Microsurgical Reconstruction of Extensive Lower Limb Defects: Latissimus Dorsi Free Flap for Circumferential Soft Tissue Loss Following High-Energy Trauma
Abstract
1. Introduction
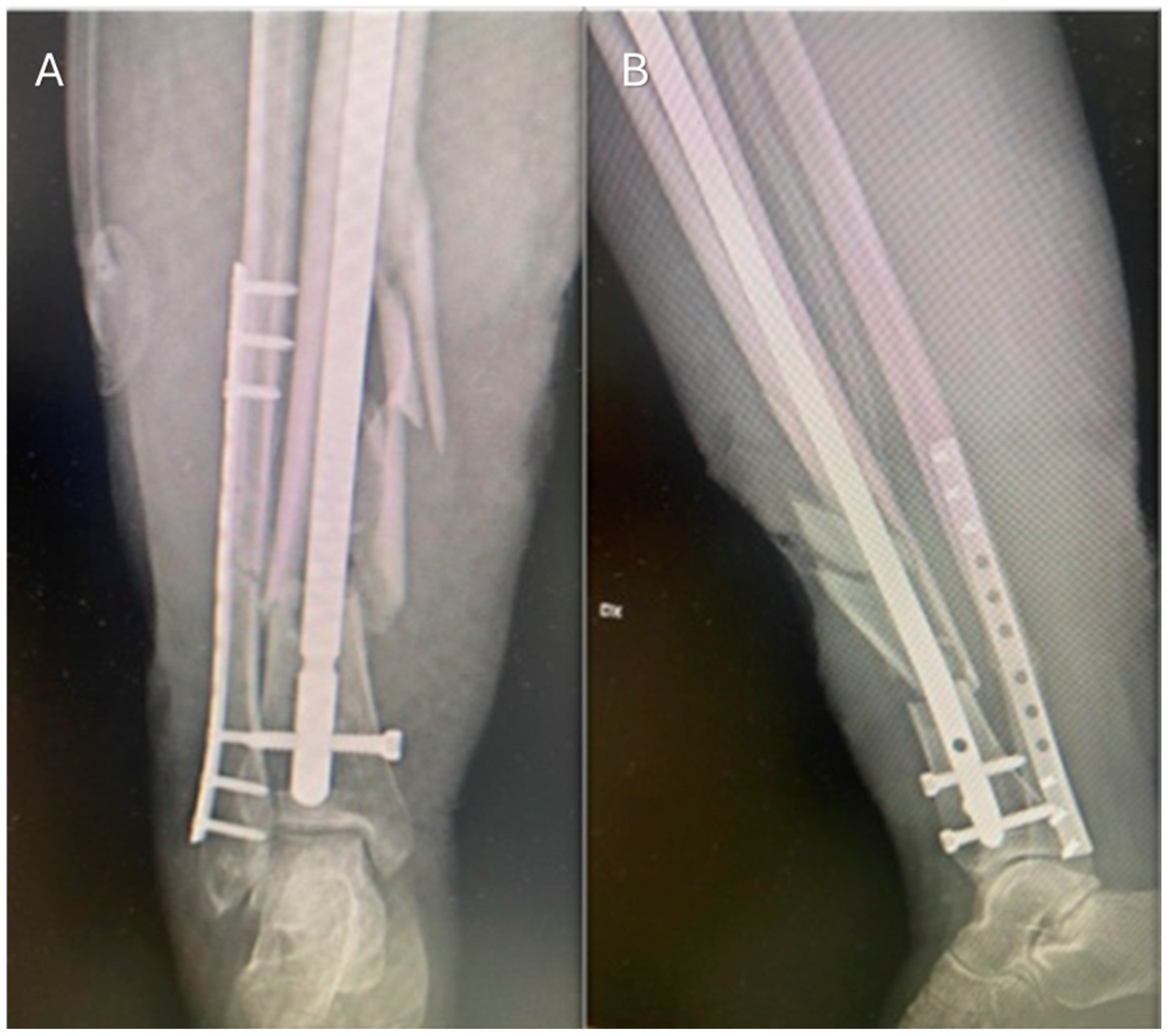
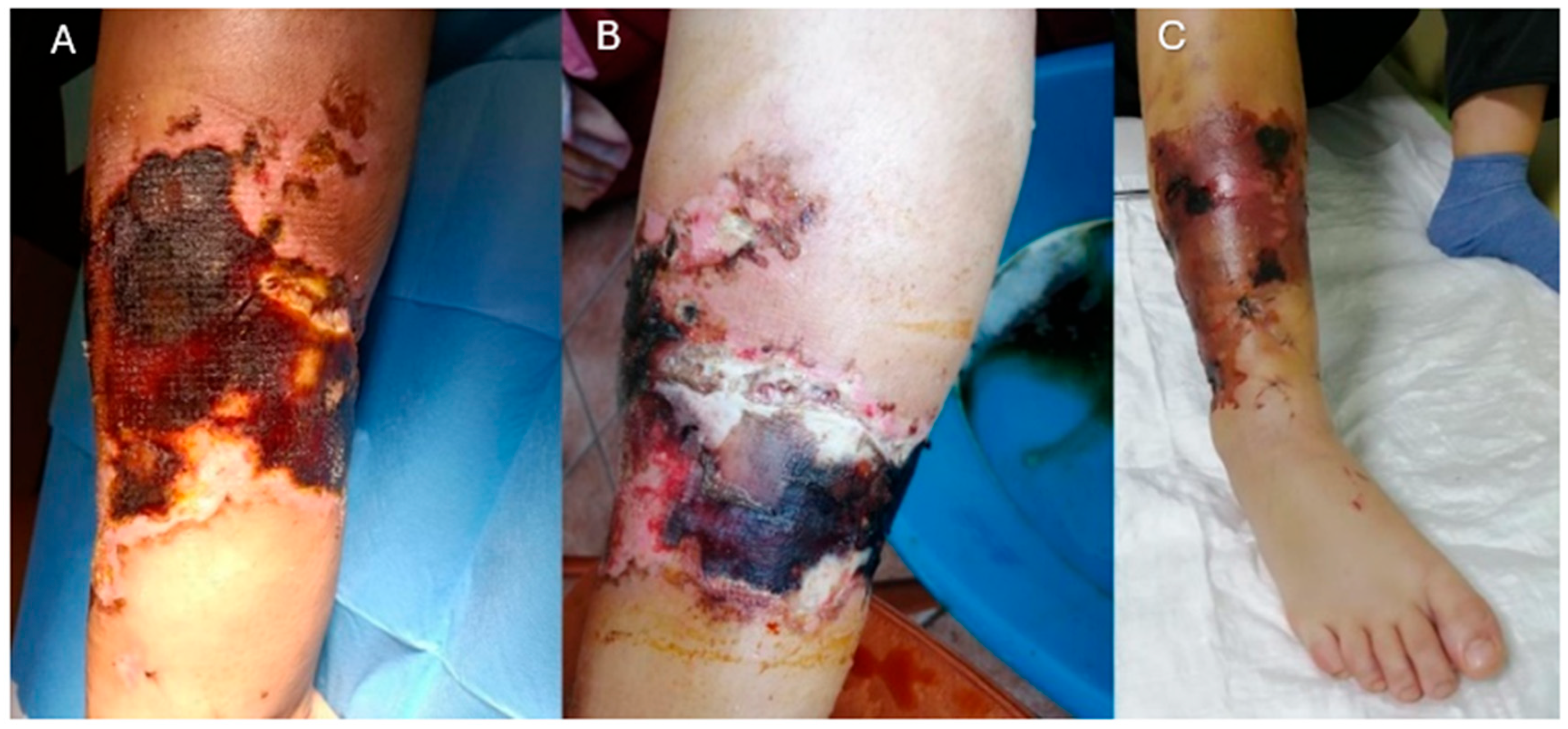
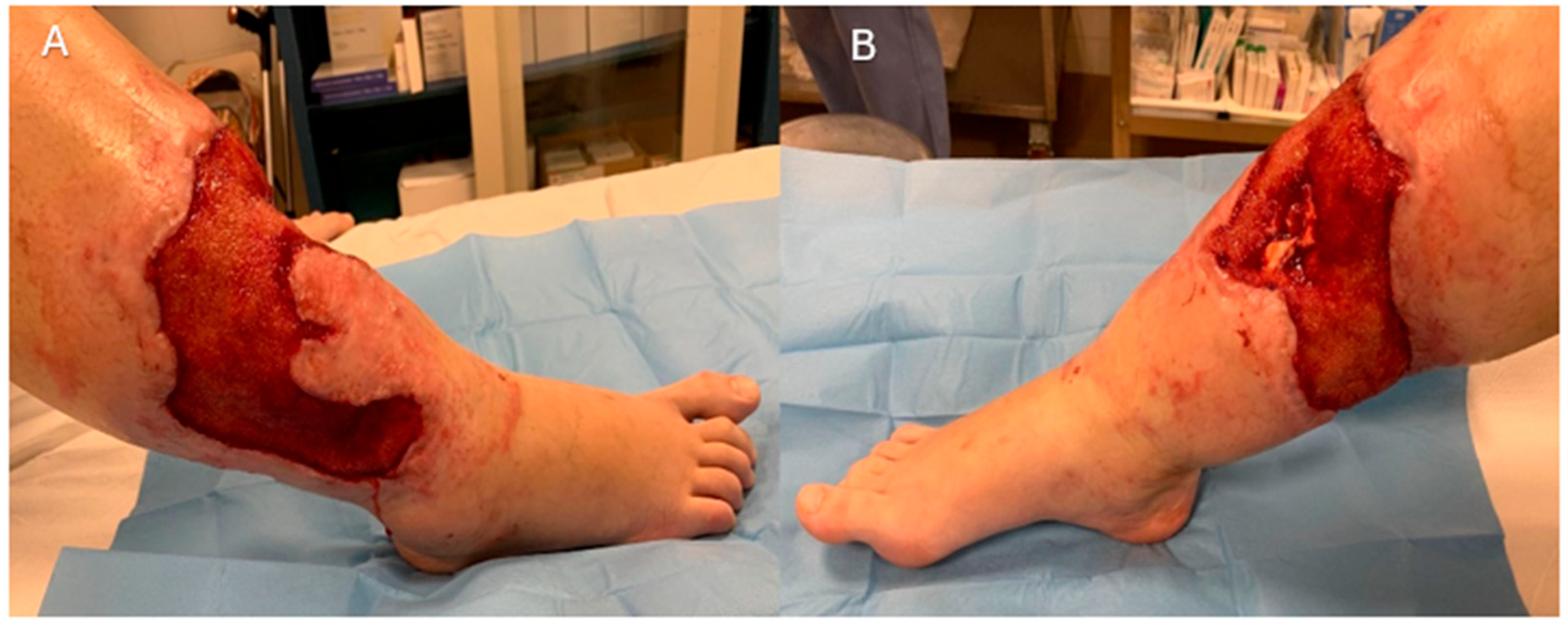
