Agreement Between the Gross Motor Ability Estimator-3 and the Reduced Gross Motor Function Measure-66 Based on Artificial Intelligence
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Statistical Analyses
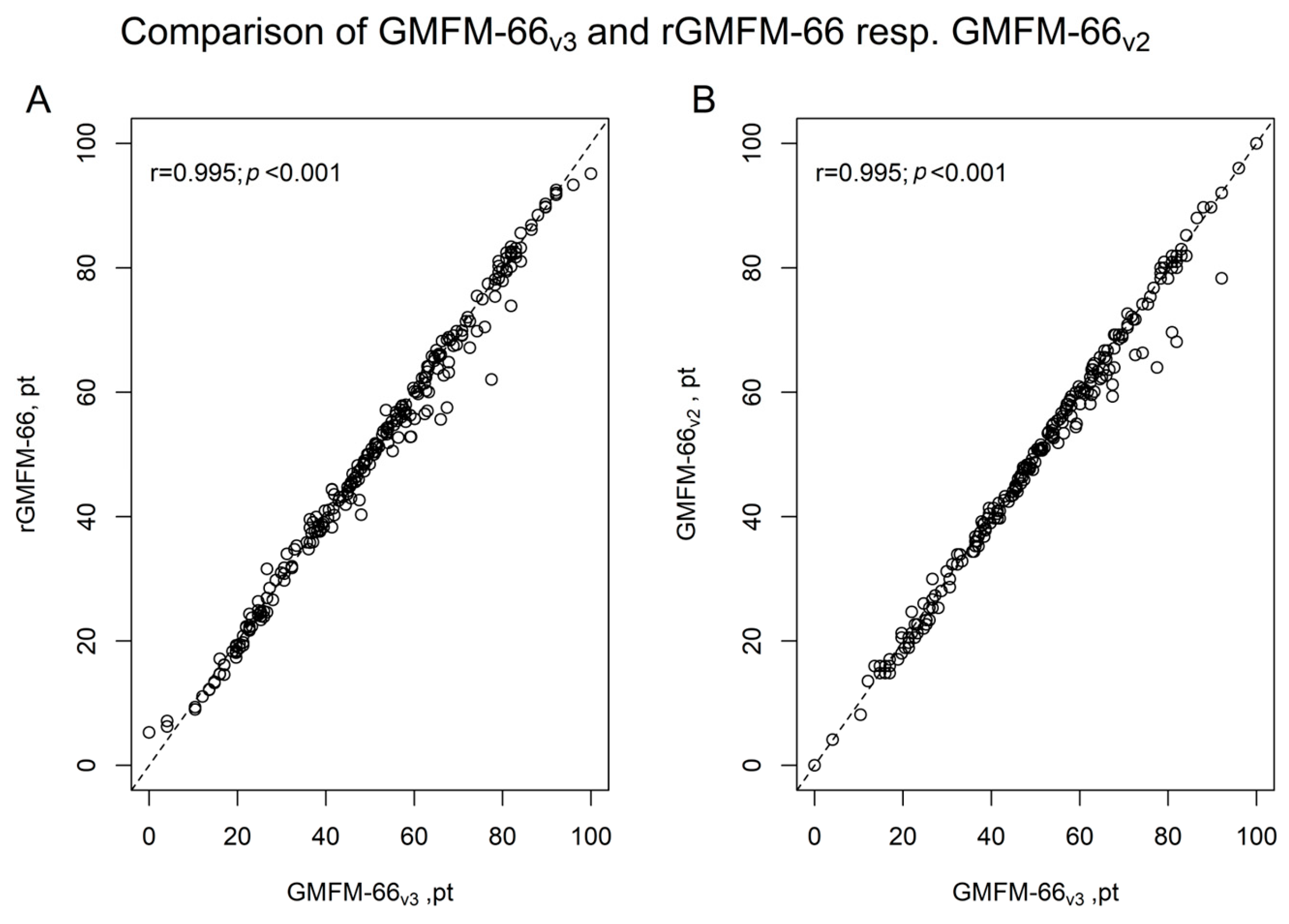
3. Results
3.1. Study Population
3.2. Comparison of GMFM-66v3 Versus rGMFM-66 and GMFM-66v3 Versus GMFM-66v2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pierce, S.R.; Skorup, J.; Kolobe, T.H.; Smith, B.A.; Prosser, L.A. Agreement between the Gross Motor Ability Estimator-2 and the Gross Motor Ability Estimator-3 in Young Children with Cerebral Palsy. Pediatr. Phys. Ther. 2024, 36, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.; Long, T.; Kennedy, E.; Bavishi, S. The efficacy of GMFM-88 and GMFM-66 to detect changes in gross motor function in children with cerebral palsy (CP): A literature review. Disabil. Rehabil. 2014, 36, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.R. The Gross Motor Function Measure (GMFM). J. Physiother. 2017, 63, 187. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.J.; Rosenbaum, P.; Wright, M.; Avery, L.M. Gross Motor Function Measure (GMFM-66 & GMFM-88) Users Manual; Mac Keith Press: London, UK, 2002. [Google Scholar]
- Russell, D.J.; Avery, L.M.; Rosenbaum, P.L.; Raina, P.S.; Walter, S.D.; Palisano, R.J. Improved Scaling of the Gross Motor Function Measure for Children With Cerebral Palsy: Evidence of Reliability and Validity. Phys. Ther. 2000, 80, 873–885. [Google Scholar] [CrossRef]
- Steven, S.; Spiess, K.; Schafmeyer, L.; Buggisch, J.; Schoenau, E.; Luedtke, K.; Duran, I. Efficiency and Validity of the AI-Based rGMFM-66 in Assessing Gross Motor Function in Children with Cerebral Palsy. Appl. Sci. 2025, 15, 6527. [Google Scholar] [CrossRef]
- Duran, I.; Stark, C.; Saglam, A.; Semmelweis, A.; Lioba Wunram, H.; Spiess, K.; Schoenau, E. Artificial intelligence to improve efficiency of administration of gross motor function assessment in children with cerebral palsy. Dev. Med. Child Neurol. 2022, 64, 228–234. [Google Scholar] [CrossRef]
- Dogruoz Karatekin, B.; Icagasioglu, A. The effects of the functional levels of children with cerebral palsy on the quality of life of caregivers. J. Surg. Med. 2022, 6, 191–195. [Google Scholar] [CrossRef]
- Jackman, M.; Sakzewski, L.; Morgan, C.; Boyd, R.N.; Brennan, S.E.; Langdon, K.; Toovey, R.A.M.; Greaves, S.; Thorley, M.; Novak, I. Interventions to improve physical function for children and young people with cerebral palsy: International clinical practice guideline. Dev. Med. Child Neurol. 2022, 64, 536–549. [Google Scholar] [CrossRef]
