ligament and intercondylar notch in the coronal oblique plane: Anatomy complemented by magnetic resonance imaging in cruciate ligament-intact knees. Arthrosc. J. Arthrosc. Relat. Surg. 1999, 15, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Pai, Y.C. Impaired proprioception and osteoarthritis. Curr. Opin. Rheumatol. 1997, 9, 253–258. [Google Scholar] [CrossRef]
- Wada, M.; Tatsuo, H.; Baba, H.; Asamoto, K.; Nojyo, Y. Femoral intercondylar notch measurements in osteoarthritic knees. Rheumatology 1999, 38, 554–558. [Google Scholar] [CrossRef]
- Hernigou, P.; Garabedian, J.M. Intercondylar notch width and the risk for anterior cruciate ligament rupture in the osteoarthritic knee: Evaluation by plain radiography and CT scan. Knee 2002, 9, 313–316. [Google Scholar] [CrossRef]
- Lund-Hanssen, H.; Gannon, J.; Engebretsen, L.; Holen, K.J.; Anda, S.; Vatten, L. Intercondylar notch width and the risk for anterior cruciate ligament rupture. A case-control study in 46 female handball players. Acta Orthop. Scand. 1994, 65, 529–532. [Google Scholar] [CrossRef]
- Shelbourne, K.D.; Kerr, B. The relationship of femoral intercondylar notch width to height, weight, and sex in patients with intact anterior cruciate ligaments. Am. J. Knee Surg. 2001, 14, 92–96. [Google Scholar]
- Shelbourne, K.D.; Facibene, W.A.; Hunt, J.J. Radiographic and intraoperative intercondylar notch width measurements in men and women with unilateral and bilateral anterior cruciate ligament tears. Knee Surg. Sports Traumatol. Arthrosc. 1997, 5, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.A.; Agel, J.; Dick, R. Anterior cruciate ligament injury patterns among collegiate men and women. J. Athl. Train. 1999, 34, 86–92. [Google Scholar]