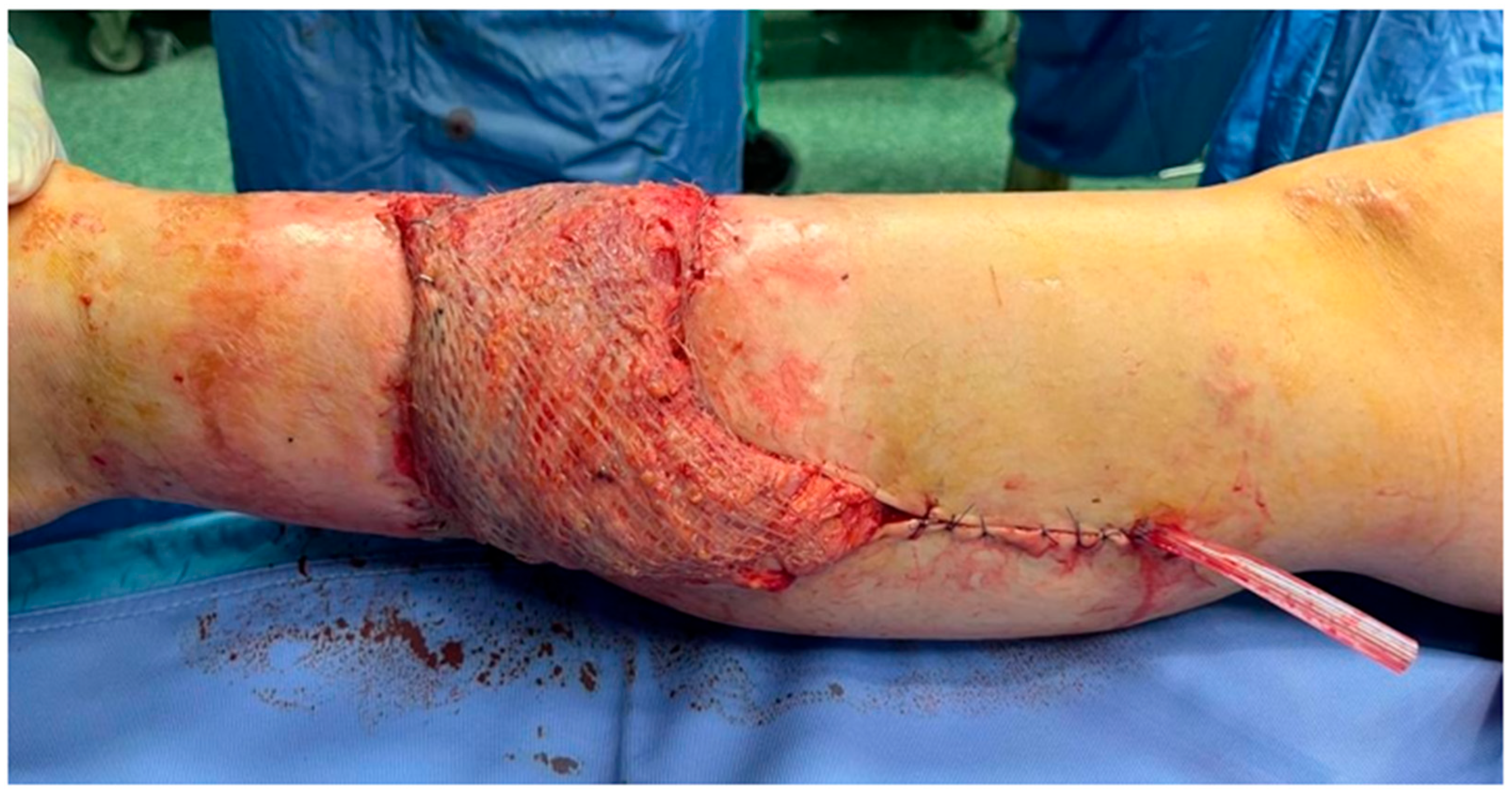
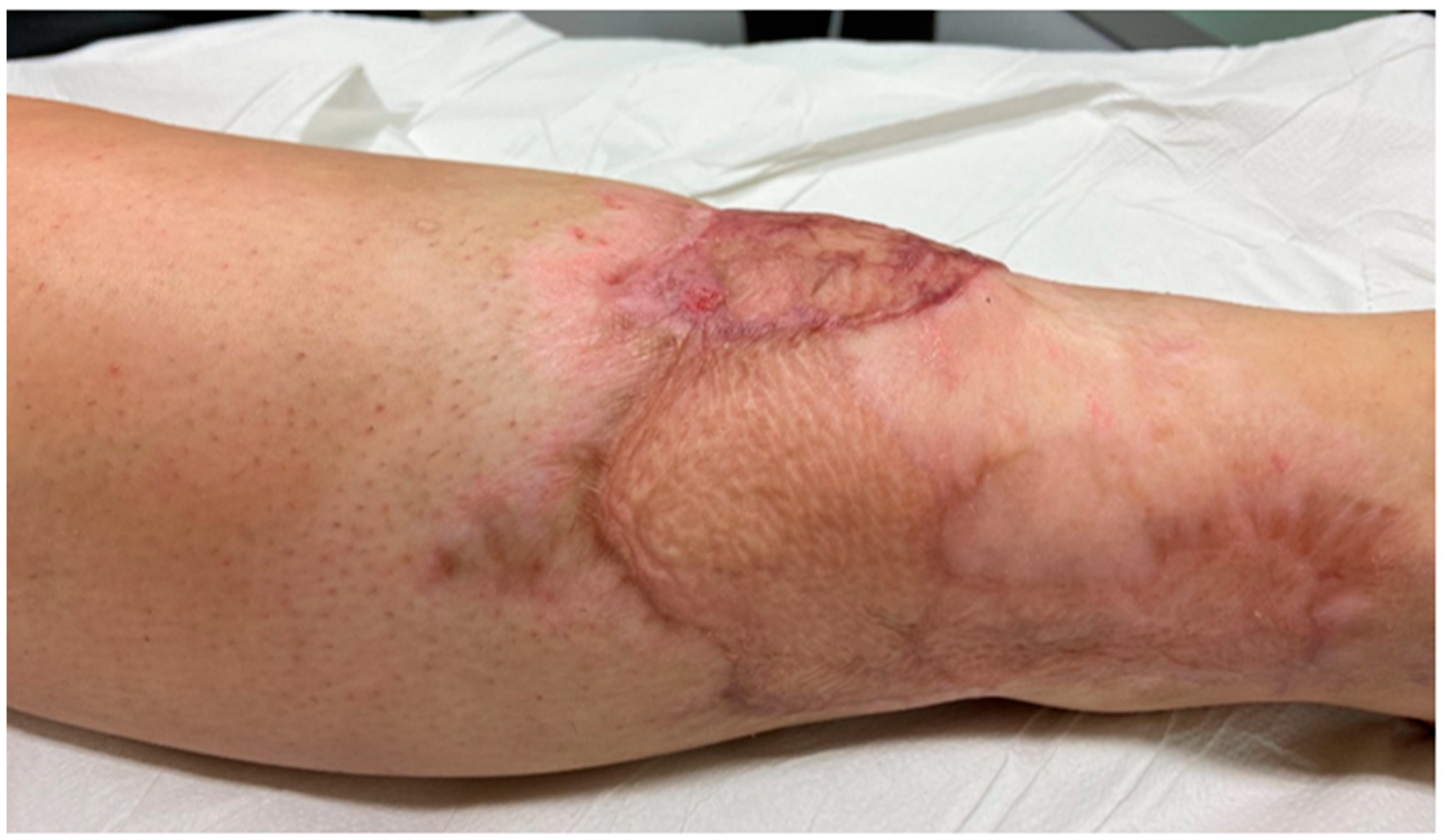
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shokrollahi, K.; Whitaker, I.S.; Nahai, F. (Eds.) Flaps: Practical Reconstructive Surgery; Thieme: New York, NY, USA, 2017. [Google Scholar]
- Pu, L.L.Q.; Medalie, D.A.; Rosenblum, W.J.; Lawrence, S.J.; Vasconez, H.C. Free Tissue Transfer to a Difficult Wound of the Lower Extremity. Ann. Plast. Surg. 2004, 53, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Hemmann, P.; Friederich, M.; Körner, D.; Klopfer, T.; Bahrs, C. Changing epidemiology of lower extremity fractures in adults over a 15-year period—A National Hospital Discharge Registry study. BMC Musculoskelet. Disord. 2021, 22, 456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bradshaw, F.; Hussain, I.; Karamatzanis, I.; Duchniewicz, M.; Krkovic, M. The Epidemiology of Lower Limb Fractures: A Major United Kingdom (UK) Trauma Centre Study. Cureus [Internet]. 2024. Available online: https://www.cureus.com/articles/188876-the-epidemiology-of-lower-limb-fractures-a-major-united-kingdom-uk-trauma-centre-study (accessed on 7 May 2025).
