SPM Differences in Gait Pattern of Women After Total Hip Replacement: A Longitudinal Study
Abstract
1. Introduction
- (1)
- Between which research periods are the most significant changes in gait patterns observed for the involved and uninvolved limbs?
- (2)
- What specific movements and joint kinematics exhibit the most significant variations over time during the monitored rehabilitation process?
- (3)
- How do changes in kinematic and kinetic gait parameters evolve across different phases of rehabilitation (preoperative, early postoperative, mid-term, and long-term recovery)?
- (4)
- What are the clinical implications of observed gait changes regarding rehabilitation effectiveness and functional recovery?
- (5)
- How does applying Statistical Parametric Mapping (SPM) contribute to identifying and quantifying significant gait pattern differences post-THR?
2. Materials and Methods
2.1. Participants, Inclusion Criteria
2.2. Measurement Set-Up
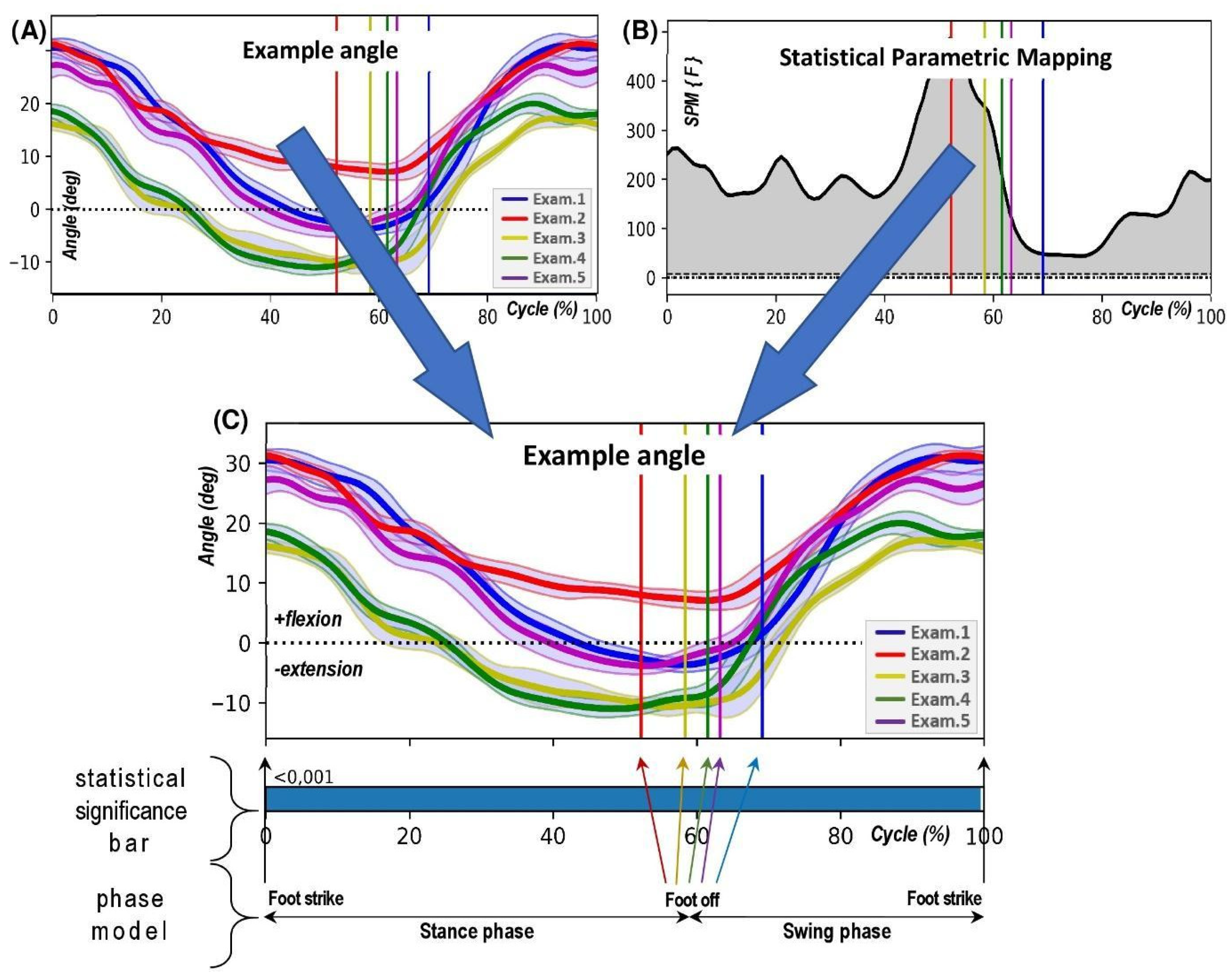
2.3. Measurement Procedure and Calculations
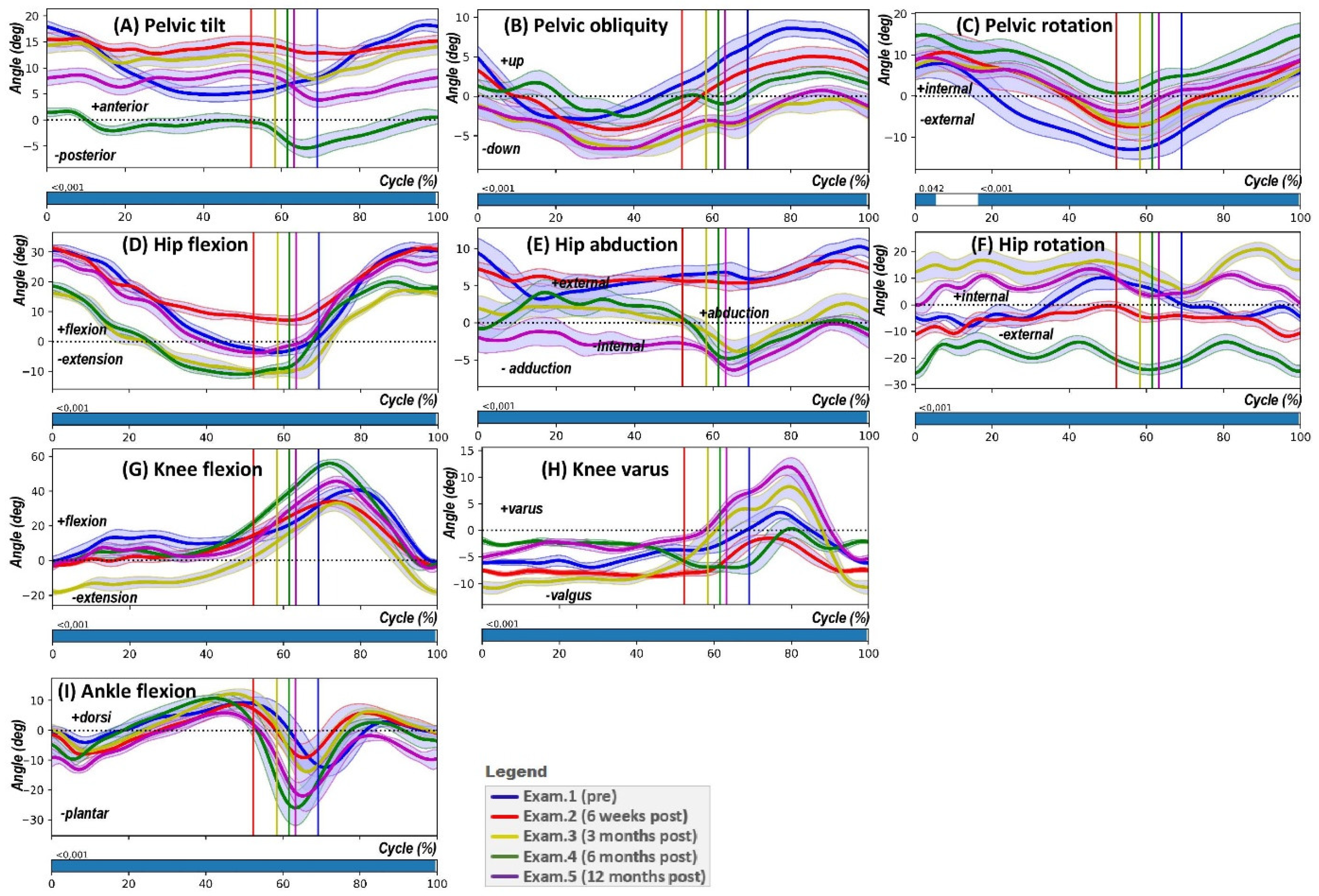
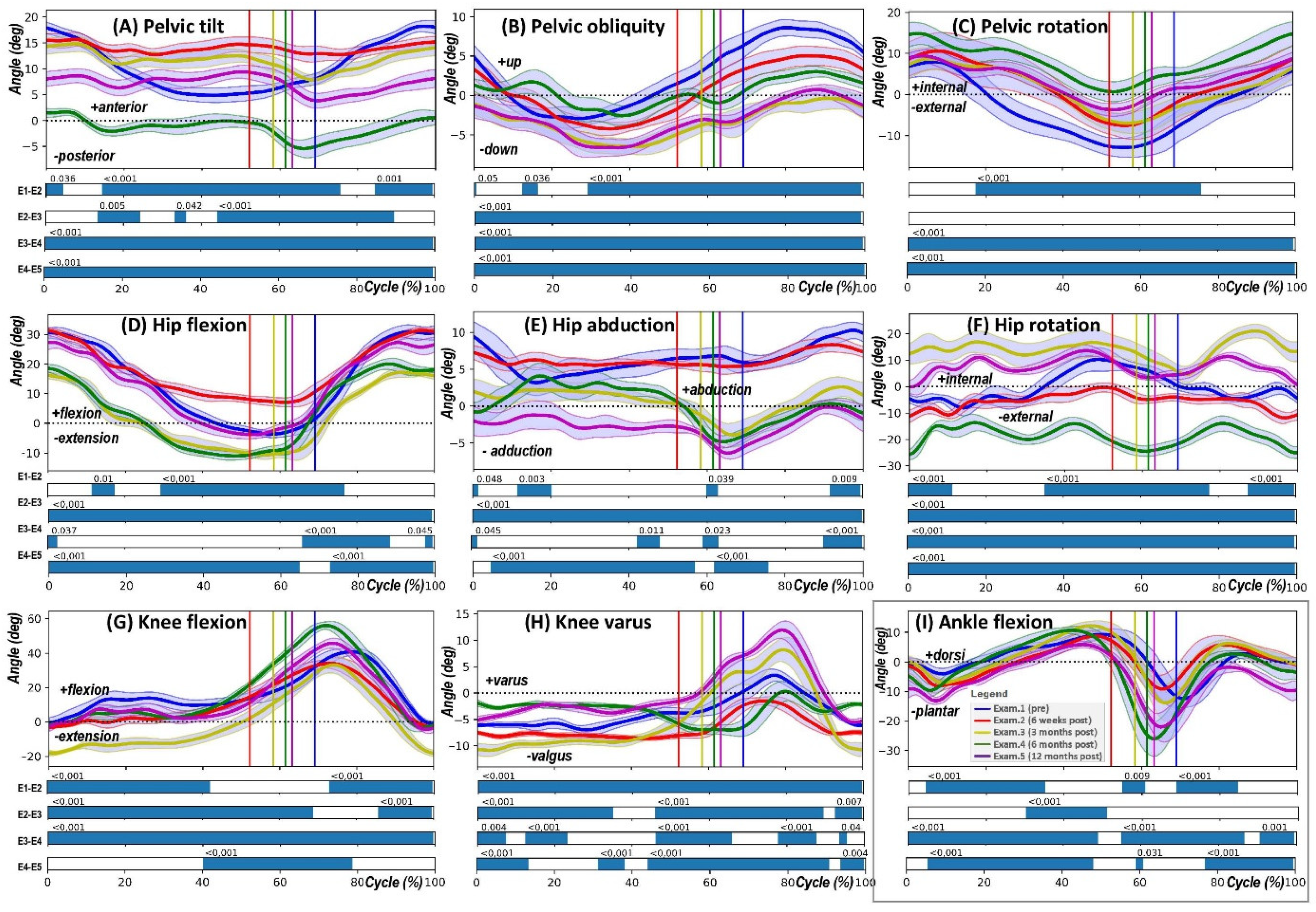
- Pelvic tilt (PTILT) involves the pelvis tilting forwards (indicating a positive tilt) or backwards (indicating a negative tilt) in the sagittal plane, which occurs due to its rotation around the mediolateral axis.
- Pelvic obliquity (POBLI) is characterised by the pelvis moving upwards (positive obliquity) or downwards (negative obliquity) relative to a global coordinate system in the coronal plane, resulting from the mediolateral axis’s rotation out of alignment with the horizontal plane.
- Pelvic rotation (PROT) refers to the inward (positive) or outward (negative) rotation of the pelvis in the transversal plane, which is due to the mediolateral axis rotating around the vertical axis.
- Hip flexion-extension (HPFE) denotes the forward (flexion, positive) or backward (extension, negative) movement of the thigh bone (femur) in the sagittal plane, as a result of its rotation around the mediolateral axis at the hip joint.
- Hip abduction-adduction (HPAA) describes the movement of the femur away from (abduction, positive) or towards (adduction, negative) the midline of the body in the coronal plane, occurring through the rotation of the proximal–distal axis away from the sagittal plane.
