Abstract
Background: Gastrointestinal endoscopy is crucial for diagnosing colorectal cancer and inflammatory bowel diseases, but its effectiveness can be impacted by peristalsis, poor bowel preparation, and inadequate withdrawal time. Conventional antispasmodics, though effective, may not be suitable for elderly patients or those with comorbidities. L-menthol, derived from peppermint oil, has emerged as a safer alternative. Through calcium channel blockade, L-menthol promotes GI smooth muscle relaxation. This study evaluated L-menthol’s efficacy and safety as a potential alternative to antispasmodic agents in endoscopy. Methods: Following PRISMA2020 guidelines and the Cochrane Handbook, we conducted a systematic review and meta-analysis of randomized controlled trials involving adults undergoing endoscopy, comparing L-menthol to placebo. The primary outcome was the adenoma detection rate, with secondary outcomes, including severity of peristalsis, safety, withdrawal time, and ease of examination. We searched five databases on 31 May 2023, with updates on 20 October 2024. Results: Fourteen studies were included. L-menthol reduced peristalsis during colonoscopy and upper endoscopy, achieving a suppression rate of 55.9% (560/1002 patients; odds ratio (OR) = 3.88, 95% confidence interval (95% CI): 2.13–7.07), which improved mucosal visualization. It improved ease of examination (OR = 2.53, 95% CI: 1.35–4.73), allowing endoscopists to perform procedures with less technical difficulty. However, L-menthol had no significant impact on the adenoma detection rate (OR = 1.06, 95% CI: 0.69–1.64), indicating no added benefit for lesion detection, and did not prolong withdrawal time (MD = 3.24 s, 95% CI: −101.05–107.53). Adverse event rates remained low and comparable to placebo (OR = 0.97, 95% CI: 0.74–1.27). Conclusions: L-menthol reduces peristalsis and enhances ease of examination without adverse events. Although its effect on the adenoma detection rate remains inconclusive, its antispasmodic properties make it a promising alternative for patients who cannot tolerate conventional agents.
1. Introduction
Gastrointestinal (GI) endoscopy is an essential diagnostic tool [1] to identify and manage various GI diseases, such as colorectal cancer (CRC) [2], inflammatory bowel disease (IBD) [3], and peptic ulcer disease (PUD) [4]. More than 7.4 million upper GI endoscopies [5] and more than 13 million colonoscopies are annually performed in the United States alone [6]. Factors such as natural peristaltic movements, inappropriate bowel preparation, or insufficient withdrawal time can affect the accuracy of endoscopic procedures by obstructing small lesions, thus decreasing diagnostic precision [7,8]. Suppressing peristalsis is crucial for high-quality and accurate outcomes during endoscopic procedures [9,10].
Hyoscine-N-butyl bromide (HBB) is widely used during endoscopy to reduce peristalsis, improving mucosal visualization and polyp detection [11,12]. However, HBB is associated with cardiovascular risks, such as tachycardia and arrhythmias, and is contraindicated in patients with conditions such as myasthenia gravis and narrow-angle glaucoma [13]. HBB requires intravenous administration, complicating its use in unsedated patients without a cannula [14]. It is costly and less suitable for elderly patients or those with co-morbidities [15,16,17,18]. There is a critical need for a safe, effective, and cost-efficient alternative to suppress peristalsis during endoscopy.
Peppermint oil and its main component, L-menthol, extracted from the Mentha × piperita L. plant, have been considered potentially safer natural antispasmodics [15,16]. Traditionally, peppermint oil has been used to alleviate irritable bowel syndrome (IBS) symptoms, such as discomfort caused by spasms [17,18]. This antispasmodic effectiveness is attributed to menthol, which acts as a calcium channel blocker to relax GI smooth muscles [19,20,21,22]. Recent studies have investigated the potential use of menthol during endoscopy and its ability to reduce peristalsis without adverse effects. The benefits of using menthol include improving the lesion detection quality while minimizing adverse events [23]. Menthol is approved for upper GI endoscopy in Japan [24]. This systematic review and meta-analysis aims to assess the efficacy and safety of menthol during upper and lower GI endoscopy.
2. Materials and Methods
This study followed the PRISMA 2020 guideline [25] (see Table S3) and the Cochrane Handbook [26]. No ethical approval was required, as all data were from peer-reviewed journals. The protocol was registered in the PROSPERO database (CRD42023430941) on 30 May 2023.
2.1. Eligibility Criteria
We used the PICO framework to answer our clinical question: Does L-Menthol improve the detection rate of lesions during endoscopy in adults compared to placebo? The population (P) included adults (>18 years) undergoing upper GI endoscopy, colonoscopy, endoscopic retrograde cholangiopancreatography (ERCP), or endoscopic ultrasound (EUS). The intervention (I) involved spraying peppermint oil or L-Menthol solution onto the GI mucosa, while controls (C) received a placebo. The primary outcome (O) included the adenoma detection rate (ADR), with secondary outcomes being peristalsis severity, withdrawal time, ease of examination, and safety.
2.2. Information Sources
Our systematic search was conducted on 31 May 2023, in five databases (Scopus, Embase, CENTRAL, MEDLINE, and Web of Science) with an update on 20 October 2024.
2.3. Search Strategy
The search used keywords related to peppermint/L-menthol and endoscopic procedures without language restrictions. Details are in Table S1.
2.4. Selection Process
Randomized controlled trials (RCTs), observational studies, case series, case-control studies, and conference abstracts were eligible. Reviews, meta-analyses, animal studies, in vitro studies, and guidelines were excluded. There were no language restrictions.
The search results were first exported to the EndNote 20 citation manager (Clarivate Analytics, Philadelphia, PA, USA). Duplicates were automatically and manually removed (DG). Then, two independent authors performed the title and abstract selection. Next, full-text selection was performed using the online screening tool Rayyan (Qatar Computing Research Institute, Hamad Bin Khalifa University, Doha, Qatar; https://www.rayyan.ai) [27] according to the inclusion criteria (DG and ATM). To resolve disagreements, a third author (GG) made the final decision. Cohen’s kappa coefficient was calculated at each selection step to evaluate the level of agreement between the authors. The references from eligible studies were manually screened for eligibility and with an automated citation chaser [28].
2.5. Data Collection Process
Two investigators (DG and UNDT) independently extracted data, resolving disagreements by consensus. Plot Digitizer (Version 2.6.9, Joseph A. Huwaldt, Minneapolis, MN, USA; https://plotdigitizer.com, 2020) converted graphics to numerical values. Microsoft Excel (Microsoft, Office 365, Redmond, WA, USA) was used for data collection. Missing data were requested from the study authors.
2.6. Data Items
The following data were extracted: study characteristics (first author, publication year, country, digital object identifier, study site, and study type), study population (sample size, gender, and age), endoscopy type, intervention type, and details (peppermint oil/L-menthol, dosage, and evaluation time point). The adenoma detection rate was the primary outcome, with the experience level of the endoscopist(s). Secondary outcomes were peristalsis levels, withdrawal time, ease of examination reported by the endoscopist, and the total number of adverse events and adverse drug reactions.