- Godina, M. Early Microsurgical Reconstruction of Complex Trauma of the Extremities. Plast. Reconstr. Surg. 1986, 78, 285–292. [Google Scholar] [CrossRef]
- Ciudad, P.; Agko, M.; Date, S.; Chang, W.; Manrique, O.J.; Huang, T.C.T.; Torto, F.L.; Trignano, E.; Chen, H. The radial forearm free flap as a “vascular bridge” for secondary microsurgical head and neck reconstruction in a vessel-depleted neck. Microsurgery 2018, 38, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Norris, B.L.; Kellam, J.F. Soft-Tissue Injuries Associated with High-Energy Extremity Trauma: Principles of Management. J. Am. Acad. Orthop. Surg. 1997, 5, 37–46. [Google Scholar] [CrossRef]
- Patterson, J.T.; Nakata, H.; Becerra, J.; Duong, A. Traumatic soft tissue defects: A perspective review on reconstruction and communication priorities from the orthopaedic trauma surgeon as a partner in care. Plast. Aesthetic Res. 2022, 9, 23. [Google Scholar] [CrossRef]
- Lin, C.H. Functional Restoration in Lower Extremity Reconstruction. Clin. Plast. Surg. 2021, 48, 289–297. [Google Scholar] [CrossRef]
- Endara, M.; Ducic, I.; Attinger, C. Free Tissue Transfer for Limb Salvage in High-Risk Patients: Worth the Risk. Adv. Wound Care. 2013, 2, 63–68. [Google Scholar] [CrossRef]
- Serra, P.L.; Boriani, F.; Khan, U.; Atzeni, M.; Figus, A. Rate of Free Flap Failure and Return to the Operating Room in Lower Limb Reconstruction: A Systematic Review. J. Clin. Med. 2024, 13, 4295. [Google Scholar] [CrossRef]
- Theodorakopoulou, E.; Mason, K.A.; Pafitanis, G.; Ghanem, A.M.; Myers, S.; Iwuagwu, F.C. Free-Tissue Transfer for the Reconstruction of War-Related Extremity Injuries: A Systematic Review of Current Practice. Mil. Med. 2016, 181, 27–34. [Google Scholar] [CrossRef]
- Vellingiri, K.; Nagakumar, J.S.; Hongaiah, D. Negative Pressure Wound Therapy with Flap Reconstruction for Extensive Soft Tissue Loss in the Foot: A Case Report. Cureus [Internet]. 2020. Available online: https://www.cureus.com/articles/38480-negative-pressure-wound-therapy-with-flap-reconstruction-for-extensive-soft-tissue-loss-in-the-foot-a-case-report (accessed on 21 February 2025).
- Krijgh, D.D.; Van Straeten, M.M.E.; Mureau, M.A.M.; Luijsterburg, A.J.M.; Schellekens, P.P.A.; Maarse, W.; Coert, J.H. Postoperative care in microvascular free flap reconstruction of the lower extremity: A systematic review. Orthoplastic Surg. 2020, 1–2, 21–26. [Google Scholar] [CrossRef]
- Hodea, F.V.; Hariga, C.S.; Bordeanu-Diaconescu, E.M.; Cretu, A.; Dumitru, C.S.; Ratoiu, V.A.; Lascar, I.; Grosu-Bularda, A. Assessing Donor Site Morbidity and Impact on Quality of Life in Free Flap Microsurgery: An Overview. Life 2024, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, S.A.; Kleipool, S.C.; Mulders, M.A.M.; Winkelhagen, J.; Schep, N.W.L.; Goslings, J.C.; Schepers, T. Normative data for the lower extremity functional scale (LEFS). Acta Orthop. 2017, 88, 422–426. [Google Scholar] [CrossRef]
- Paro, J.; Chiou, G.; Sen, S.K. Comparing Muscle and Fasciocutaneous Free Flaps in Lower Extremity Reconstruction—Does It Matter? Ann. Plast. Surg. 2016, 76 (Suppl. S3), S213–S215. [Google Scholar] [CrossRef]