- Choi, J.Y. Motor Function Measurement in Children: Gross Motor Function Measure (GMFM). Ann. Rehabil. Med. 2024, 48, 301–304. [Google Scholar] [CrossRef]
- Russel, D.J.; Rosenbaum, P.L.; Avery, L.M.; Lane, M. GMFM und GMFCS—Messung und Klassifikation Motorischer Funktionen, 1st ed.; Buch; Huber: Bern, Switzerland, 2006; ISBN 3-456-84230-9. [Google Scholar]
- Russell, D.J.; Avery, L.M.; Walter, S.D.; Hanna, S.E.; Bartlett, D.J.; Rosenbaum, P.L.; Palisano, R.J.; Gorter, J.W. Development and validation of item sets to improve efficiency of administration of the 66-item Gross Motor Function Measure in children with cerebral palsy. Dev. Med. Child Neurol. 2010, 52, e48–e54. [Google Scholar] [CrossRef]
- Russell, D.J.; Rosenbaum, P.L.; Cadman, D.T.; Gowland, C.; Hardy, S.; Jarvis, S. The Gross Motor Function Measure: A Means to Evaluate the Effects of Physical Therapy. Dev. Med. Child Neurol. 1989, 31, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.E.; Rosenbaum, P.L.; Bartlett, D.J.; Palisano, R.J.; Walter, S.D.; Avery, L.; Russell, D.J. Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev. Med. Child Neurol. 2009, 51, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Han, O.; Tan, H.W.; Julious, S.; Sutton, L.; Jacques, R.; Lee, E.; Lewis, J.; Walters, S. A descriptive study of samples sizes used in agreement studies published in the PubMed repository. BMC Med. Res. Methodol. 2022, 22, 242. [Google Scholar] [CrossRef] [PubMed]
- Bonett, D.G. Sample size requirements for estimating intraclass correlations with desired precision. Stat. Med. 2002, 21, 1331–1335. [Google Scholar] [CrossRef]
- Mall, V.; Heinen, F.; Michaelis, U. Klassifikation der motorischen Fähigkeiten von Kindern mit Zerebralparese. Monatsschr. Kinderheilkd. 2009, 157, 1096–1097. [Google Scholar] [CrossRef][Green Version]
- Gamer, M.; Lemon, J.; Singh, I.F.P. irr: Various Coefficients of Interrater Reliability and Agreement, Version 0.84.1. 2019. Available online: https://cran.r-project.org/web/packages/irr/index.html (accessed on 15 April 2025). [CrossRef]
- Cans, C. Surveillance of Cerebral Palsy in Europe Surveillance of cerebral palsy in Europe: A collaboration of cerebral palsy surveys and registers. Dev. Med. Child Neurol. 2000, 42, 816–824. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Duran, I.; Stark, C.; Martakis, K.; Hamacher, S.; Semler, O.; Schoenau, E. Reference centiles for the gross motor function measure and identification of therapeutic effects in children with cerebral palsy. J. Eval. Clin. Pract. 2019, 25, 78–87. [Google Scholar] [CrossRef]