The adenoma detection rate (ADR), a key performance indicator for colonoscopy, is analyzed as the proportion of procedures in which at least one adenoma is detected [29]. Peristalsis severity in upper endoscopy was assessed using Niwa’s Classification, a five-point scale where Grade 1 indicates no peristalsis, Grade 2 mild peristalsis, Grade 3 moderate peristalsis, Grade 4 vigorous peristalsis, and Grade 5 markedly vigorous peristalsis. For analysis, data were dichotomized as ‘Grade 1’ versus ‘Grades 2–5’ or ‘Grades 1–2’ versus ‘Grades 3–5’. In colonoscopy, peristalsis or spasm severity was evaluated using the method described by Asao et al. (2001) [30]. The ease of examination is crucial for ensuring the quality of the endoscopy, as endoscopist fatigue may negatively impact outcomes [31]. The ease of examination is assessed by the endoscopist using a standardized four-grade scale. This scale evaluates the degree to which gastric peristalsis interfered with the endoscopic procedure, and includes the following categories: ‘very easy’ (no peristalsis, no interference with observation), ‘easy’ (mild peristalsis, observation performed without interference), ‘slightly difficult’ (peristalsis slightly interfered with observation), and ‘difficult’ (marked peristalsis, observation significantly impaired). For statistical analysis, results were dichotomized as ‘very easy’ and ‘easy’ versus ‘slightly difficult’ and ‘difficult’. Withdrawal time was defined as the duration from cecal intubation to scope withdrawal, including biopsy time. Adverse events and adverse drug reactions were assessed as the proportion of patients experiencing symptoms such as abdominal pain, nausea, diarrhea, rash, fever, or abnormal laboratory findings during or immediately after the endoscopic procedure.
2.7. Study Risk of Bias Assessment
Two authors (DG and UNDT) independently assessed the risk of bias using the Cochrane risk-of-bias tool (RoB2) Excel tool, version 9 (Cochrane Methods Group, London, UK; https://www.riskofbias.info/) [32], applying the tool to assess the intention-to-treat effect in the included randomized controlled trials. Disagreements were resolved by consensus. Domains were evaluated, such as the randomization process, deviations from intended interventions, missing data, outcome measurement, and result selection. The risk was categorized as ‘low’, ‘some concerns’, or ‘high’, visualized using the robvis tool [33].
2.8. Quality of Evidence
The GRADEpro GDT tool (McMaster University [developed by Evidence Prime], Hamilton, ON, Canada; https://www.gradepro.org) [34,35] was used to evaluate the quality of evidence. Two authors (DG and UNDT) independently graded each outcome. Discrepancies in GRADE ratings were resolved through discussion or inclusion of a third author (GG). Grading started at ‘high’ for randomized trials and downgraded for risk of bias, inconsistency, indirectness, imprecision, or publication bias. Upgrading the certainty of evidence did not apply to randomized controlled trials. The final certainty was categorized as ‘very low’, ‘low’, ‘moderate’, or ‘high’.
2.9. Synthesis Methods and Statistical Analysis
Both qualitative and quantitative syntheses were performed. At least three studies that reported clinically poolable effect sizes were a prerequisite for statistical poolability. Meta-analyses were conducted using the ‘meta’ [36] and ‘dmetar’ [37] packages in R (version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/) [38].
For dichotomous outcomes, odds ratios (ORs) with 95% confidence intervals (95% CI) were used to measure the effect of the intervention. If not reported, these were calculated from participant numbers and event occurrences [39,40,41]. For continuous outcomes, the mean difference (MD) between intervention and control groups was used with a 95% CI.
The random effects model was chosen for the meta-analyses. Confidence intervals were adjusted using the Hartung–Knapp method [42,43]. For pooled results, the exact Mantel–Haenszel method without continuity correction was applied to manage zero cell counts [44,45]. The τ2 estimate utilized the Paule–Mandel method, and the confidence interval for τ2 was determined using the Q profile method [46,47].
Statistical heterogeneity was assessed using the Cochrane Q test and I2 values, with statistical significance at p < 0.1 [48]. For subgroup analyses, if heterogeneity in a subgroup was low (I2 < 25%), a fixed-effects model (Mantel–Haenszel method) was used for that subgroup, otherwise, random-effects models were applied. Publication bias was assessed using funnel plots and Egger’s test was performed for outcomes with at least 10 studies. Outlier and influence analyses followed methodologies by Harrer et al. (2021) [37] and Viechtbauer and Cheung (2010) [49]. For subgroup analysis, we used a fixed-effects ‘plural’ model (aka mixed-effects model), stratified by procedure type (colonoscopy vs. upper endoscopy) and by time point of antiperistaltic effect measurement. We assumed that subgroups share common τ2 values. A Cochrane Q test assessed the difference between the subgroups. The null hypothesis was rejected at a significance level of 5%.
Our analysis included studies using both spraying and direct application of peppermint oil. These methods were combined because the included studies used comparable peppermint oil concentrations and reported similar antiperistaltic effects, regardless of the delivery mechanism.
Peristalsis severity was assessed using Niwa’s Classification [50], which categorizes peristalsis into five grades: Grade 1 (no peristalsis), Grade 2 (mild), Grade 3 (moderate), Grade 4 (vigorous), and Grade 5 (markedly vigorous). Two separate analyses were performed: data were dichotomized as ‘Grade 1’ versus ‘Grades 2–5’ or ‘Grades 1 and 2’ versus ‘Grades 3–5,’ with ORs calculated.
The ease of intragastric examination was evaluated on a four-grade scale: ‘very easy’, ‘easy’, ‘slightly difficult’, and ‘difficult’ [30]. The data were dichotomized as ‘very easy’ and ‘easy’ versus ‘slightly difficult’ and ‘difficult,’ with ORs calculated.
Withdrawal time was defined as the duration to remove the colonoscope from the cecum to the anus, including biopsy time. Adverse events and reactions were assessed as the proportion of patients experiencing symptoms.