- Hip rotation (HPROT) involves the internal (positive) or external (negative) rotation of the femur in the transversal plane, which happens due to rotation about the proximal–distal axis.
- Knee flexion-extension (KFE) is the bending (flexion, positive) or straightening (extension, negative) movement of the lower leg (tibia) in relation to the femur in the sagittal plane, caused by the rotation of the proximal–distal axis around the mediolateral axis.
- Knee varus/valgus indicates the movement of the lower leg (tibia) towards (varus, positive) or away from (valgus, negative) the midline of the body in the coronal plane.
- Ankle dorsiflexion-plantarflexion (AFE) represents the upward (dorsiflexion, positive) or downward (plantarflexion, negative) movement of the foot in relation to the lower leg (tibia) in the sagittal plane.
2.4. Statistical Calculations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| THR | Total Hip Replacement |
| SPM | Statistical Parametric Mapping |
| SPM{F} | SPM F-test continuum |
| SPM{t} | SPM t-test continuum |
| Ex1–Ex5 | Examination 1 to 5 (time points of gait analysis) |
| ROM | Range of Motion |
References
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2019, 396, 1204–1222. [Google Scholar]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global Estimates of the Need for Rehabilitation Based on the Global Burden of Disease Study 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; Peeters, G.M.; Dennison, E.M.; Zambon, S.; Nikolaus, T.; Sanchez-Martinez, M.; Musacchio, E.; Van Schoor, N.M.; Deeg, D.J.H. European Project on Osteoarthritis (EPOSA): Methodological Challenges in Harmonization of Existing Data from Five European Population-Based Cohorts on Aging. BMC Musculoskelet. Disord. 2011, 12, 272. [Google Scholar] [CrossRef]
- Dziedzic, K.S.; Allen, K.D. Challenges and Controversies of Complex Interventions in Osteoarthritis Management: Recognizing Inappropriate and Discordant Care. Rheumatology 2018, 57, iv88–iv98. [Google Scholar] [CrossRef]
- Okafor, L.; Chen, A.F. Patient Satisfaction and Total Hip Arthroplasty: A Review. Arthroplasty 2019, 1, 6. [Google Scholar] [CrossRef]
- Winiarski, S.; Aleksandrowicz, K.; Jarząb, S.; Pozowski, A.; Rutkowska-Kucharska, A. Assessment of Gait after Bilateral Hip Replacement. Case Study. Ortop. Traumatol. Rehabil. 2014, 16, 197–208. [Google Scholar]
- Ahmed, F.; Islam, M.S.; Hassan, M.N.; Ahmed, M.S.; Nahid, Z.B.S.; Rasel, M.A.H. Physiotherapy Interventions in Treating Patients Following Total Hip Arthroplasty: A Narrative Review. Physiother. Q. 2024, 32, 1–6. [Google Scholar] [CrossRef]
- Bahadori, S.; Davenport, P.; Immins, T.; Wainwright, T.W. Validation of Joint Angle Measurements: Comparison of a Novel Low-Cost Marker-Less System with an Industry Standard Marker-Based System. J. Med. Eng. Technol. 2019, 43, 19–24. [Google Scholar] [CrossRef]
- van Drongelen, S.; Stetter, B.J.; Böhm, H.; Stief, F.; Stein, T.; Meurer, A. Identification of Patients with Similar Gait Compensating Strategies Due to Unilateral Hip Osteoarthritis and the Effect of Total Hip Replacement: A Secondary Analysis. J. Clin. Med. 2021, 10, 2167. [Google Scholar] [CrossRef]
- Winiarski, S.; Rutkowska-Kucharska, A.; Pozowski, A.; Aleksandrowicz, K. A New Method of Evaluating the Symmetry of Movement Used to Assess the Gait of Patients After Unilateral Total Hip Replacement. Appl. Bionics Biomech. 2019, 2019, 7863674. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; D’Addio, G.; De Campi, M.; Donisi, L.; Biancardi, A.; Cesarelli, M. Rehabilitation Outcome in Patients Undergone Hip or Knee Replacement Surgery Using Inertial Technology for Gait Analysis. In Proceedings of the 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 1 June–1 July 2020. [Google Scholar] [CrossRef]