- Calderon, W.; Chang, N.; Mathes, S.J. Comparison of the Effect of Bacterial Inoculation in Musculocutaneous and Fasciocutaneous Flaps. Plast. Reconstr. Surg. 1986, 77, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Ziani, F.; Rubino, C.; Manconi, A.; Arrica, G.; Trignano, C.; Ginatempo, I.; Tettamanzi, M.; Trignano, E. Complex Post-Traumatic Reconstruction of the Lower Limb: A Case Report on Managing Soft Tissue Defects and Deltoid Ligament Damage Using an ALT Flap With Fascia Lata Extension and Fascia Lata Graft. Ann. Ital. Chir. 2025, 96, 579–588. [Google Scholar] [CrossRef]
- Demirtas, Y.; Kelahmetoglu, O.; Cifci, M.; Tayfur, V.; Demir, A.; Guneren, E. Comparison of free anterolateral thigh flaps and free muscle-musculocutaneous flaps in soft tissue reconstruction of lower extremity. Microsurgery 2010, 30, 24–31. [Google Scholar] [CrossRef]
- Philandrianos, C.; Moullot, P.; Gay, A.M.; Bertrand, B.; Legré, R.; Kerfant, N.; Casanova, D. Soft Tissue Coverage in Distal Lower Extremity Open Fractures: Comparison of Free Anterolateral Thigh and Free Latissimus Dorsi Flaps. J. Reconstr. Microsurg. 2018, 34, 121–129. [Google Scholar]
- Rodriguez, E.D.; Bluebond-Langner, R.; Copeland, C.; Grim, T.N.; Singh, N.K.; Scalea, T. Functional Outcomes of Posttraumatic Lower Limb Salvage: A Pilot Study of Anterolateral Thigh Perforator Flaps Versus Muscle Flaps. J. Trauma-Inj. Infect. Crit. Care 2009, 66, 1311–1314. [Google Scholar] [CrossRef]
- Cho, E.H.; Shammas, R.L.; Carney, M.J.; Weissler, J.M.; Bauder, A.R.; Glener, A.D.; Kovach, S.J.; Hollenbeck, S.T.; Levin, L.S. Muscle versus Fasciocutaneous Free Flaps in Lower Extremity Traumatic Reconstruction: A Multicenter Outcomes Analysis. Plast. Reconstr. Surg. 2018, 141, 191–199. [Google Scholar] [CrossRef]
- Kaur, A.; Ang, K.L.; Ali, S.; Dobbs, T.; Pope-Jones, S.; Harry, L.; Whitaker, I.; Emam, A.; Marsden, N. Free flaps for lower limb soft tissue reconstruction—A systematic review of complications in ‘Silver Trauma’ patients. Injury 2023, 54, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Yazar, S.; Lin, C.H.; Lin, Y.T.; Ulusal, A.E.; Wei, F.C. Outcome Comparison between Free Muscle and Free Fasciocutaneous Flaps for Reconstruction of Distal Third and Ankle Traumatic Open Tibial Fractures. Plast. Reconstr. Surg. 2006, 117, 2468–2475. [Google Scholar] [CrossRef]
- Koster, I.T.S.; Borgdorff, M.P.; Jamaludin, F.S.; De Jong, T.; Botman, M.; Driessen, C. Strategies Following Free Flap Failure in Lower Extremity Trauma: A Systematic Review. JPRAS Open 2023, 36, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Ciudad, P.; Kaciulyte, J.; Torto, F.L.; Vargas, M.I.; Bustamante, A.; Chen, H.; Maruccia, M.; Zulueta, J.; Trignano, E.; Bolletta, A. The profunda artery perforator free flap for lower extremity reconstruction. Microsurgery 2022, 42, 13–21. [Google Scholar] [CrossRef]
- Trignano, E.; Serra, P.L.; Grieco, F.; Rodio, M.; Rampazzo, S.; Pili, N.; Trignano, C.; Rubino, C. Heel reconstruction with ALT free flap in a 4-year-old patient after a severe lawnmower injury. A case report. Case Rep. Plast. Surg. Hand Surg. 2023, 10, 2157280. [Google Scholar] [CrossRef]