3. Results
3.1. Search and Selection
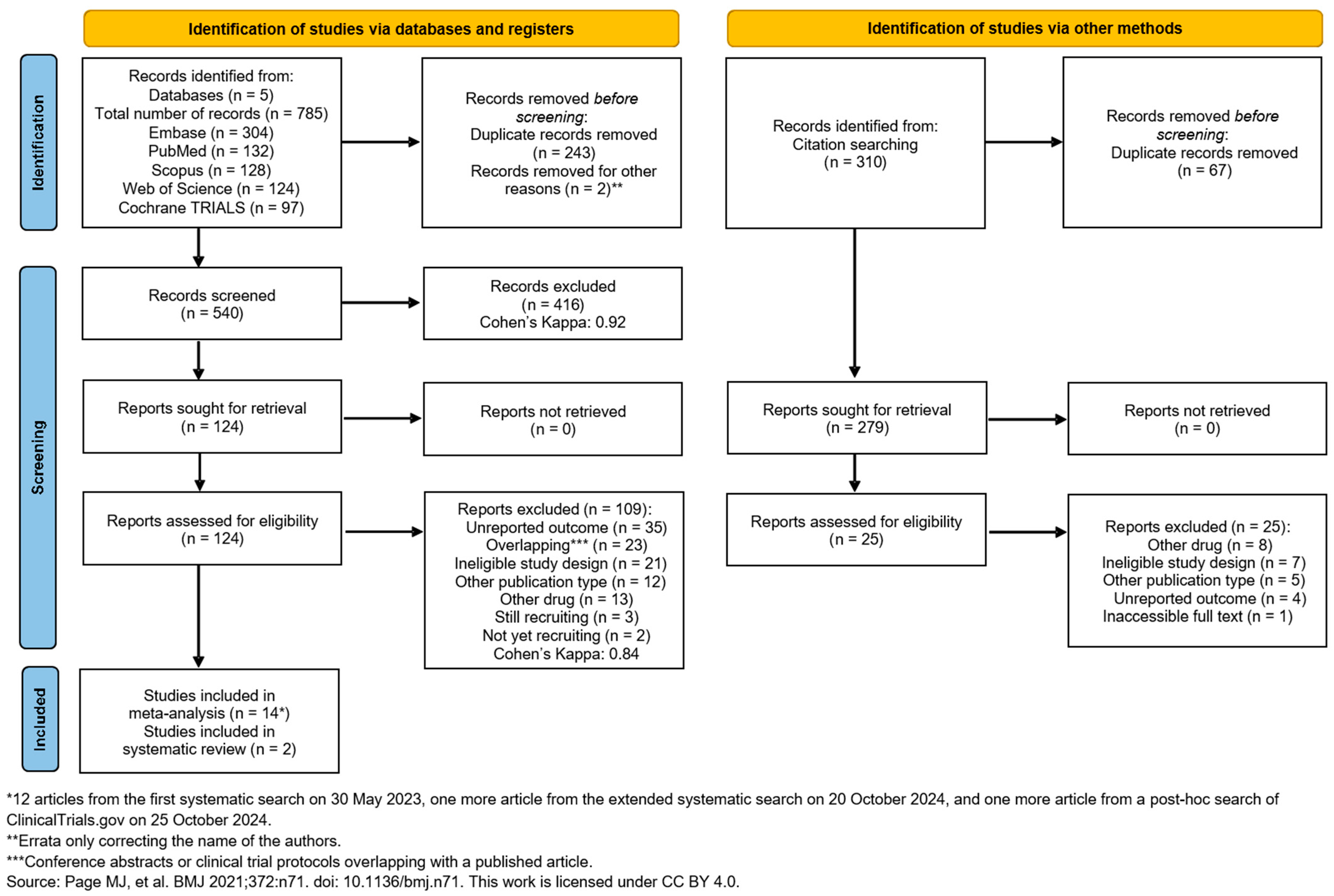
The systematic search on 31 May 2023, identified 785 studies from five databases: EMBASE (n = 304), Scopus (n = 128), PubMed (n = 132), Web of Science (n = 124), and Cochrane Library (Trials) (n = 97). After removing 245 duplicates, 540 unique studies remained. Following title and abstract selection, 416 studies were excluded. After our renewed systematic search on 20 October 2024, we found one more eligible study. We also conducted a post hoc search of ClinicalTrials.gov on 25 October 2024. This search identified one additional eligible study.
Sixteen studies were included: 14 studies for quantitative analysis (colonoscopy: [30,51,52,53,54,55,56], upper endoscopy: [57,58,59,60,61,62,63]) and two studies for qualitative analysis (upper endoscopy: [64], colonoscopy: [65])
We used the ‘citationchaser’ tool [28] to explore more relevant studies; however, none were added. No meta-analyses were possible for ERCP and EUS due to the lack of available studies in the literature [66,67,68,69,70]. The search and selection process is summarized in the PRISMA-Flowchart 2020 (Figure 1).
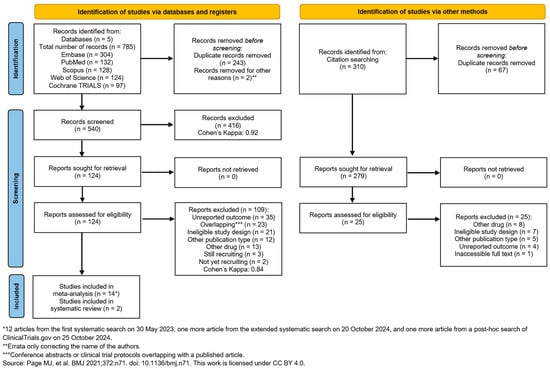
Figure 1.
PRISMA 2020 flow diagram for searches in databases and other sources [25].
3.2. Basic Characteristics of Included Studies
Of the included studies, the majority were conducted in Asia, with 10 studies in Japan [30,53,54,57,58,59,61,63,64,65], one in China [60], one in Taiwan [62], one in Thailand [55], and one in Iran [71]. Three studies were conducted in North America: two in the USA [52,56] and one in Canada [51].
Gastric peristalsis was evaluated using Niwa’s Classification [50] or the modified version of Niwa’s classification [58]. Colonic peristalsis was assessed using the method reported by Asao et al. (2001) [30]. The baseline characteristics of each study are detailed in Table 1.

Table 1.
Baseline characteristics of the RCTs with colonoscopy and upper endoscopy patients investigated in the systematic review and meta-analysis.