- Cha, J.H.; Lee, S.H.; Shin, M.J.; Choi, B.K.; Bin, S.I. Relationship between mucoid hypertrophy of the anterior cruciate ligament (ACL) and morphologic change of the intercondylar notch: MRI and arthroscopy correlation. Skelet. Radiol. 2008, 37, 821–826. [Google Scholar] [CrossRef]
- Amiel, D.; Woo, S.L.; Harwood, F.L.; Akeson, W.H. The effect of immobilization on collagen turnover in connective tissue: A biochemical-biomechanical correlation. Acta Orthop. Scand. 1982, 53, 325–332. [Google Scholar] [CrossRef]
- Cowin, S.C. The mechanical and stress adaptive properties of bone. Ann. Biomed. Eng. 1983, 11, 263–295. [Google Scholar] [CrossRef]
- Maffulli, N.; King, J.B. Effects of physical activity on some components of the skeletal system. Sports Med. 1992, 13, 393–407. [Google Scholar] [CrossRef]
- Newton, P.O.; Woo, S.L.; MacKenna, D.A.; Akeson, W.H. Immobilization of the knee joint alters the mechanical and ultrastructural properties of the rabbit anterior cruciate ligament. J. Orthop. Res. 1995, 13, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Noyes, F.R.; Torvik, P.J.; Hyde, W.B.; DeLucas, J.L. Biomechanics of ligament failure. II. An analysis of immobilization, exercise, and reconditioning effects in primates. J. Bone Jt. Surg. Am. 1974, 56, 1406–1418. [Google Scholar] [CrossRef]
- Rasch, P.J.; Maniscalco, R.; Pierson, W.R.; Logan, G.A. Effect of exercise, immobilization and intermittent stretching on strength of knee ligaments of albino rats. J. Appl. Physiol. 1960, 15, 289–290. [Google Scholar] [CrossRef]
- Tipton, C.M.; James, S.L.; Mergner, W.; Tcheng, T.K. Influence of exercise on strength of medial collateral knee ligaments of dogs. Am. J. Physiol. 1970, 218, 894–902. [Google Scholar] [CrossRef]