- Besmens, I.S.; Frueh, F.S.; Gehrke, C.; Knipper, S.; Giovanoli, P.; Calcagni, M. 10-Year single center experience in lower limb reconstruction with free muscle flaps—Factors influencing complications in 266 consecutive cases. J. Plast. Surg. Hand Surg. 2023, 57, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, A.; Gazyakan, E.; Schmidt, V.; Bauer, C.; Germann, G.; Radu, C.; Kneser, U.; Hirche, C. Long-Term Outcome after Successful Lower Extremity Free Flap Salvage. J. Reconstr. Microsurg. 2019, 35, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Trignano, E.; Fallico, N.; Fiorot, L.; Bolletta, A.; Maffei, M.; Ciudad, P.; Maruccia, M.; Chen, H.; Gian Vittorio Campus, G.V. Flap monitoring with continuous oxygen partial tension measurement in breast reconstructive surgery: A preliminary report. Microsurgery 2018, 38, 402–406. [Google Scholar] [CrossRef]
- Othman, S.; Stranix, J.T.; Piwnica-Worms, W.; Bauder, A.; Azoury, S.C.; Elfanagely, O.; Klifto, K.M.; Levin, L.S.; Kovach, S.J. Microvascular free tissue transfer for reconstruction of complex lower extremity trauma: Predictors of complications and flap failure. Microsurgery 2023, 43, 5–12. [Google Scholar] [CrossRef]
- Stannard, J.P.; Volgas, D.A.; McGwin, G.; Stewart, R.L.; Obremskey, W.; Moore, T.; O Anglen, J. Incisional Negative Pressure Wound Therapy After High-Risk Lower Extremity Fractures. J. Orthop. Trauma. 2012, 26, 37–42. [Google Scholar] [CrossRef]
- Raju, A.; Ooi, A.; Ong, Y.; Tan, B. Traumatic Lower Limb Injury and Microsurgical Free Flap Reconstruction with the Use of Negative Pressure Wound Therapy: Is Timing Crucial? J. Reconstr. Microsurg. 2014, 30, 427–430. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filigheddu, E.; Ziani, F.; Arrica, G.; De Riso, S.; Manconi, A.; Rubino, C.; Trignano, E. Microsurgical Reconstruction of Extensive Lower Limb Defects: Latissimus Dorsi Free Flap for Circumferential Soft Tissue Loss Following High-Energy Trauma. J. Clin. Med. 2025, 14, 4424. https://doi.org/10.3390/jcm14134424
Filigheddu E, Ziani F, Arrica G, De Riso S, Manconi A, Rubino C, Trignano E. Microsurgical Reconstruction of Extensive Lower Limb Defects: Latissimus Dorsi Free Flap for Circumferential Soft Tissue Loss Following High-Energy Trauma. Journal of Clinical Medicine. 2025; 14(13):4424. https://doi.org/10.3390/jcm14134424
Chicago/Turabian StyleFiligheddu, Edoardo, Federico Ziani, Giovanni Arrica, Sofia De Riso, Anna Manconi, Corrado Rubino, and Emilio Trignano. 2025. "Microsurgical Reconstruction of Extensive Lower Limb Defects: Latissimus Dorsi Free Flap for Circumferential Soft Tissue Loss Following High-Energy Trauma" Journal of Clinical Medicine 14, no. 13: 4424. https://doi.org/10.3390/jcm14134424
APA StyleFiligheddu, E., Ziani, F., Arrica, G., De Riso, S., Manconi, A., Rubino, C., & Trignano, E. (2025). Microsurgical Reconstruction of Extensive Lower Limb Defects: Latissimus Dorsi Free Flap for Circumferential Soft Tissue Loss Following High-Energy Trauma. Journal of Clinical Medicine, 14(13), 4424. https://doi.org/10.3390/jcm14134424