3.3. Meta-Analysis of the Findings
3.3.1. Efficacy of L-Menthol on Adenoma Detection Rate
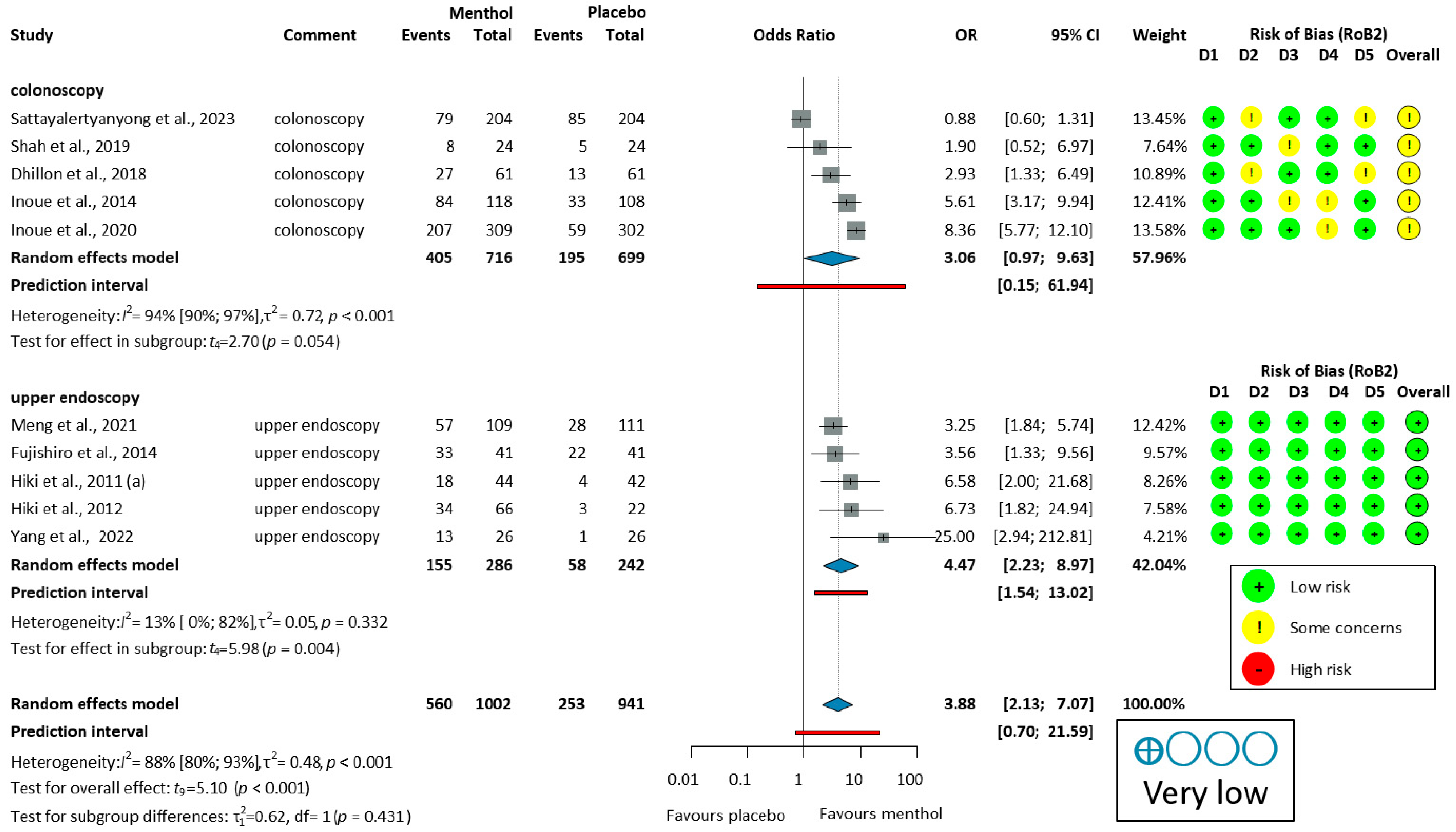
The ADR was analyzed in six colonoscopy studies [51,52,53,54,55,56]. Only one study (Inoue, 2014 [53]) demonstrated a significant improvement in the ADR with L-menthol. Due to moderate heterogeneity (I2 = 51%, p = 0.070), a random-effects model was used to calculate OR. L-menthol had no significant effect on the ADR in patients undergoing colonoscopy (OR = 1.06, 95% CI: 0.69 to 1.64, p = 0.734), as shown in Figure 2.
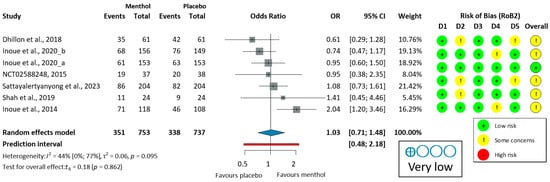
Figure 2.
Forest plot of adenoma detection rate in colonoscopy showing pooled ORs with 95% CIs for menthol versus placebo. Risk-of-bias assessments are shown as colored circles for each domain and overall. “Very low” indicates GRADE certainty of evidence. OR = odds ratio, CI = confidence interval, D = domain [51,52,53,54,55,56].
Leave-one-out (LOO) sensitivity analysis (Figure S1) showed some variability in pooled ADR estimates (OR 0.91–1.16) and heterogeneity (I2 = 0–61%). Excluding Inoue et al. (2014) [53]—the only study with a significant ADR benefit—reduced heterogeneity to zero and yielded the lowest OR. Excluding other studies maintained moderate-to-high heterogeneity (I2 = 47–61%) and ORs of 1.04–1.16. All confidence intervals confirming the overall lack of ADR benefit are robust, although heterogeneity is largely due to the single positive study.
Subgroup analysis suggested that sedation status might influence efficacy, with deeper sedation protocols (e.g., propofol and/or fentanyl) showing more favorable trends [55] compared to minimally sedated procedures [51,54]. However, traditional patient factors, such as age, comorbidities, or gender, did not significantly influence ADR outcomes.
3.3.2. Antiperistaltic Effect of L-Menthol
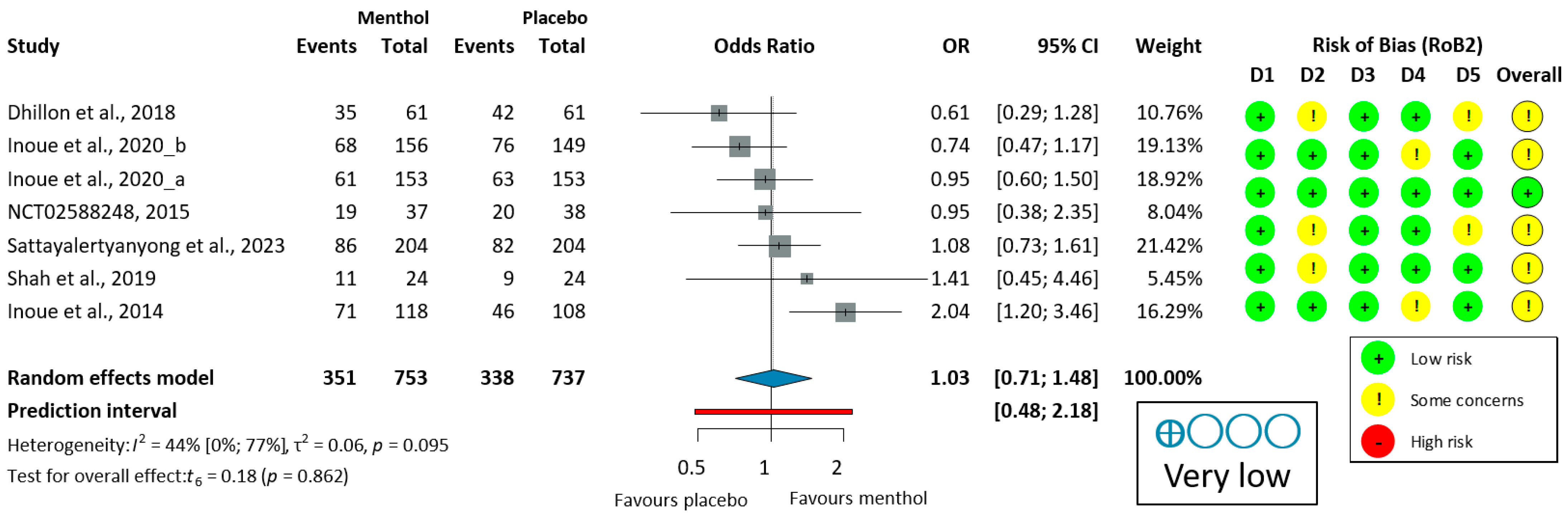
Ten studies [51,53,54,55,56,57,58,59,60,62] reported on the proportion of no peristalsis (PNP). PNP had a high overall heterogeneity (I2 = 88%, p < 0.001). For colonoscopy, the analysis of PNP data (405/716, 56.6%, OR = 3.06, 95% CI: 0.97 to 9.63, p = 0.054) indicates a trend toward efficacy that did not reach statistical significance. In studies of upper endoscopy (155/286, 54.2%, OR = 4.47, 95% CI: 2.23 to 8.97, p = 0.004), and overall PNP (560/1002, 55.9%, OR = 3.88, 95% CI: 2.13 to 7.07, p < 0.001), L-menthol demonstrated a significantly higher antispasmodic effect compared to placebo, as shown in Figure 3.