- Yasuda, K.; Hayashi, K. Changes in biomechanical properties of tendons and ligaments from joint disuse. Osteoarthr. Cartil. 1999, 7, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Quasnichka, H.L.; Anderson-MacKenzie, J.M.; Tarlton, J.F.; Sims, T.J.; Billingham, M.E.; Bailey, A.J. Cruciate ligament laxity and femoral intercondylar notch narrowing in early-stage knee osteoarthritis. Arthritis Rheum. 2005, 52, 3100–3109. [Google Scholar] [CrossRef]
- Anderson, A.F.; Lipscomb, A.B.; Liudahl, K.J.; Addlestone, R.B. Analysis of the intercondylar notch by computed tomography. Am. J. Sports Med. 1987, 15, 547–552. [Google Scholar] [CrossRef]
- Houseworth, S.W.; Mauro, V.J.; Mellon, B.A.; Kieffer, D.A. The intercondylar notch in acute tears of the anterior cruciate ligament: A computer graphics study. Am. J. Sports Med. 1987, 15, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Ireland, M.L.; Ballantyne, B.T.; Little, K.; McClay, I.S. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee Surg. Sports Traumatol. Arthrosc. 2001, 9, 200–205. [Google Scholar] [CrossRef]
- Hoteya, K.; Kato, Y.; Motojima, S.; Ingham, S.J.; Horaguchi, T.; Saito, A.; Tokuhashi, Y. Association between intercondylar notch narrowing and bilateral anterior cruciate ligament injuries in athletes. Arch. Orthop. Trauma Surg. 2011, 131, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Keays, S.L.; Keays, R.; Newcombe, P.A. Femoral intercondylar notch width size: A comparison between siblings with and without anterior cruciate ligament injuries. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 672–679. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Archbold, P.; Cucurulo, T.; Fayard, J.M.; Bortolletto, J.; Thaunat, M.; Prost, T.; Chambat, P. The influence of the tibial slope and the size of the intercondylar notch on rupture of the anterior cruciate ligament. J. Bone Jt. Surg. Br. Vol. 2011, 93, 1475–1478. [Google Scholar] [CrossRef]
