Ophthalmic Artery Doppler at 11–13 Weeks’ Gestation and Birth of Small-for-Gestational-Age Neonates
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants:
2.2. Ophthalmic Artery Doppler Indices
2.3. Outome Collection
2.4. Data Analysis
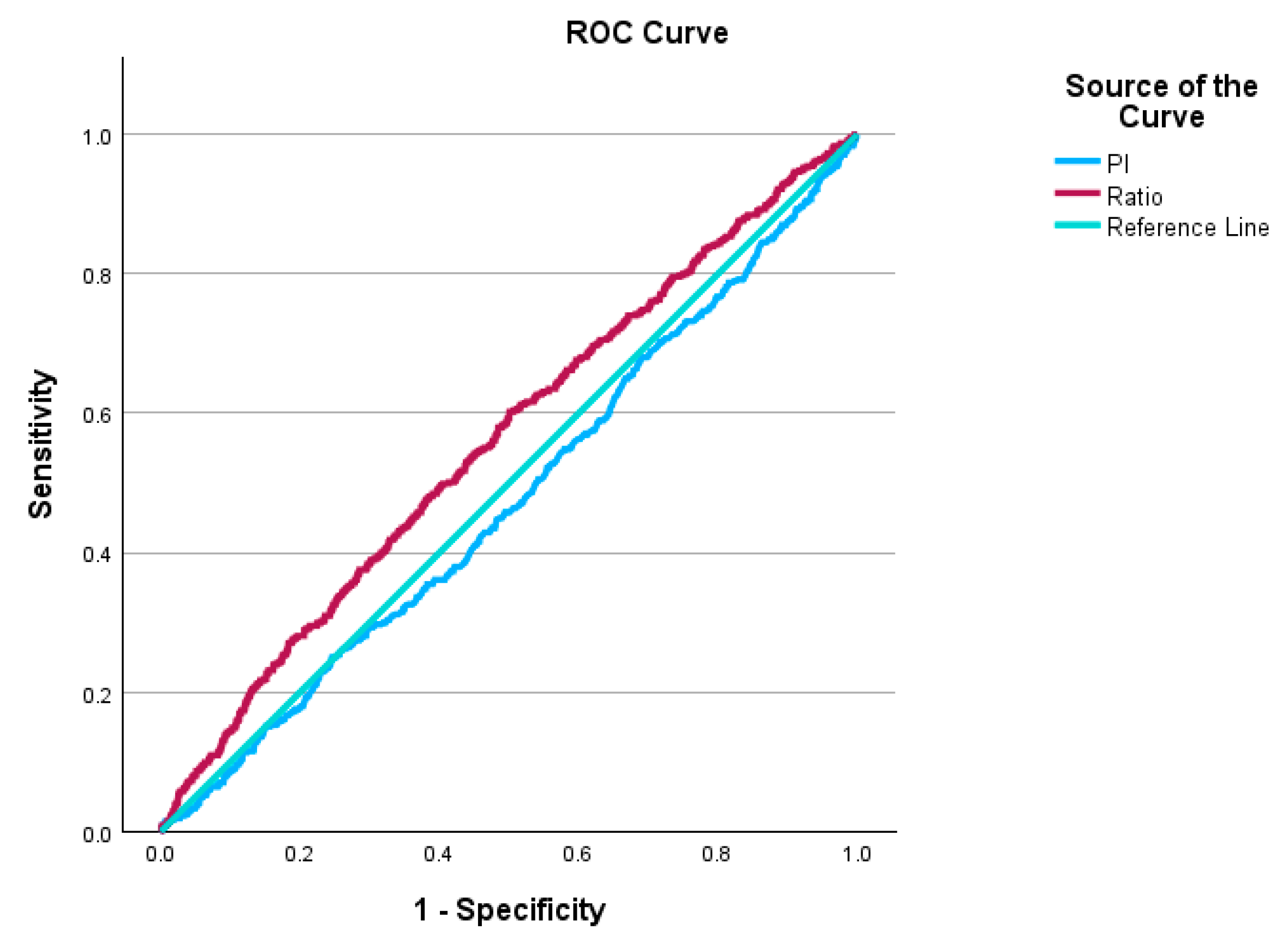
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BMI | Body mass index |
| CI | Confidence interval |
| FMF | Fetal Medicine Foundation |
| IVF | In vitro fertilization |
| MAP | Mean arterial pressure |
| MOM | Multiples of median |
| PAPP-A | Pregnancy-associated plasma protein-A |
| PE | Preeclampsia |
| PI | Pulsatility index |
| PlGF | Placental growth factor |
| PSV | Peak systolic velocity |
| ROC | Receiver operating characteristic curve |
| SD | Standard deviation |
| SGA | Small for gestational age |
| Ut-A-PI | Uterine artery pulsatility index |
References
- Gonser, M.; Vonzun, L.; Ochsenbein-Kölble, N. Ophthalmic artery Doppler in prediction of pre-eclampsia: Insights from hemodynamic considerations. Ultrasound Obs. Gynecol. 2021, 58, 145–147. [Google Scholar] [CrossRef]
- Mynard, J.P.; Kowalski, R.; Cheung, M.M.; Smolich, J.J. Beyond the aorta: Partial transmission of reflected waves from aortic coarctation into supra-aortic branches modulates cerebral hemodynamics and left ventricular load. Biomech. Model. Mechanobiol. 2017, 16, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Curtis, S.L.; Zambanini, A.; Mayet, J.; McGThom, S.A.; Foale, R.; Parker, K.H.; Hughes, A.D. Reduced systolic wave generation and increased peripheral wave reflection in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H557–H562. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.T.P.; Martins-Costa, S.; Oppermann, M.L.D.R.; Palma Dias, R.; Magno, V.; Peña, J.A.; Ramos, J.G.L. Maternal ophthalmic artery Doppler ultrasonography in preeclampsia and pregnancy outcomes. Pregnancy Hypertens. 2017, 10, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.Y.; De Wolf, F.; Robertson, W.B.; Brosens, I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br. J. Obs. Gynaecol. 1986, 93, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Sławek-Szmyt, S.; Kawka-Paciorkowska, K.; Ciepłucha, A.; Lesiak, M.; Ropacka-Lesiak, M. Preeclampsia and Fetal Growth Restriction as Risk Factors of Future Maternal Cardiovascular Disease-A Review. J. Clin. Med. 2022, 11, 6048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reijnders, I.F.; Mulders, A.G.M.G.J.; Koster, M.P.H. Placental development and function in women with a history of placenta-related complications: A systematic review. Acta Obs. Gynecol. Scand. 2018, 97, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Amelia, M.; Siahaan, W.H.; Wijaya, N.; Harahap, A.M.; Rivany, M.A.; Lumbanraja, S.N. Pre-eclampsia screening with maternal risk factors and ophthalmic Doppler artery: A systematic review. Hypertens. Pregnancy 2025, 44, 2506451. [Google Scholar] [CrossRef]