- Stolarczyk, A.; Stolarczyk, M.; Oleksy, Ł.; Maciąg, G.J.; Stępiński, P.; Szymczak, J.; Świercz, M.; Żarnovsky, K.; Mostowy, M.; Maciąg, B.M. Analysis of Biomechanical Gait Parameters in Patients after Total Hip Replacement Operated via Anterolateral Approach Depending on Size of the Femoral Head Implant: Retrospective Matched-Cohort Study. Arch. Orthop. Trauma Surg. 2022, 142, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.L.; Baker, R.; Wolfe, R.; Morris, M.E. The Reliability of Three-Dimensional Kinematic Gait Measurements: A Systematic Review. Gait Posture 2009, 29, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Allali, G.; Sekhon, H.; Verghese, J.; Guilain, S.; Steinmetz, J.P.; Kressig, R.W.; Barden, J.M.; Szturm, T.; Launay, C.P.; et al. Guidelines for Assessment of Gait and Reference Values for Spatiotemporal Gait Parameters in Older Adults: The Biomathics and Canadian Gait Consortiums Initiative. Front. Hum. Neurosci. 2017, 11, 251365. [Google Scholar] [CrossRef]
- Rojek, I.; Prokopowicz, P.; Dorożyński, J.; Mikołajewski, D. Novel Methods of AI-Based Gait Analysis in Post-Stroke Patients. Appl. Sci. 2023, 13, 6258. [Google Scholar] [CrossRef]
- Lovecchio, N.; Zago, M.; Sforza, C. Gait Analysis in the Rehabilitation Process. In Rehabilitation After Limb Salvage Surgery; Springer: Berlin/Heidelberg, Germany, 2021; pp. 109–118. [Google Scholar] [CrossRef]
- De Marchis, C.; Ranaldi, S.; Varrecchia, T.; Serrao, M.; Castiglia, S.F.; Tatarelli, A.; Ranavolo, A.; Draicchio, F.; Lacquaniti, F.; Conforto, S. Characterizing the Gait of People with Different Types of Amputation and Prosthetic Components Through Multimodal Measurements: A Methodological Perspective. Front. Rehabil. Sci. 2022, 3, 804746. [Google Scholar] [CrossRef]
- Pieruccini-Faria, F.; Montero-Odasso, M.; Hausdorff, J.M. Gait Variability and Fall Risk in Older Adults: The Role of Cognitive Function. In Falls and Cognition in Older Persons; Springer: Berlin/Heidelberg, Germany, 2020; pp. 107–138. [Google Scholar] [CrossRef]
- Beck Jepsen, D.; Robinson, K.; Ogliari, G.; Montero-Odasso, M.; Kamkar, N.; Ryg, J.; Freiberger, E.; Tahir, M. Predicting Falls in Older Adults: An Umbrella Review of Instruments Assessing Gait, Balance, and Functional Mobility. BMC Geriatr. 2022, 22, 615. [Google Scholar] [CrossRef]
- Pataky, T.C. One-Dimensional Statistical Parametric Mapping in Python. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 295–301. [Google Scholar] [CrossRef]
- Booth, B.G.; Keijsers, N.L.W.; Sijbers, J.; Huysmans, T. STAPP: Spatiotemporal Analysis of Plantar Pressure Measurements Using Statistical Parametric Mapping. Gait Posture 2018, 63, 268–275. [Google Scholar] [CrossRef]
- Bańkosz, Z.; Winiarski, S. The Application of Statistical Parametric Mapping to Evaluate Differences in Topspin Backhand between Chinese and Polish Female Table Tennis Players. Appl. Bionics Biomech. 2021, 2021, 5555874. [Google Scholar] [CrossRef]
- Mestanza Mattos, F.G.; Luciano, F.; Lencioni, T.; Gervasoni, E.; Jonsdottir, J.; Anastasi, D.; Pavei, G.; Clerici, M.; Cattaneo, D. Complementary Use of Statistical Parametric Mapping and Gait Profile Score to Describe Walking Alterations in Multiple Sclerosis: A Cross-Sectional Study. Sci. Rep. 2023, 13, 10465. [Google Scholar] [CrossRef] [PubMed]