- Good, L.; Odensten, M.; Gillquist, J. Intercondylar notch measurements with special reference to anterior cruciate ligament surgery. Clin. Orthop. Relat. Res. 1991, 263, 185–189. [Google Scholar] [CrossRef]
- Swami, V.G.; Mabee, M.; Hui, C.; Jaremko, J.L. Three-dimensional intercondylar notch volumes in a skeletally immature pediatric population: A magnetic resonance imaging-based anatomic comparison of knees with torn and intact anterior cruciate ligaments. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 1954–1962. [Google Scholar] [CrossRef]
- Whitney, D.C.; Sturnick, D.R.; Vacek, P.M.; DeSarno, M.J.; Gardner-Morse, M.; Tourville, T.W.; Smith, H.C.; Slauterbeck, J.R.; Johnson, R.J.; Shultz, S.J.; et al. Relationship Between the Risk of Suffering a First-Time Noncontact ACL Injury and Geometry of the Femoral Notch and ACL: A Prospective Cohort Study with a Nested Case-Control Analysis. Am. J. Sports Med. 2014, 42, 1796–1805. [Google Scholar] [CrossRef]
- Lambert, K.L. Vascularized patellar tendon graft with rigid internal fixation for anterior cruciate ligament insufficiency. Clin. Orthop. Relat. Res. 1983, 172, 85–89. [Google Scholar] [CrossRef]
- Amling, M.; Posl, M.; Wening, V.J.; Ritzel, H.; Hahn, M.; Delling, G. Structural heterogeneity within the axis: The main cause in the etiology of dens fractures. A histomorphometric analysis of 37 normal and osteoporotic autopsy cases. J. Neurosurg. 1995, 83, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Alentorn-Geli, E.; Pelfort, X.; Mingo, F.; Lizano-Diez, X.; Leal-Blanquet, J.; Torres-Claramunt, R.; Hinarejos, P.; Puig-Verdie, L.; Monllau, J.C. An Evaluation of the Association Between Radiographic Intercondylar Notch Narrowing and Anterior Cruciate Ligament Injury in Men: The Notch Angle Is a Better Parameter Than Notch Width. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Sturnick, D.R.; Vacek, P.M.; DeSarno, M.J.; Gardner-Morse, M.G.; Tourville, T.W.; Slauterbeck, J.R.; Johnson, R.J.; Shultz, S.J.; Beynnon, B.D. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am. J. Sports Med. 2015, 43, 839–847. [Google Scholar] [CrossRef]