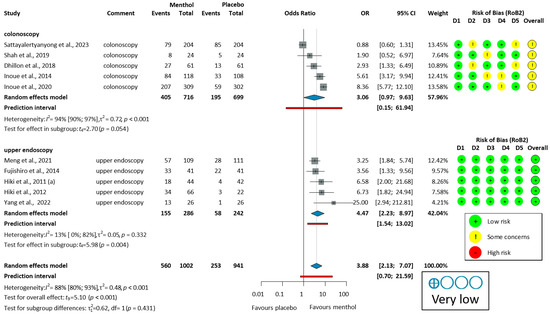
Figure 3.
Forest plot of the proportion of no peristalsis (PNP) in colonoscopy and upper endoscopy showing pooled ORs with 95% CIs for menthol versus placebo. Risk-of-bias assessments are shown as colored circles for each domain and overall. “Very low” indicates GRADE certainty of evidence. OR = odds ratio, CI = confidence interval, D = domain [51,53,54,55,56,57,58,59,60,62].
Eleven studies [30,53,54,55,56,57,58,59,60,61,62] reported data on PNMP. Heterogeneity was high (I2 = 85%, p < 0.001). For colonoscopy, the analysis of PNMP data (649/690, 94.1%, OR = 3.36, 95% CI: 0.27 to 42.41, p = 0.255) indicated no statistically significant antiperistaltic effect of L-menthol compared to placebo. In studies of upper endoscopy (251/335, 74.9%, OR = 3.79, 95% CI: 0.91 to 15.82, p = 0.062), a trend toward efficacy was observed, though this did not reach statistical significance. In the overall PNP (900/1025, 87.8%, OR = 3.70, 95% CI 1.27 to 10.76, p = 0.021), L-menthol demonstrated a significantly higher antispasmodic effect compared to placebo, as shown in Figure S2.
Yoshida et al. (2014) [65] demonstrated a rapid antispasmodic effect of L-Menthol. It reduced peristalsis in 60.0% (39/65) of cases within 30 s and 70.8% (46/65) within 1 min, compared to 22.2% (6/27) and 29.6% (8/27) in the control group. Imagawa et al. (2012) [64] assessed spasm severity using antispasmodic scores (1–5, where 5 indicates no spasm). This study reported higher scores in the peppermint oil group (4.025 (0.925), n = 1893) compared to the placebo group (3.846 (1.073), n = 156), indicating effective peristalsis reduction.
LOO sensitivity analysis (Figure S3) showed moderate variability in pooled estimates for the proportion of no peristalsis (OR 3.42–4.47) and consistently high heterogeneity (I2 = 50–90%). Excluding any study maintained a significant antiperistaltic effect, with all confidence intervals favoring L-menthol. The most conservative estimate occurred when omitting Inoue et al. (2020) [54], while the highest estimate resulted from omitting the study of Fujishiro et al. (2014) [57]. Persistent high heterogeneity suggests variability is distributed among studies, confirming L-menthol’s antiperistaltic benefit is robust.
Subgroup analysis identified specific patient groups where L-menthol demonstrates superior efficacy. Unsedated upper GI procedures showed the strongest effects in elderly patients (mean age 81.7 vs. 82.6 years) [62], and in middle-aged participants (mean age 51.64 vs. 51.44 years) [60]. These findings suggest that L-menthol may benefit those with contraindications to antispasmodic drugs or sedation. In contrast, colonoscopy studies yielded more modest effects, likely due to greater procedural complexity and more common use of sedation or general anesthesia.
3.3.3. Ease of Examination for the Operator in Upper Endoscopy
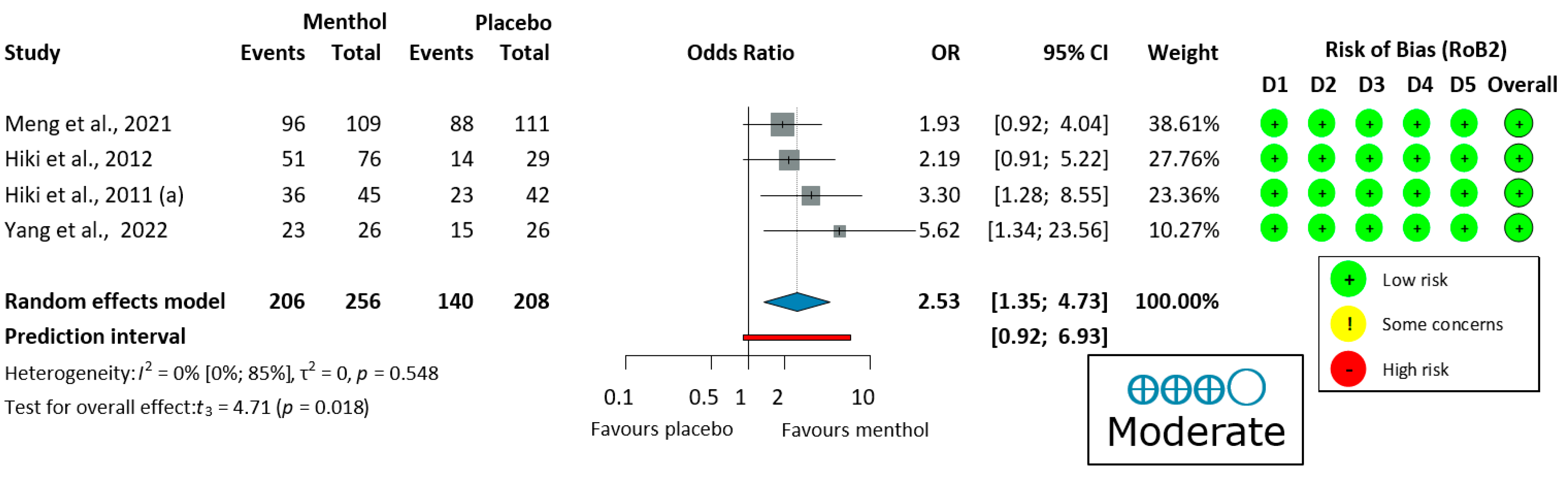
Four upper endoscopy studies [58,59,60,62] evaluated ease of examination, using a four-grade scale (very easy, easy, slightly difficult, and difficult) shown in Figure 4. There was no heterogeneity (I2 = 0%, p = 0.548). Thus, a fixed-effects model was used to pool data. The endoscopic procedure was significantly easier for the operator when L-menthol was applied (OR = 2.53, 95% CI: 1.35 to 4.73, 206/256 subjects, p = 0.018).