- Gyokova, E.; Hristova-Atanasova, E.; Iskrov, G. Preeclampsia Management and Maternal Ophthalmic Artery Doppler Measurements between 19 and 23 Weeks of Gestation. J. Clin. Med. 2024, 13, 950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lees, C.C.; Stampalija, T.; Baschat, A.A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obs. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Morris, R.K.; Johnstone, E.; Lees, C.; Morton, V.; Smith, G. The Royal College of Obstetricians and Gynaecologists. Investigation and Care of a Small-for-Gestational-Age Fetus and a Growth Restricted Fetus (Green-top Guideline No. 31). BJOG 2024, 131, e31–e80. [Google Scholar] [CrossRef] [PubMed]
- Roeckner, J.T.; Pressman, K.; Odibo, L.; Duncan, J.R.; Odibo, A.O. Outcome-based comparison of SMFM and ISUOG definitions of fetal growth restriction. Ultrasound Obs. Gynecol. 2021, 57, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.C.G.; Odibo, L.; Zientara, S.; Običan, S.G.; Rodriguez, A.; Stout, M.; Odibo, A.O. Validation of Delphi procedure consensus criteria for defining fetal growth restriction. Ultrasound Obs. Gynecol. 2020, 56, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (international federation of Gynecology and obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 2021, 152 (Suppl. S1), 3–57. [Google Scholar] [CrossRef]
- Chaemsaithong, P.; Sahota, D.S.; Poon, L.C. First trimester preeclampsia screening and prediction. Am. J. Obs. Gynecol. 2022, 226, S1071–S1097.e2. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, D.L.; Wright, D.; Poon, L.C.Y.; Syngelaki, A.; O’Gorman, N.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. ASPRE trial: Performance of screening for preterm pre-eclampsia. Ultrasound Obs. Gynecol. 2017, 50, 492–495, Erratum in Ultrasound Obs. Gynecol. 2017, 50, 807. https://doi.org/10.1002/uog.18950. [Google Scholar] [CrossRef] [PubMed]
- Bonacina, E.; Armengol-Alsina, M.; Hurtado, I.; Garcia-Manau, P.; Ferrer-Oliveras, R.; Monreal, S.; Pancorbo, M.; Mendoza, M.; Carreras, E. sFlt-1 to PlGF ratio cut-offs to predict adverse pregnancy outcomes in early-onset FGR and SGA: A prospective observational study. J. Obs. Gynaecol. 2022, 42, 2840–2845. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, A.; Reisch, B.; Mavarani, L.; Darkwah Oppong, M.; Kimmig, R.; Mach, P.; Schmidt, B.; Köninger, A.; Gellhaus, A. Soluble endoglin versus sFlt-1/PlGF ratio: Detection of preeclampsia, HELLP syndrome, and FGR in a high-risk cohort. Hypertens. Pregnancy 2022, 41, 159–172. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, X.; Yang, L.; Wang, M.; Hu, D.; Ren, M. Ultrasound Evaluation of the Changes of Ophthalmic Artery Doppler and Optic Nerve Sheath in Pregnant Women With FGR. J. Ultrasound Med. 2025, 44, 1007–1015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melo, N.A.; Araujo Júnior, E.; Helfer, T.M.; Caetano, A.C.R.; Zamarian, A.C.P.; Moron, A.F.; Diniz, A.L.D.; Nardozza, L.M.M. Assessment of maternal Doppler parameters of ophthalmic artery in fetuses with growth restriction in the third trimester of pregnancy: A case—Control study. J. Obs. Gynaecol. Res. 2015, 41, 1330–1336. [Google Scholar] [CrossRef]
- Dai, X.; Kang, L.; Ge, H. Doppler parameters of ophthalmic artery in women with preeclampsia: A meta-analysis. J. Clin. Hypertens. 2023, 25, 5–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.L. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Front. Endocrinol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zur, R.L.; Kingdom, J.C.; Parks, W.T.; Hobson, S.R. The Placental Basis of Fetal Growth Restriction. Obs. Gynecol. Clin. N. Am. 2020, 47, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Sasi, A.; Abraham, V.; Davies-Tuck, M.; Polglase, G.R.; Jenkin, G.; Miller, S.L.; Malhotra, A. Impact of intrauterine growth restriction on preterm lung disease. Acta Paediatr. 2015, 104, e552–e556. [Google Scholar] [CrossRef]
- Unterscheider, J.; O’Donoghue, K.; Daly, S.; Geary, M.P.; Kennelly, M.M.; McAuliffe, F.M.; Hunter, A.; Morrison, J.J.; Burke, G.; Dicker, P.; et al. Fetal growth restriction and the risk of perinatal mortality–case studies from the multicentre PORTO study. BMC Pregnancy Childbirth 2014, 14, 63. [Google Scholar] [CrossRef]
- Page, J.M.; Blue, N.R.; Silver, R.M. Fetal Growth and Stillbirth. Obs. Gynecol. Clin. North Am. 2021, 48, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.F.; Wang, Y.; Wang, H.W.; Liu, Y. The incidence rate, high-risk factors, and short- and long-term adverse outcomes of fetal growth restriction: A report from Mainland China. Medicine 2014, 93, e210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adam-Raileanu, A.; Miron, I.; Lupu, A.; Bozomitu, L.; Sasaran, M.O.; Russu, R.; Rosu, S.T.; Nedelcu, A.H.; Salaru, D.L.; Baciu, G.; et al. Fetal Growth Restriction and Its Metabolism-Related Long-Term Outcomes-Underlying Mechanisms and