- Yona, T.; Kamel, N.; Cohen-Eick, G.; Ovadia, I.; Fischer, A. Scoping Review of One-Dimension Statistical Parametric Mapping in Lower Limb Biomechanical Analysis. medRxiv 2023, 109, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Alhossary, A.; Pataky, T.; Ang, W.T.; Chua, K.S.G.; Kwong, W.H.; Donnelly, C.J. Versatile Clinical Movement Analysis Using Statistical Parametric Mapping in MovementRx. Sci. Rep. 2023, 13, 2414. [Google Scholar] [CrossRef] [PubMed]
- Fusca, M.; Negrini, F.; Perego, P.; Magoni, L.; Molteni, F.; Andreoni, G. Validation of a Wearable IMU System for Gait Analysis: Protocol and Application to a New System. Appl. Sci. 2018, 8, 1167. [Google Scholar] [CrossRef]
- Zahradka, N.; Verma, K.; Behboodi, A.; Bodt, B.; Wright, H.; Lee, S.C.K. An Evaluation of Three Kinematic Methods for Gait Event Detection Compared to the Kinetic-Based ‘Gold Standard’. Sensors 2020, 20, 5272. [Google Scholar] [CrossRef]
- Wu, G.; Siegler, S.; Allard, P.; Kirtley, C.; Leardini, A.; Rosenbaum, D.; Whittle, M.; D’Lima, D.D.; Cristofolini, L.; Witte, H.; et al. ISB Recommendation on Definitions of Joint Coordinate System of Various Joints for the Reporting of Human Joint Motion—Part I: Ankle, Hip, and Spine. J. Biomech. 2002, 35, 543–548. [Google Scholar] [CrossRef]
- Wu, G.; van der Helm, F.C.T.; (DirkJan) Veeger, H.E.J.; Makhsous, M.; Van Roy, P.; Anglin, C.; Nagels, J.; Karduna, A.R.; McQuade, K.; Wang, X.; et al. ISB Recommendation on Definitions of Joint Coordinate Systems of Various Joints for the Reporting of Human Joint Motion—Part II: Shoulder, Elbow, Wrist and Hand. J. Biomech. 2005, 38, 981–992. [Google Scholar] [CrossRef]
- Wu, G.; Cavanagh, P.R. ISB Recommendations for Standardization in the Reporting of Kinematic Data. J. Biomech. 1995, 28, 1257–1261. [Google Scholar] [CrossRef]
- Armand, S.; Sawacha, Z.; Goudriaan, M.; Horsak, B.; van der Krogt, M.; Huenaerts, C.; Daly, C.; Kranzl, A.; Boehm, H.; Petrarca, M.; et al. Current Practices in Clinical Gait Analysis in Europe: A Comprehensive Survey-Based Study from the European Society for Movement Analysis in Adults and Children (ESMAC) Standard Initiative. Gait Posture 2024, 111, 65–74. [Google Scholar] [CrossRef]
- Bańkosz, Z.; Winiarski, S. Statistical Parametric Mapping Reveals Subtle Gender Differences in Angular Movements in Table Tennis Topspin Backhand. Int. J. Environ. Res. Public Health 2020, 17, 6996. [Google Scholar] [CrossRef]
- Pataky, T.C.; Vanrenterghem, J.; Robinson, M.A. Zero- vs. One-Dimensional, Parametric vs. Non-Parametric, and Confidence Interval vs. Hypothesis Testing Procedures in One-Dimensional Biomechanical Trajectory Analysis. J. Biomech. 2015, 48, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Pataky, T.C.; Caravaggi, P.; Savage, R.; Parker, D.; Goulermas, J.Y.; Sellers, W.I.; Crompton, R.H. New Insights into the Plantar Pressure Correlates of Walking Speed Using Pedobarographic Statistical Parametric Mapping (PSPM). J. Biomech. 2008, 41, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Agostini, V.; Ganio, D.; Facchin, K.; Cane, L.; Moreira Carneiro, S.; Knaflitz, M.; Carneiro, S.M.; Knaflitz, M. Gait Parameters and Muscle Activation Patterns at 3, 6 and 12 Months after Total Hip Arthroplasty. J. Arthroplast. 2014, 29, 1265–1272. [Google Scholar] [CrossRef]
- Horstmann, T.; Listringhaus, R.; Haase, G.-B.; Grau, S.; Mündermann, A. Changes in Gait Patterns and Muscle Activity Following Total Hip Arthroplasty: A Six-Month Follow-Up. Clin. Biomech. 2013, 28, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Ramadanov, N.; Ostojic, M.; Lazaru, P.; Liu, K.; Hable, R.; Marinova-Kichikova, P.; Dimitrov, D.; Becker, R. Risk Factors and Predictors for Functional Outcome and Complication Rate in Total Hip Arthroplasty through Minimally Invasive and Conventional Approaches: A Systematic Review and Meta-Regression Analysis of 41 Randomized Controlled Trials. J. Clin. Med. 2023, 12, 5895. [Google Scholar] [CrossRef]