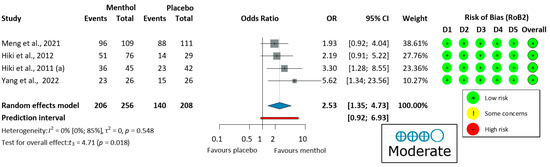
Figure 4.
Forest plot of ease of examination for the operator in upper endoscopy showing pooled ORs with 95% CIs for menthol versus placebo. Risk-of-bias assessments are shown as colored circles for each domain and overall. “Moderate” indicates GRADE certainty of evidence. OR = odds ratio, CI = confidence interval, D = domain [58,59,60,62].
LOO sensitivity analysis (Figure S4) showed minimal variability in pooled estimates (OR 2.31–3.00) with zero heterogeneity (I2 = 0%). Excluding any individual study maintained the benefit of L-menthol for procedural ease. The most conservative estimate occurred when omitting Yang et al. (2022) [62], while the highest estimate resulted from omitting Meng et al. (2021) [60]. The zero heterogeneity indicates great homogeneity among studies. These findings confirm that L-menthol’s benefit for ease of examination is robust and not dependent on any single study.
This benefit was most evident in unsedated patients (improved ease for 88.1% vs. 79.3% patients [60]). Neither age, comorbidities, nor gender moderated this effect, supporting L-menthol’s broad applicability.
3.3.4. Withdrawal Time in Colonoscopy
Five colonoscopy studies provided withdrawal times [52,53,54,55,56]. Our analysis showed that L-menthol did not significantly reduce withdrawal time compared to placebo in patients undergoing colonoscopy (MD = 3.24 s, 95% CI: −101.05 to 107.53, p = 0.935), as shown in Figure S5.
3.3.5. Total Adverse Events
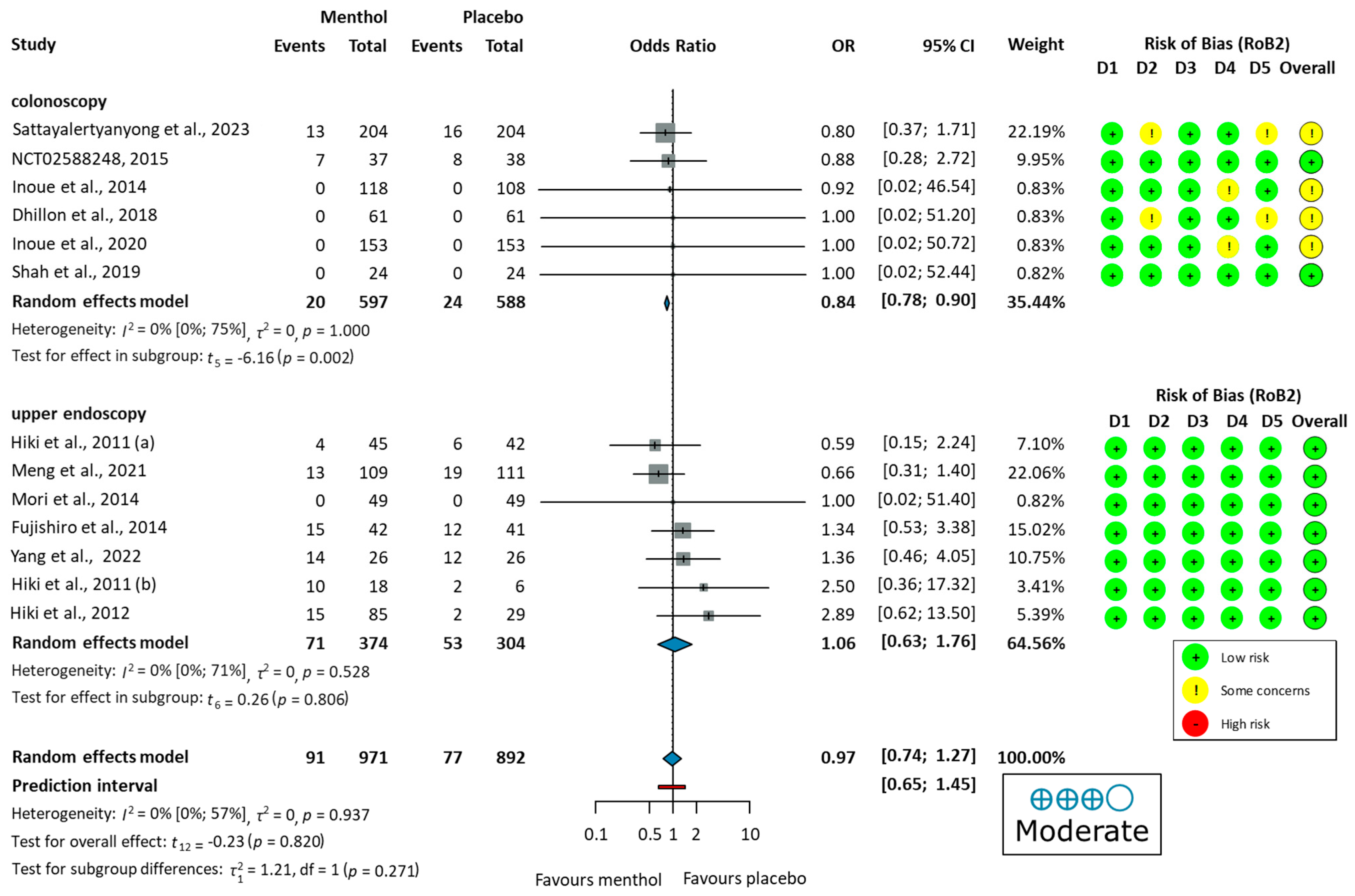
Four studies reported no major [51], serious [61], or any [53,54], adverse events during the procedure. Nine studies [52,55,56,57,58,59,60,62,63] reported the number of adverse events. Commonly reported GI symptoms included abdominal pain, nausea, diarrhea, heartburn, and bloating, observed in both colonoscopy and upper endoscopy studies. Some studies also noted dizziness, headache, and fever. Additionally, cardiovascular changes, including electrocardiogram ST-T changes, palpitations, and ventricular premature beats, were observed. Urinary tract infections or respiratory infections were observed as well. The pooled OR did not indicate a significant difference in total adverse events between the L-Menthol and the placebo groups (OR = 0.97, 95% CI: 0.74 to 1.27, p = 0.937), as shown in Figure 5.