Clinical Implications. Nutrients 2025, 17, 555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sacchi, C.; Marino, C.; Nosarti, C.; Vieno, A.; Visentin, S.; Simonelli, A. Association of Intrauterine Growth Restriction and Small for Gestational Age Status with Childhood Cognitive Outcomes: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020, 174, 772–781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ali, S.; Mukasa, D.C.; Lukakamwa, D.; Nakayenga, A.; Namagero, P.; Biira, J.; Byamugisha, J.; Papageorghiou, A.T. Relationship of maternal ophthalmic artery Doppler with uterine artery Doppler, hemodynamic indices and gestational age: Prospective MATERA study. Ultrasound Obs. Gynecol. 2025, 65, 163–172. [Google Scholar] [CrossRef]
- Gana, N.; Sarno, M.; Vieira, N.; Wright, A.; Charakida, M.; Nicolaides, K.H. Ophthalmic artery Doppler at 11–13 weeks’ gestation in prediction of pre-eclampsia. Ultrasound Obs. Gynecol. 2022, 59, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Gerstmair, A.; Worsley, C.; Murphy, A. As low as reasonably achievable (ALARA). Reference Article. Available online: https://radiopaedia.org/articles/35183 (accessed on 12 April 2025).
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obs. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.H.; Wright, D.; Syngelaki, A.; Wright, A.; Akolekar, R. Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound Obs. Gynecol. 2018, 52, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Frérot, A.; Novais, A.R.B.; Baud, O. Neonatal and Long-Term Consequences of Fetal Growth Restriction. Curr. Pediatr. Rev. 2018, 14, 212–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monteiro, V.N.P.; de Oliveira, C.A.; Gomes Junior, S.C.; do Cima, L.C.; Naves, W.U.; Diniz, A.L.D.; Araujo Júnior, E.; de Sá, R.A.M. Ophthalmic Artery Doppler as a Predictor of Adverse Neonatal Outcomes in Women with Preeclampsia. J. Clin. Ultrasound 2025, 53, 504–509. [Google Scholar] [CrossRef]
- Hata, T.; Miyazaki, K. Maternal ophthalmic artery Doppler velocimetry in normotensive pregnancies with small-for-gestational-age infants. Ultrasound Obs. Gynecol. 1998, 11, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, I.; Wright, D.; Syngelaki, A.; Souretis, K.; Chrysanthopoulou, E.; Nicolaides, K.H. Competing-risks model for prediction of small-for-gestational-age neonate from biophysical and biochemical markers at 11–13 weeks’ gestation. Ultrasound Obs. Gynecol. 2021, 57, 52–61. [Google Scholar] [CrossRef]
- Baschat, A.A. Arterial and venous Doppler in the diagnosis and management of early-on set fetal growth restriction. Early Hum. Dev. 2005, 81, 877–887. [Google Scholar] [CrossRef]