- Schafmeyer, L.; Losch, H.; Bossier, C.; Lanz, I.; Wunram, H.L.; Schoenau, E.; Duran, I. Using artificial intelligence-based technologies to detect clinically relevant changes of gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 2023, 66, 226–232. [Google Scholar] [CrossRef]
- Davids, J.; Lidströmer, N.; Ashrafian, H. Artificial Intelligence for Physiotherapy and Rehabilitation. In Artificial Intelligence in Medicine; Lidströmer, N., Ashrafian, H., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1789–1807. ISBN 978-3-030-64572-4. [Google Scholar]
- Berweck, S. GMFM und GMFCS—Messung und Klassifikation motorischer Funktionen. CD-ROM: The Gross motor function estimator. In GMFM und GMFCS Messung und Klassifikation motorischer Funktionen; Russel, D.J., Rosenbaum, P.L., Avery, L.M., Lane, M., Eds.; Huber: Bern, Switzerland, 2006; pp. 109–124. ISBN 3-456-84230-9. [Google Scholar]
- Wang, H.-Y.; Yang, Y.H. Evaluating the Responsiveness of 2 Versions of the Gross Motor Function Measure for Children With Cerebral Palsy. Arch. Phys. Med. Rehabil. 2006, 87, 51–56. [Google Scholar] [CrossRef]
- Wei, S.; Su-Juan, W.; Yuan-Gui, L.; Hong, Y.; Xiu-Juan, X.; Xiao-Mei, S. Reliability and Validity of the GMFM-66 in 0- to 3-Year-Old Children with Cerebral Palsy. Am. J. Phys. Med. Rehabil. 2006, 85, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Erickson, S.M.; Rockwern, B.; Koltov, M.; McLean, R.M.; für das Medical Practice and Quality Committee des American College of Physicians. Putting Patients First by Reducing Administrative Tasks in Health Care: A Position Paper of the American College of Physicians. Ann. Intern. Med. 2017, 166, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Gerke, O. Reporting Standards for a Bland–Altman Agreement Analysis: A Review of Methodological Reviews. Diagnostics 2020, 10, 334. [Google Scholar] [CrossRef] [PubMed]


| GMFCS Level | ||||||
|---|---|---|---|---|---|---|
| I–V (n = 250) | I (n = 50) | II (n = 50) | III (n = 50) | IV (n = 50) | V (n = 50) | |
| female, n | 107 (42.8) | 24 (44.0) | 24 (42.0) | 17 (34.0) | 25 (50.0) | 17 (34.0) |
| Age, years | 6.9 (3.4) | 7.6 (3.6) | 7.4 (3.9) | 6.6 (3.3) | 6.6 (3.2) | 6.3 (2.7) |
| Height, cm | 114.0 (19.5) | 122.3 (23.4) a | 119.4 (23.3) | 110.3 (16.1) | 110.4 (15.9) | 107.4 (12.1) a |
| Weight, kg | 21.2 (11.4) | 25.6 (12.2) b | 24.4 (14.3) c | 20.3 (9.5) | 19.1 (11.2) b | 16.5 (5.8) b,c |
| CP subtype, % | ||||||
| Spastic bilateral | 68.0 | 56.0 | 74.0 | 82.0 | 74.0 | 54.0 |
| Spastic unilateral | 11.2 | 38.0 | 12.0 | 4.0 | 2.0 | 0 |
| Dyskinetic | 7.2 | 0 | 0 | 8.0 | 8.0 | 20.0 |
| Ataxic | 2.0 | 4.0 | 4.0 | 2.0 | 0 | 0 |
| Mixed type | 11.6 | 2.0 | 10.0 | 4.0 | 16.0 | 26.0 |
| GMFM-66vs3 Versus | ||
|---|---|---|
| rGMFM-66 | GMFM-66v2 | |