- Simon, R.A.; Everhart, J.S.; Nagaraja, H.N.; Chaudhari, A.M. A case-control study of anterior cruciate ligament volume, tibial plateau slopes and intercondylar notch dimensions in ACL-injured knees. J. Biomech. 2010, 43, 1702–1707. [Google Scholar] [CrossRef]
- Fernandez-Jaen, T.; Lopez-Alcorocho, J.M.; Rodriguez-Inigo, E.; Castellan, F.; Hernandez, J.C.; Guillen-Garcia, P. The Importance of the Intercondylar Notch in Anterior Cruciate Ligament Tears. Orthop. J. Sports Med. 2015, 3, 2325967115597882. [Google Scholar] [CrossRef]
- Al-Saeed, O.; Brown, M.; Athyal, R.; Sheikh, M. Association of femoral intercondylar notch morphology, width index and the risk of anterior cruciate ligament injury. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Kusayama, T.; Harner, C.D.; Carlin, G.J.; Xerogeanes, J.W.; Smith, B.A. Anatomical and biomechanical characteristics of human meniscofemoral ligaments. Knee Surg. Sports Traumatol. Arthrosc. 1994, 2, 234–237. [Google Scholar] [CrossRef]
- Poynton, A.; Moran, C.J.; Moran, R.; O’Brien, M. The meniscofemoral ligaments influence lateral meniscal motion at the human knee joint. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 365–371. [Google Scholar] [CrossRef]
- Gupte, C.M.; Bull, A.M.; Atkinson, H.D.; Thomas, R.D.; Strachan, R.K.; Amis, A.A. Arthroscopic appearances of the meniscofemoral ligaments: Introducing the “meniscal tug test”. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, M.R.; Chung, C.B.; Trudell, D.; Resnick, D. Meniscofemoral ligaments: Patterns of tears and pseudotears of the menisci using cadaveric and clinical material. Skelet. Radiol. 