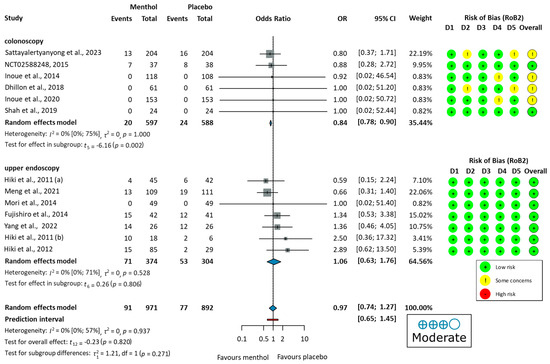
Figure 5.
Forest plot of adverse events showing pooled ORs with 95% CIs for menthol versus placebo. Risk-of-bias assessments are shown as colored circles for each domain and overall. “Moderate” indicates GRADE certainty of evidence. OR = odds ratio, CI = confidence interval, D = domain [51,52,53,54,55,56,57,58,59,60,61,62,63].
Differences in adverse event proportions were observed when analyzing the data separately for colonoscopy and upper endoscopy groups. In the colonoscopy group, the proportion of patients experiencing adverse events was 3.35% (20/597 patients) in the L-menthol group and 4.08% (24/588 patients) in the placebo group. In the upper endoscopy group, adverse events were reported in 18.98% (71/374 patients) in the L-menthol group and 17.43% (53/304 patients) in the placebo group.
3.3.6. Total Adverse Drug Reactions
One study (Inoue et al. (2014)) [53] reported no adverse drug reactions. Four studies [57,58,59,60] provided exact numbers and were included in the quantitative synthesis. Reported adverse events included procedural pain, rash, fever, abdominal pain, diarrhea, and abnormal laboratory examination. The pooled OR identified a significant difference between the two groups (OR = 0.57, 95% CI: 0.37 to 0.87, p = 0.021) favouring the usage of L-menthol, as shown in Figure S6.
3.4. Studies with Peppermint Capsules
Three studies evaluated peppermint oil in orally administered formulations, specifically IBGard™ [74] and Colpermin® [75], both of which are frequently used to manage symptoms of irritable bowel syndrome (IBS) [76,77]. Although these products and studies were not included in our main analysis, they are presented here to provide a more comprehensive overview of the therapeutic profile of peppermint essential oil.
Han et al. (2021) [73] found no significant difference in the ADR between the two groups (IBGard™: 47.8% vs. placebo: 43.1%; p = 0.51). Shavakhi et al. (2012) [71] found significantly lower spasm scores in the Colpermin® compared to placebo (no movement: 25 vs. 0; any movement: 8 vs. 32). Al Moussawi et al. (2017) [72], however, reported no significant difference between the groups. In the ease of examination scores, Han et al. (2021) [73] reported no significant difference (p = 0.23). Similarly, Al Moussawi et al. (2017) [72] found no significant differences in endoscopist satisfaction scores between Colpermin® and placebo groups (p = 0.8). Han et al. (2021) [73] observed slightly shorter withdrawal times in the placebo group compared to the IBGard™ group (14.5 min (10.3) vs. 16.5 min (8.3 min)), although this difference was not significant (p = 0.31). For adverse events, Shavakhi et al. (2012) [71] observed isolated cases of abdominal discomfort, nausea, blurred vision, and heartburn. Meanwhile, Al Moussawi et al. (2017) [72] and Han et al. (2021) [73] reported no adverse events in either group.
3.5. Risk of Bias and GRADE Assessment
The risk of bias was assessed for each outcome using the RoB2 tool, and GRADE certainty was evaluated per outcome. For the adenoma detection rate, most studies were rated as having “some concerns,” primarily due to deviations from intended interventions, measurement of the outcome, and selection of the reported result. The assessment of the proportion of no peristalsis showed greater variability; several studies exhibited “some concerns” or “high risk”, while others demonstrated low risk across all domains. Outcomes such as ease of examination and adverse events were generally associated with low risk of bias, though a few studies still showed “some concerns”. Withdrawal time was often rated as having “some concerns,” mainly due to issues with adherence to the intervention and outcome measurement. The RoB2 judgements are provided in Figure S7. The overall certainty for each outcome is summarized in Table S3, with detailed explanations provided.
3.6. Publication Bias and Heterogeneity
We included conference abstracts in our analysis and also attempted to contact the corresponding authors of included studies to identify unpublished data, although no additional information was obtained. To further mitigate publication bias, we conducted citation chasing using the Citationchaser tool [28] to identify relevant studies from the reference lists of eligible articles.