- Kachakayala, M.; Vavilala, S. Stage-based FGR (Barcelona Protocol): Perinatal Outcome in SGA and FGR. Int. J. Infertil. Fetal Med. 2022, 13, 101–110. [Google Scholar] [CrossRef]

| a | ||||
| Characteristics | Total (n = 4054) | BW ≤ 3rd Percentile (n = 196) | BW > 3rd Percentile (n = 3858) | p-Value |
| Maternal age (years) | 32.8 ± 4.83 years | 31.64 ± 5.48 | 32.86 ± 4.8 | <0.001 * |
| Weight (kg) | 70.1 ± 15.17 | 66.19 ± 13.85 | 70.3 ± 15.21 | <0.001 * |
| Height (cm) | 165.84 ± 5.29 | 162.78 ± 6.79 | 166 ± 6.77 | <0.001 * |
| BMI (kg/m2) | 25.48 ± 5.29 kg/m2 | 24.98 ± 5.04 | 25.51 ± 5.31 | 0.174 |
| PAPPA MoM | 1.21 ± 0.66 | 1.06 ± 0.72 | 1.22 ± 0.66 | <0.001 * |
| UtA-PI MoM | 1.03 ± 0.31 | 1.13 ± 0.36 | 1.03 ± 0.31 | 0.001 * |
| MAP MoM | 1.01 ± 0.08 | 1.01 ± 0.09 | 1.02 ± 0.08 | 0.137 |
| Gestation at birth (weeks) | 39.52 ± 1.83 | 37.69 ± 2.95 | 39.62 ± 1.7 | <0.001 * |
| Conception (number) | 0.47 | |||
| IVF | 184 (4.5%) | 11 (6%) | 173 (94%) | |
| Ovulation drugs | 19 (0.5%) | 0 | 19 (100%) | |
| Spontaneous | 3851 (95%) | 185 (4.8%) | 3666 (95.2%) | |
| Ethnicity (number) | <0.001 * | |||
| Black | 531 (13.1%) | 40 (7.5%) | 491 (92.5%) | |
| East Asian | 93 (2.3%) | 3 (10%) | 90 (96.8%) | |
| Mixed | 145 (3.6%) | 10 (6.9%) | 135 (93.1%) | |
| South Asian | 274 (6.8%) | 28 (10.2%) | 246 (89.8%) | |
| White | 3011 (74.3%) | 115 (3.8%) | 2896 (96.2%) | |
| Smoking (number) | 0.032 * | |||
| Yes | 92 (2.3%) | 9 (9.8%) | 83 (90.2%) | |
| No | 3962 (97.3%) | 187 (4.7%) | 3775 (95.3%) | |
| Low-dose Aspirin (number) | 0.44 | |||
| No | 3924 (96.8%) | 189 (4.8%) | 3735 (95.2%) | |
| Yes | 130 (3.2%) | 7 (5.4%) | 123 (94.6%) | |
| Previous SGA (number) | <0.001 * | |||
| Multipara-no SGA | 1842 (45.4%) | 55 (3%) | 1787 (97%) | |
| Multipara-SGA | 126 (3.1%) | 17 (13.5%) | 109 (86.5%) | |
| Nullipara | 2086 (51.5%) | 124 (5.9%) | 1962 (94.1%) | |
| * p < 0.05 | ||||
| b | ||||
| Characteristics | Total (n = 4054) | BW ≤ 10th Percentile (n = 485) | BW > 10th Percentile (n = 3569) | p-Value |
| Maternal age (years) | 32.8 ± 4.83 | 32.2 ± 5.13 | 32.89 ± 4.79 | 0.003 |
| Weight (kg) | 70.1 ± 15.17 | 66.07 ± 14.44 | 70.65 ± 15.19 | <0.001 * |
| Height (cm) | 165.84 ± 5.29 | 163.28 ± 7.06 | 166.19 ± 6.69 | <0.001 * |
| BMI (kg/m2) | 25.48 ± 5.29 | 24.75 ± 5.01 | 25.58 ± 5.33 | 0.001 * |
| PAPPA MoM | 1.21 ± 0.66 | 1.1 ± 0.7 | 1.23 ± 0.66 | <0.001 * |
| UtA-PI MoM | 1.03 ± 0.31 | 1.09 ± 034 | 1.02 ± 0.31 | <0.001 * |
| MAP MoM | 1.01 ± 0.08 | 1.02 ± 0.09 | 1.02 ± 0.08 | 0.523 |