2007, 36, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.C.; Felle, P. The menisco-femoral ligaments. Clin. Anat. 1995, 8, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Hirohata, K. Anatomical study on the menisco-femoral ligaments of the knee. Kobe J. Med. Sci. 1991, 37, 209–226. [Google Scholar]
- Harner, C.D.; Baek, G.H.; Vogrin, T.M.; Carlin, G.J.; Kashiwaguchi, S.; Woo, S.L. Quantitative analysis of human cruciate ligament insertions. Arthrosc. J. Arthrosc. Relat. Surg. 1999, 15, 741–749. [Google Scholar] [CrossRef]
- Cross, M.B.; Raphael, B.S.; Maak, T.G.; Plaskos, C.; Egidy, C.C.; Pearle, A.D. Characterization of the orientation and isometry of Humphrey’s ligament. Knee 2013, 20, 515–519. [Google Scholar] [CrossRef]
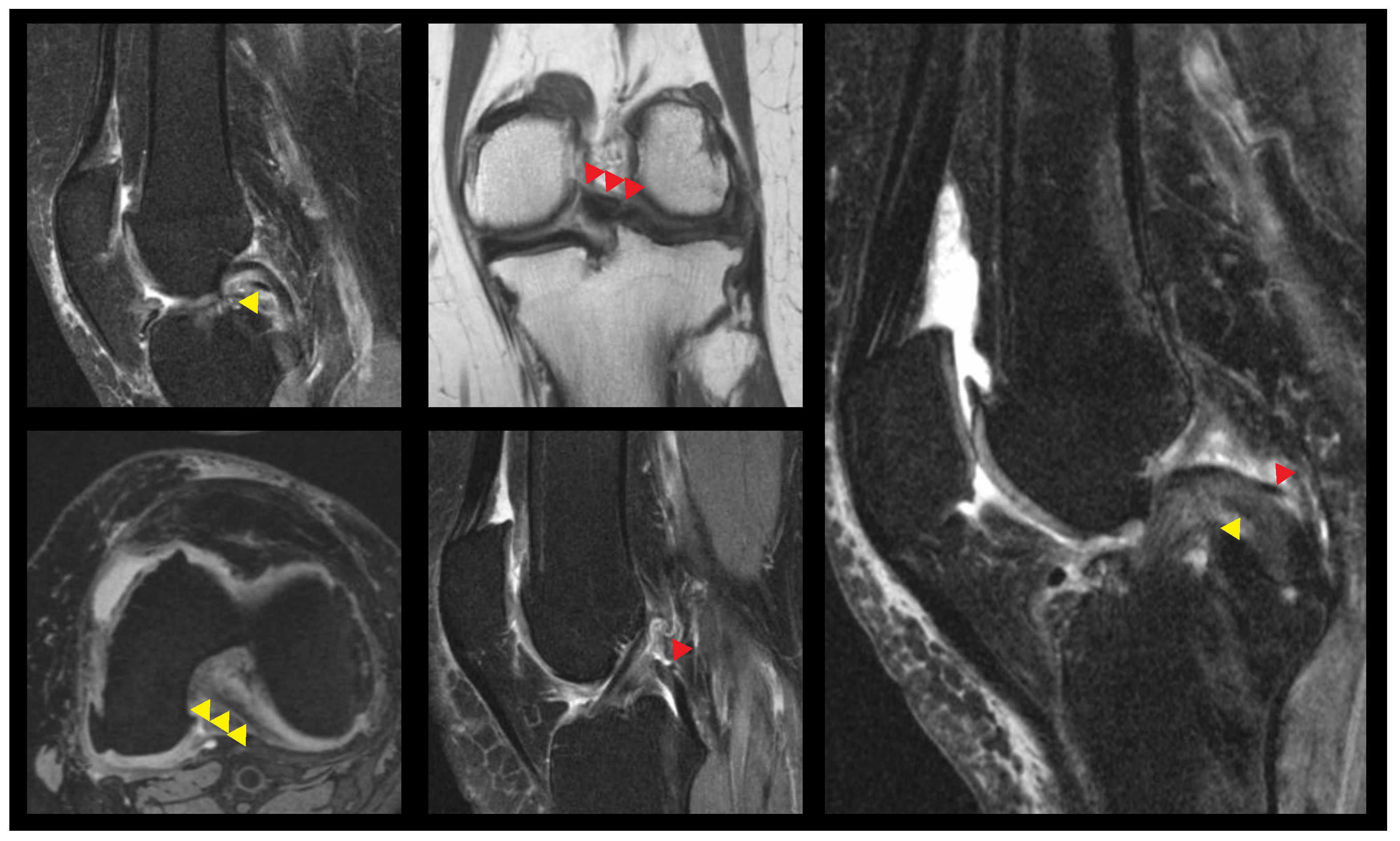
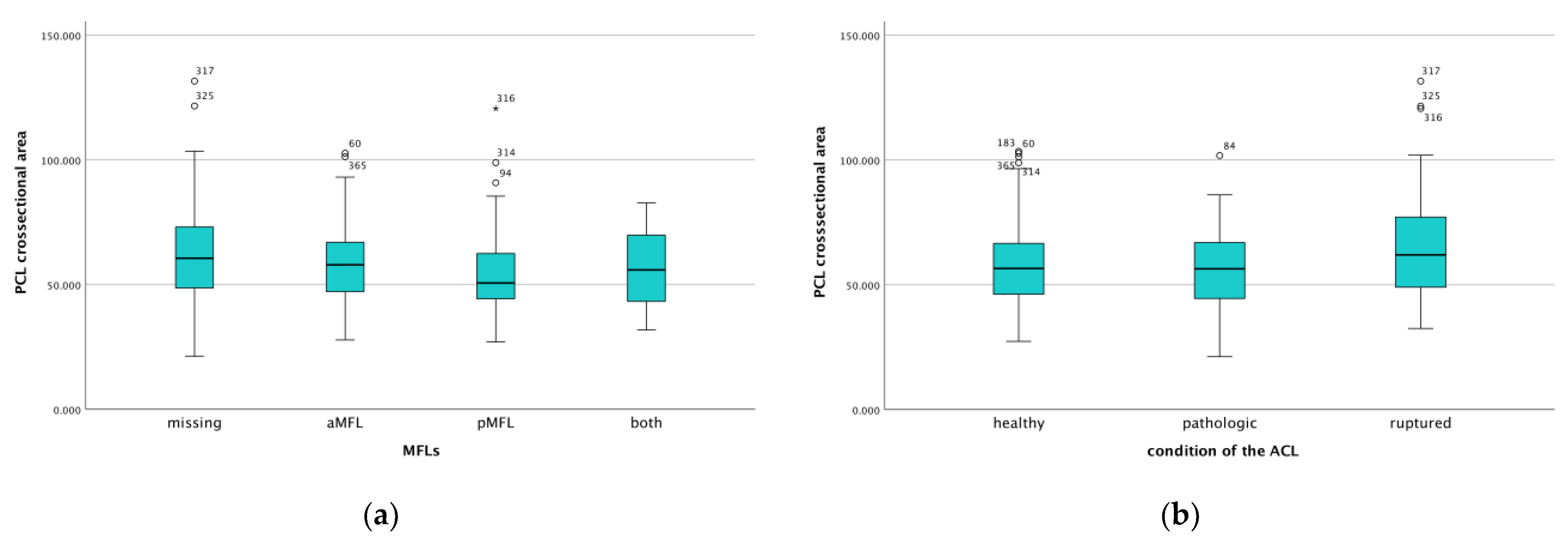
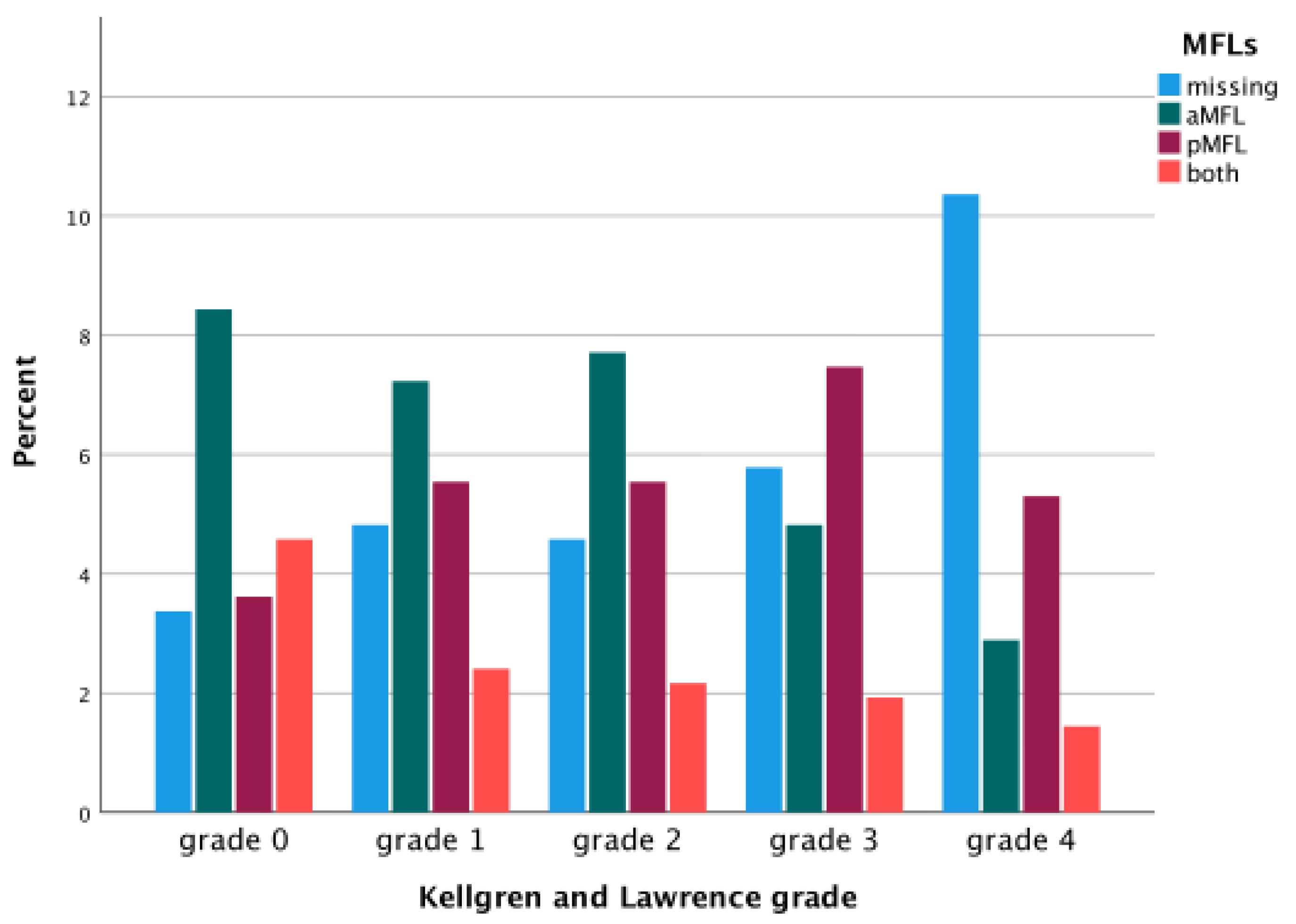
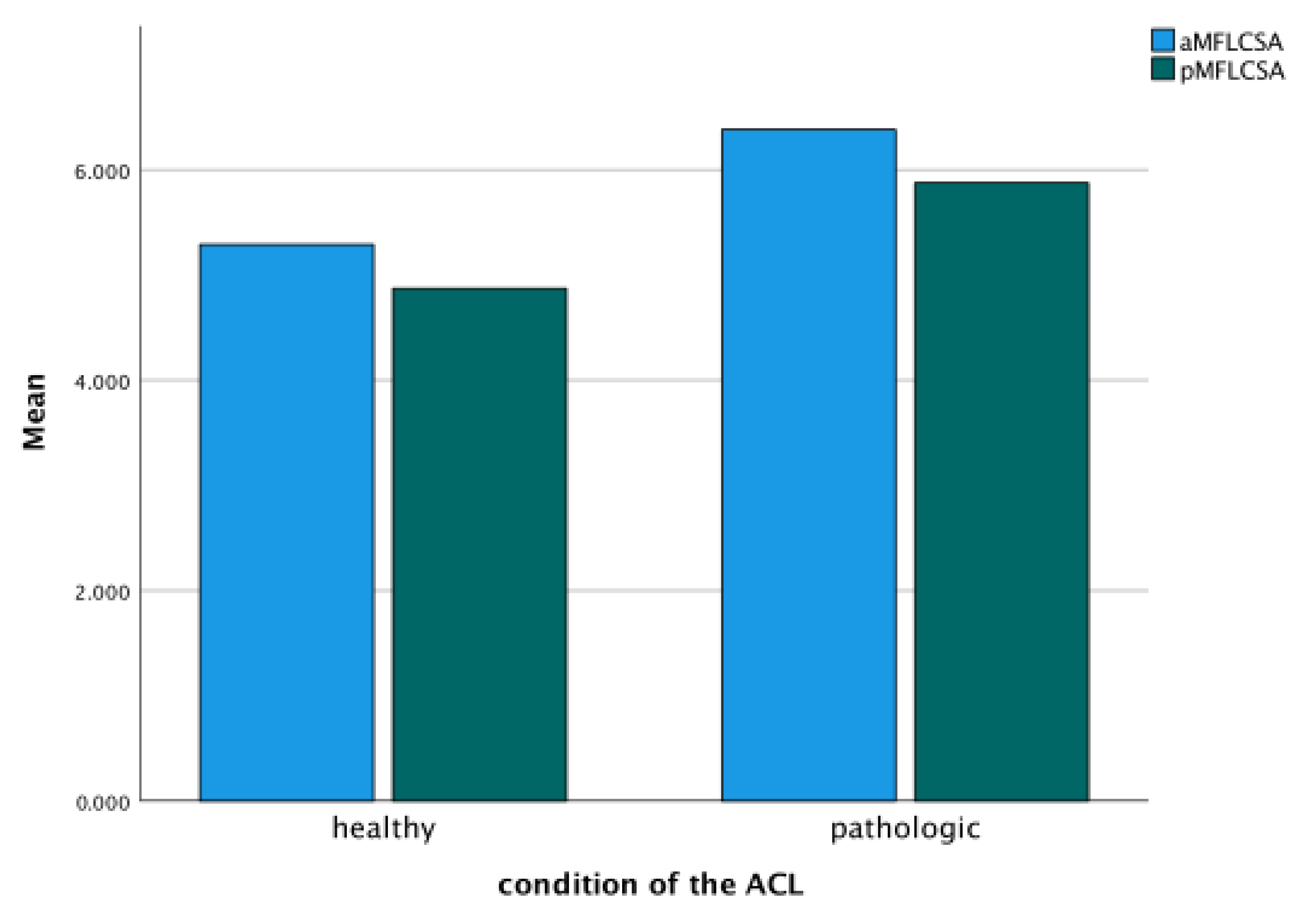

| Variable | Average ± SD (Min–Max) |
|---|---|
| Age (years) | 61.93 ± 9.22 (45–79) |
| Sex * | 265 male (63.9%) 150 female (36.1%) |
| Side * | 210 right (50.6%) 205 left (49.4%) |
| Race | 295 (71.1%) Caucasian 108 (26.0%) black or African American 9 (2.2%) other non-white 3 (0.7%) Asian |
| Height (m) | 1.72 ± 0.09 (1.48–1.90) |
| Weight (kg) | 87.15 ± 16.57 (50.20–135.50) |
| BMI | 29.47 ± 4.68 (19.50–44.60) |
| Ligament | Variable | Morphometrics |
|---|---|---|
| ACL | morphology | healthy 214 (51.6%) pathologic 158 (38.1%) * completely ruptured 43 (10.4%) |
| anteroposterior diameter (mm) | 9.94 ± 4.15 (2.22–22.65) | |
| mediolateral diameter (mm) | 5.73 ± 2.20 (1.51–18.72) | |
| cross-sectional area (mm2) | 43.68 ± 27.14 (4.91–245.20) | |
| PCL | anteroposterior diameter (mm) | 6.47 ± 1.58 (3.71–13.73) |
| mediolateral diameter (mm) | 11.48 ± 2.07 (5.63–19.10) | |