- Hendrickson, J.; Patterson, K.K.; Inness, E.L.; McIlroy, W.E.; Mansfield, A. Relationship between Asymmetry of Quiet Standing Balance Control and Walking Post-Stroke. Gait Posture 2014, 39, 177–181. [Google Scholar] [CrossRef]
- Wada, O.; Asai, T.; Hiyama, Y.; Nitta, S.; Mizuno, K. Gait Variability in Women with Hip Osteoarthritis before and after Total Hip Replacement. Am. J. Phys. Med. Rehabil. 2019, 98, 866–871. [Google Scholar] [CrossRef]
- Pincheira, P.A.; De La Maza, E.; Silvestre, R.; Guzmán-Venegas, R.; Becerra, M. Comparison of Total Hip Arthroplasty Surgical Approaches by Statistical Parametric Mapping. Clin. Biomech. 2019, 62, 7–14. [Google Scholar] [CrossRef]
- Rao, R.P.; Sara, L.K.; Perkins, Z.E.; Dwyer, M.K.; Lewis, C.L. Females with Hip Pain Walk with Altered Kinematics at Peaks and throughout the Gait Cycle. Clin. Biomech. 2024, 118, 106314. [Google Scholar] [CrossRef]
- Loppini, M.; Temporiti, F.; Furone, R.; Galli, M.; Grappiolo, G.; Gatti, R. Static and Dynamic Pelvic Kinematics after One-Stage Bilateral or Unilateral Total Hip Arthroplasty. HIP Int. 2021, 31, 729–734. [Google Scholar] [CrossRef]
- Alves, S.A.; Preuße, M.; Hommel, H.; Duda, G.N.; Agres, A.N. The Recovery of Weight-Bearing Symmetry After Total Hip Arthroplasty Is Activity-Dependent. Front. Bioeng. Biotechnol. 2022, 10, 813345. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.S.; Kowalski, E.; Antoniades, S.; Catelli, D.S.; Beaulé, P.E.; Lamontagne, M.; Grammatopoulos, G. Do 3-Dimensional Spinopelvic Characteristics Normalize After THA? A Prospective, Comparative Study Using Motion Capture Analysis. Clin. Orthop. Relat. Res. 2024, 482, 1642–1655. [Google Scholar] [CrossRef]
- Wesseling, M.; Meyer, C.; Corten, K.; Desloovere, K.; Jonkers, I. Longitudinal Joint Loading in Patients before and up to One Year after Unilateral Total Hip Arthroplasty. Gait Posture 2018, 61, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Vasiljeva, K.; Lunn, D.; Chapman, G.; Redmond, A.; Wang, L.; Thompson, J.; Williams, S.; Wilcox, R.; Jones, A. Dynamic Acetabular Cup Orientation during Gait: A Study of Fast- and Slow-Walking Total Hip Replacement Patients. Bioengineering 2024, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.C.; Xiao, D. Efficacy of Proprioceptive Training on the Recovery of Total Joint Arthroplasty Patients: A Meta-Analysis. J. Orthop. Surg. Res. 2020, 15, 505. [Google Scholar] [CrossRef]
- Mumbleau, A.M.; Schilaty, N.D.; Hewett, T.E. Hip Muscle Inhibition After Hip Arthroscopy: A Role for Neuromuscular Electrical Stimulation. Int. J. Sports Phys. Ther. 2020, 15, 1222–1228. [Google Scholar] [CrossRef]
- Jin, X.; Chen, G.; Chen, M.; Riaz, M.N.; Wang, J.; Yang, S.; Xu, W. Comparison of Postoperative Outcomes Between Bikini-Incision via Direct Anterior Approach and Posterolateral Approach in Simultaneous Bilateral Total Hip Arthroplasty: A Randomized Controlled Trial. Sci. Rep. 2023, 13, 7023. [Google Scholar] [CrossRef]
- Hjorth, M.H.; Stilling, M.; Lorenzen, N.D.; Jakobsen, S.S.; Soballe, K.; Mechlenburg, I. Block-Step Asymmetry 5 Years After Large-Head Metal-on-Metal Total Hip Arthroplasty Is Related to Lower Muscle Mass and Leg Power on the Implant Side. Clin. Biomech. 2014, 29, 684–690. [Google Scholar] [CrossRef]
- Adler, K.L.; Cook, P.C.; Geisler, P.R.; Yen, Y.M.; Giordano, B.D. Current Concepts in Hip Preservation Surgery: Part II—Rehabilitation. Sports Health 2016, 8, 57. [Google Scholar] [CrossRef]