| Gestation at birth (weeks) | 39.52 ± 1.83 | 38.57 ± 2.4 | 39.66 ± 1.7 | <0.001 * |
| Conception | 0.773 | |||
| IVF | 184 (4.5%) | 25 (13.6%) | 159 (86.4%) | |
| Ovulation drugs | 19 (0.5%) | 2 (10.5%) | 17 (89.5%) | |
| Spontaneous | 3851 (95%) | 458 (11.9%) | 3393 (88.1%) | |
| Ethnicity (number) | <0.001 * | |||
| Black | 531 (13.1%) | 84 (15.8%) | 447 (84.2%) | |
| East Asian | 93 (2.3%) | 10 (10.8%) | 83 (89.2%) | |
| Mixed | 145 (3.6%) | 19 (13.1%) | 126 (86.8%) | |
| South Asian | 274 (6.8%) | 70 (25.5%) | 204 (74.5%) | |
| White | 3011 (74.3%) | 302 (10%) | 2709 (90%) | |
| Smoking (number) | 0.003 * | |||
| Yes | 92 (2.3%) | 21 (22.8%) | 71 (77.2%) | |
| No | 3962 (97.3%) | 464 (11.7%) | 3498 (88.3%) | |
| Low-dose Aspirin (number) | 0.14 | |||
| No | 3924 (96.8%) | 465 (11.9%) | 3459 (88.1%) | |
| Yes | 130 (3.2%) | 20 (15.4%) | 110 (84.6%) | |
| Previous SGA (number) | <0.001 * | |||
| Multipara-no SGA | 1842 (45.4%) | 152 (8.3%) | 1690 (91.7%) | |
| Multipara-SGA | 126 (3.1%) | 37 (29.4%) | 89 (70.6%) | |
| Nullipara | 2086 (51.5%) | 296 (14.2%) | 1790 (95.8%) | |
| Group | BW ≤ 3rd Centile (n = 196) | BW > 3rd Centile (n = 3858) | p-Value |
|---|---|---|---|
| PI (mean ± SD) | 1.86 ± 0.37 | 1.88 ± 1.39 | 0.475 |
| PSV ratio (mean ± SD) | 0.65 ± 0.10 | 0.63 ± 0.10 | 0.038 * |
| Group | BW ≤ 10th Percentile (n = 485) | BW > 10th Percentile (n = 3569) | p-Value |
|---|---|---|---|
| PI (mean ± SD) | 1.85 ± 0.40 | 1.88 ± 0.39 | 0.113 |
| PSV ratio (mean ± SD) | 0.65 ± 0.10 | 0.63 ± 0.10 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gana, N.; Ianosev, D.; Allafi, N.; Impis Oglou, M.; Nicolaides, K.H. Ophthalmic Artery Doppler at 11–13 Weeks’ Gestation and Birth of Small-for-Gestational-Age Neonates. J. Clin. Med. 2025, 14, 4425. https://doi.org/10.3390/jcm14134425
Gana N, Ianosev D, Allafi N, Impis Oglou M, Nicolaides KH. Ophthalmic Artery Doppler at 11–13 Weeks’ Gestation and Birth of Small-for-Gestational-Age Neonates. Journal of Clinical Medicine. 2025; 14(13):4425. https://doi.org/10.3390/jcm14134425
Chicago/Turabian StyleGana, Nicoleta, Dragana Ianosev, Nima Allafi, Mechmet Impis Oglou, and Kypros H. Nicolaides. 2025. "Ophthalmic Artery Doppler at 11–13 Weeks’ Gestation and Birth of Small-for-Gestational-Age Neonates" Journal of Clinical Medicine 14, no. 13: 4425. https://doi.org/10.3390/jcm14134425
APA StyleGana, N., Ianosev, D., Allafi, N., Impis Oglou, M., & Nicolaides, K. H. (2025). Ophthalmic Artery Doppler at 11–13 Weeks’ Gestation and Birth of Small-for-Gestational-Age Neonates. Journal of Clinical Medicine, 14(13), 4425. https://doi.org/10.3390/jcm14134425