| cross-sectional area (mm2) | 57.98 ± 16.42 (21.19–131.60) | |
| internal angle (°) | 109.73 ± 12.71 (34.88–140.71) | |
| MFL | one present 243 (58.6%) | aMFL 129 (31.1%) pMFL 114 (27.5%) |
| both present 52 (12.5%) | ||
| completely missing 128 (28.9%) | ||
| aMFL | missing 234 (56.4%) present 181 (43.6%) | |
| length (mm) | 31.96 ± 3.71 (22.77–42.25) | |
| anteroposterior width (mm) | 2.83 ± 0.94 (0.99–6.13) | |
| cross-sectional area (mm2) | 7.62 ± 4.73 (1.76–18.29) | |
| pMFL | missing 249 (60.0%) present 166 (40.0%) | |
| length (mm) | 32.67 ± 4.44 (22.70–44.97) | |
| anteroposterior width (mm) | 2.92 ± 1.03 (1.05–8.48) | |
| cross-sectional area (mm2) | 7.70 ± 4.08 (1.56–20.90) | |
| angle (°) | 31.27 ± 5.27 (17.67–48.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandler, E.; Kainberger, F.; Hirtler, L. Changes to the Intercondylar Ligaments of the Knee in Different Stages of Osteoarthritis—A Retrospective Cross-Sectional Study. J. Clin. Med. 2025, 14, 4513. https://doi.org/10.3390/jcm14134513
Mandler E, Kainberger F, Hirtler L. Changes to the Intercondylar Ligaments of the Knee in Different Stages of Osteoarthritis—A Retrospective Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(13):4513. https://doi.org/10.3390/jcm14134513
Chicago/Turabian StyleMandler, Elisabeth, Franz Kainberger, and Lena Hirtler. 2025. "Changes to the Intercondylar Ligaments of the Knee in Different Stages of Osteoarthritis—A Retrospective Cross-Sectional Study" Journal of Clinical Medicine 14, no. 13: 4513. https://doi.org/10.3390/jcm14134513
APA StyleMandler, E., Kainberger, F., & Hirtler, L. (2025). Changes to the Intercondylar Ligaments of the Knee in Different Stages of Osteoarthritis—A Retrospective Cross-Sectional Study. Journal of Clinical Medicine, 14(13), 4513. https://doi.org/10.3390/jcm14134513