| GMFCS Level | ICC | ICC |
| I–V (n = 250) | 0.994 (0.992; 0.996) | 0.994 (0.991; 0.996) |
| I (n = 50) | 0.945 (0.893; 0.971) | 0.920 (0.855; 0.955) |
| II (n = 50) | 0.955 (0.897; 0.978) | 0.967 (0.931; 0.983) |
| III (n = 50) | 0.958 (0.926; 0.976) | 0.987 (0.976; 0.993) |
| IV (n = 50) | 0.986 (0.976; 0.992) | 0.989 (0.979; 0.994) |
| V (n = 50) | 0.983 (0.970; 0.990) | 0.982 (0.961; 0.991) |
| Study | Test Version/Focus | Population/Design | Key Findings |
|---|---|---|---|
| Wei et al. (2006) [26] | GMFM-66 in <3-year-olds | Children < 3 y with CP | ICC = 0.966–0.978; concurrent validity r = 0.985 |
| Russell et al. (2010) [12] | GMFM-66 Item Set (GMFM-66 IS) | Validation sample (varied ages) | Algorithm-based item selection; ICC = 0.994 (single test); 0.92 (retest) |
| Duran et al. (2022) [7] | rGMFM-66 | Retrospective sample with CP | Accurate prediction of GMFM-66; ICC = 0.997 (95% CI: 0.996–0.997) |
| Pierce et al. (2024) [1] | GMAE-2 vs. GMAE-3 (software only) | Software output comparison | High agreement between versions; confirms scoring equivalence |
| Steven et al. (2025) [6] | rGMFM-66 | Children with CP (prospective sample) | Validated rGMFM-66; ICC = 0.970 (95% CI: 0.942–0.983); agreement with GMAE-2 |
| Current study | rGMFM vs. GMFM-66v3 | Children with CP (retrsospective sample) | Validated rGMFM66 with GMFM-66v3; ICC = 0.994 (95% CI: 0.992–0.996) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steven, S.; Müller, C.; Spiess, K.; Bossier, C.; Schönau, E.; Duran, I. Agreement Between the Gross Motor Ability Estimator-3 and the Reduced Gross Motor Function Measure-66 Based on Artificial Intelligence. J. Clin. Med. 2025, 14, 4512. https://doi.org/10.3390/jcm14134512
Steven S, Müller C, Spiess K, Bossier C, Schönau E, Duran I. Agreement Between the Gross Motor Ability Estimator-3 and the Reduced Gross Motor Function Measure-66 Based on Artificial Intelligence. Journal of Clinical Medicine. 2025; 14(13):4512. https://doi.org/10.3390/jcm14134512
Chicago/Turabian StyleSteven, Stefanie, Carlotta Müller, Karoline Spiess, Christiane Bossier, Eckhard Schönau, and Ibrahim Duran. 2025. "Agreement Between the Gross Motor Ability Estimator-3 and the Reduced Gross Motor Function Measure-66 Based on Artificial Intelligence" Journal of Clinical Medicine 14, no. 13: 4512. https://doi.org/10.3390/jcm14134512
APA StyleSteven, S., Müller, C., Spiess, K., Bossier, C., Schönau, E., & Duran, I. (2025). Agreement Between the Gross Motor Ability Estimator-3 and the Reduced Gross Motor Function Measure-66 Based on Artificial Intelligence. Journal of Clinical Medicine, 14(13), 4512. https://doi.org/10.3390/jcm14134512