- Bahadori, S. The Application of Commercial Wearable Technology and Smartphone Rehabilitation Applications for Enhancing Individuals’ Level of Activity After Hip Replacement Surgery. Ph.D. Thesis, Bournemouth University, Poole, UK, 2023. [Google Scholar]
- Hodt-Billington, C.; Helbostad, J.L.; Moe-Nilssen, R. Should Trunk Movement or Footfall Parameters Quantify Gait Asymmetry in Chronic Stroke Patients? Gait Posture 2008, 27, 552–558. [Google Scholar] [CrossRef]
- Freddolini, M.; Esposito, F.; Marcucci, M.; Corvi, A.; Braccio, P.; Latella, L. Does Crutch Length Influence Gait Parameters After Total Hip Replacement Surgery? Gait Posture 2018, 60, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Alan Valenzuela, K.; Elizabeth Schroeder, L.; Trueblood Weinhandl, J.; Earl Cates, H.; Zhang, S. Strength and Balance Deficits Affecting Patient Satisfaction with Total Knee Replacements. Hum. Mov. 2021, 22, 83–92. [Google Scholar] [CrossRef]
- Ciunelis, K.; Borkowski, R.; Błażkiewicz, M. The Impact of Induced Acceleration Perturbations in Selected Phases of the Gait Cycle on Kinematic and Kinetic Parameters. Appl. Sci. 2024, 14, 4849. [Google Scholar] [CrossRef]
- Winiarski, S.; Kubiak, A.; Paluszak, A. Statistical Parametric Mapping Differences in Muscle Recruitment Patterns Between Comfort- and Performance-Oriented Saddle Positions. Appl. Sci. 2025, 15, 753. [Google Scholar] [CrossRef]
- Colgan, G.; Walsh, M.; Bennett, D.; Rice, J.; O’Brien, T. Gait Analysis and Hip Extensor Function Early Post Total Hip Replacement. J. Orthop. 2016, 13, 171–176. [Google Scholar] [CrossRef]
- De Pieri, E.; Lunn, D.E.; Chapman, G.J.; Rasmussen, K.P.; Ferguson, S.J.; Redmond, A.C. Patient Characteristics Affect Hip Contact Forces during Gait. Osteoarthr. Cartil. 2019, 27, 895–905. [Google Scholar] [CrossRef]
- Bahadori, S.; Middleton, R.G.; Wainwright, T.W. Using Gait Analysis to Evaluate Hip Replacement Outcomes—Its Current Use, and Proposed Future Importance: A Narrative Review. Healthcare 2022, 10, 2018. [Google Scholar] [CrossRef]
| Parameter | Ex1 (Pre-Op) | Ex2 (6 Weeks Post-Op) | Ex3 (3 Months Post-Op) | Ex4 (6 Months Post-Op) | Ex5 (12 Months Post-Op) | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Velocity (m/s) | 0.42 ± 0.10 | 0.48 ± 0.08 | 0.55 ± 0.09 | 0.62 ± 0.08 | 0.72 ± 0.06 | 177.7 | 0.00 |
| Stride Length (m) | 0.85 ± 0.12 | 0.92 ± 0.10 | 1.01 ± 0.09 | 1.08 ± 0.08 | 1.15 ± 0.07 | 195.5 | 0.03 |
| Step Length—Involved Leg (m) | 0.32 ± 0.08 | 0.36 ± 0.07 | 0.40 ± 0.06 | 0.44 ± 0.06 | 0.48 ± 0.05 | 61.5 | 0.03 |
| Cycle Time (s) | 1.50 ± 0.20 | 1.40 ± 0.18 | 1.32 ± 0.15 | 1.28 ± 0.12 | 1.22 ± 0.10 | 141.7 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrowicz, K.; Kosowski, W.; Michalska, A.; Winiarski, S. SPM Differences in Gait Pattern of Women After Total Hip Replacement: A Longitudinal Study. J. Clin. Med. 2025, 14, 4316. https://doi.org/10.3390/jcm14124316
Aleksandrowicz K, Kosowski W, Michalska A, Winiarski S. SPM Differences in Gait Pattern of Women After Total Hip Replacement: A Longitudinal Study. Journal of Clinical Medicine. 2025; 14(12):4316. https://doi.org/10.3390/jcm14124316
Chicago/Turabian StyleAleksandrowicz, Krzysztof, Wojciech Kosowski, Agata Michalska, and Sławomir Winiarski. 2025. "SPM Differences in Gait Pattern of Women After Total Hip Replacement: A Longitudinal Study" Journal of Clinical Medicine 14, no. 12: 4316. https://doi.org/10.3390/jcm14124316
APA StyleAleksandrowicz, K., Kosowski, W., Michalska, A., & Winiarski, S. (2025). SPM Differences in Gait Pattern of Women After Total Hip Replacement: A Longitudinal Study. Journal of Clinical Medicine, 14(12), 4316. https://doi.org/10.3390/jcm14124316