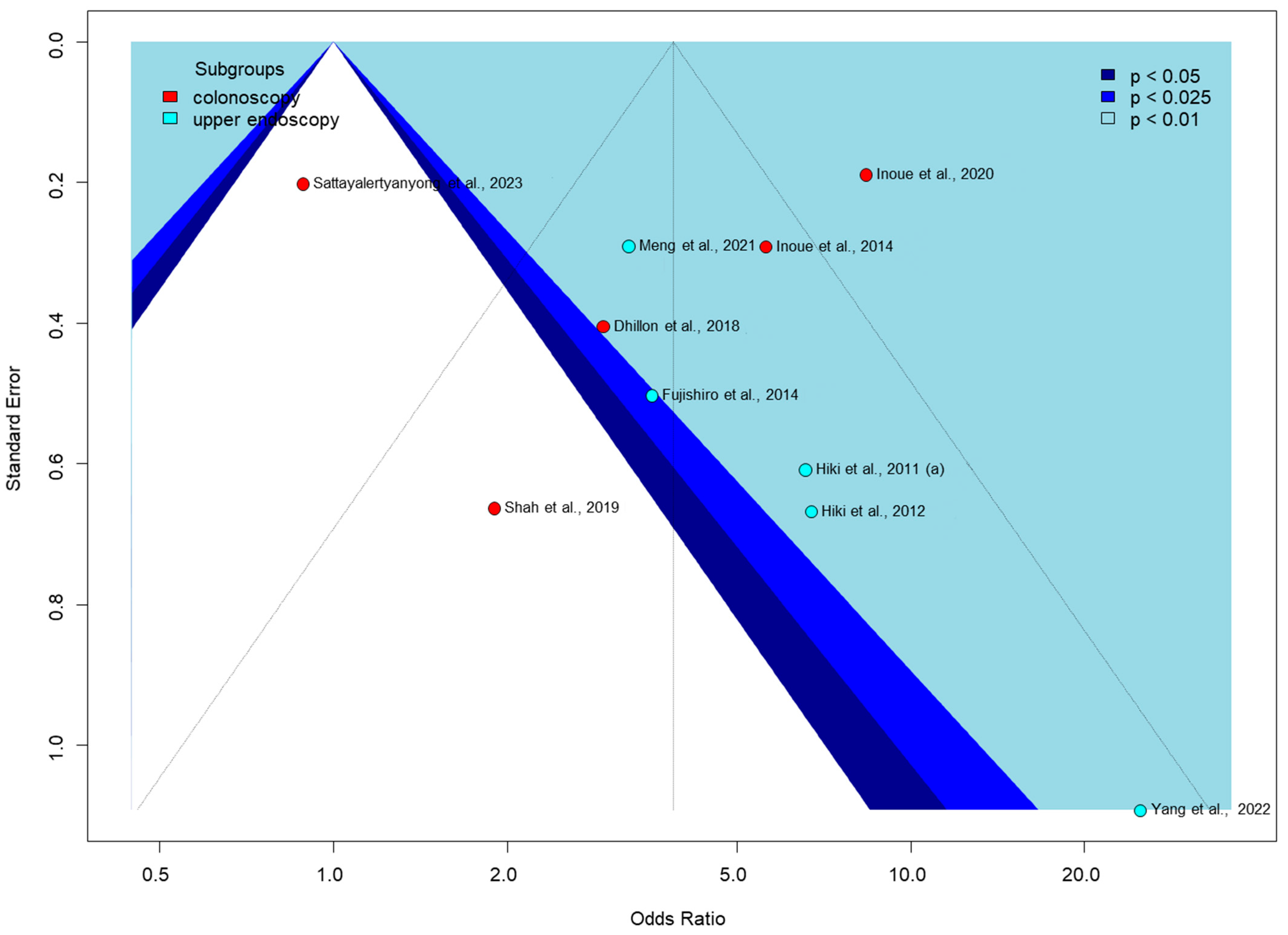
Egger’s test for publication bias was only performed for the proportion of no peristalsis outcome in Figure 6, as it was the only one with enough studies (at least 10) to meet the test’s requirements. The funnel plot shows significant asymmetry, with some studies appearing as outliers, such as Shah et al., 2019, with a low odds ratio [56], and Yang et al., 2022, with a very high odds ratio [62]. Colonoscopy studies show a wider spread of effect sizes, while upper endoscopy studies are mostly clustered in the positive effect area. The pattern of study distribution and variability in effect sizes may indicate some publication bias or true intervention heterogeneity.
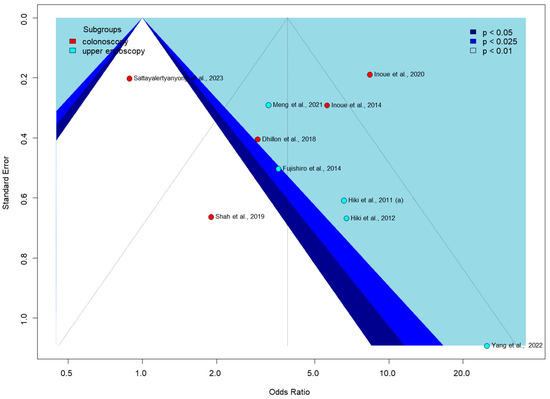
Figure 6.
Funnel plot of proportion of no peristalsis [51,53,54,55,56,57,58,59,60,62].
Additionally, we found no evidence of publication bias for the rest of the outcomes. However, our analysis was underpowered by the small sample size.
4. Discussion
This systematic review and meta-analysis evaluated the efficacy and safety of L-menthol in both upper endoscopy and colonoscopy. Although L-menthol did not consistently improve the ADR in colonoscopy, it may have beneficial effects in other areas, such as suppressing peristalsis and potentially improving the ease of examination for operators, particularly in upper endoscopy. However, L-menthol did not significantly impact withdrawal time in colonoscopy. Regarding safety, L-menthol showed a favorable profile, with adverse events comparable to placebo and fewer adverse drug reactions. Although L-menthol induces spasmolytic effects in colon circular muscle by directly inhibiting gastrointestinal smooth muscle contractility, the detailed mechanism remains unclear [20,78].
L-menthol’s antispasmodic properties make it a promising alternative for patients who cannot tolerate conventional agents, such as hyoscine butylbromide or glucagon, which are associated with systemic side effects, including dry mouth, urinary retention, and hyperglycemia [11,13,17,18]. Emerging alternatives to L-menthol include topical lidocaine and cool water irrigation, both of which have shown efficacy in reducing gastrointestinal spasm. Topical lidocaine (2–4%) blocks mucosal sodium channels, offering more prolonged action and less rebound than peppermint oil without adverse events. However, its effect is superficial, while L-menthol directly targets smooth muscle for more profound, sustained relief. Cool water (15–24 °C) reduces peristalsis via TRPM8 activation but is less potent and more technique-dependent than L-menthol’s targeted suppression [79,80].
The ADR is a critical quality indicator for colonoscopy, reflecting the ability to detect adenomas, the precursors to CRC [29]. We analyzed data from six studies on L-menthol in colonoscopy, but only one (Inoue et al., (2014) [53]) reported a significant improvement in the ADR, suggesting that applying L-menthol does not consistently improve lesion detection. However, this outcome must be interpreted cautiously due to the primarily negative results on the ADR. The ADR may be affected by various factors, such as the number and experience level of endoscopists (years of practice and the number of procedures performed), the quality of equipment (e.g., high- or lower-definition scopes) [81], withdrawal time (more or less than 6 min), and procedural factors, e.g., bowel preparation) [82], etc. While procedural efficiency benefits from L-menthol’s antiperistaltic effects—improving mucosal visualization and potentially reducing missed adenomas —the lack of ADR improvement limits its clinical utility as an additional measure to colonoscopy. Although the ADR remains the gold standard, recent studies suggest that complementary metrics, such as adenoma per colonoscopy (APC) or procedural efficiency, may more comprehensively reflect the benefits of intervention, especially given the recognized limitations of the ADR as a standalone metric [83,84,85]. Although there is no evidence based on the studies analysed, ease of examination may affect the performance of the examiner when he or she performs several examinations in succession. Investigating this relationship may be the subject of future studies. While L-menthol’s robust antiperistaltic effects and improved procedural conditions are well supported, we acknowledge the need for further research to establish its impact on lesion detection and colorectal cancer prevention.
L-menthol may improve endoscopic visibility during the procedure. Pooled analyses revealed a robust antispasmodic effect of L-menthol compared to placebo in both colonoscopy and upper endoscopy. While these procedures differ anatomically, the antispasmodic mechanism of L-menthol—mediated via TRPM8 activation and calcium channel blockade—is consistent across the gastrointestinal tract [86,87]. To ensure transparency, we conducted subgroup analyses by procedure type. The overlapping confidence intervals support similar efficacy across gastrointestinal segments (Figure 3). However, clinical heterogeneity, such as mucosal differences or procedural duration, may still influence outcomes.
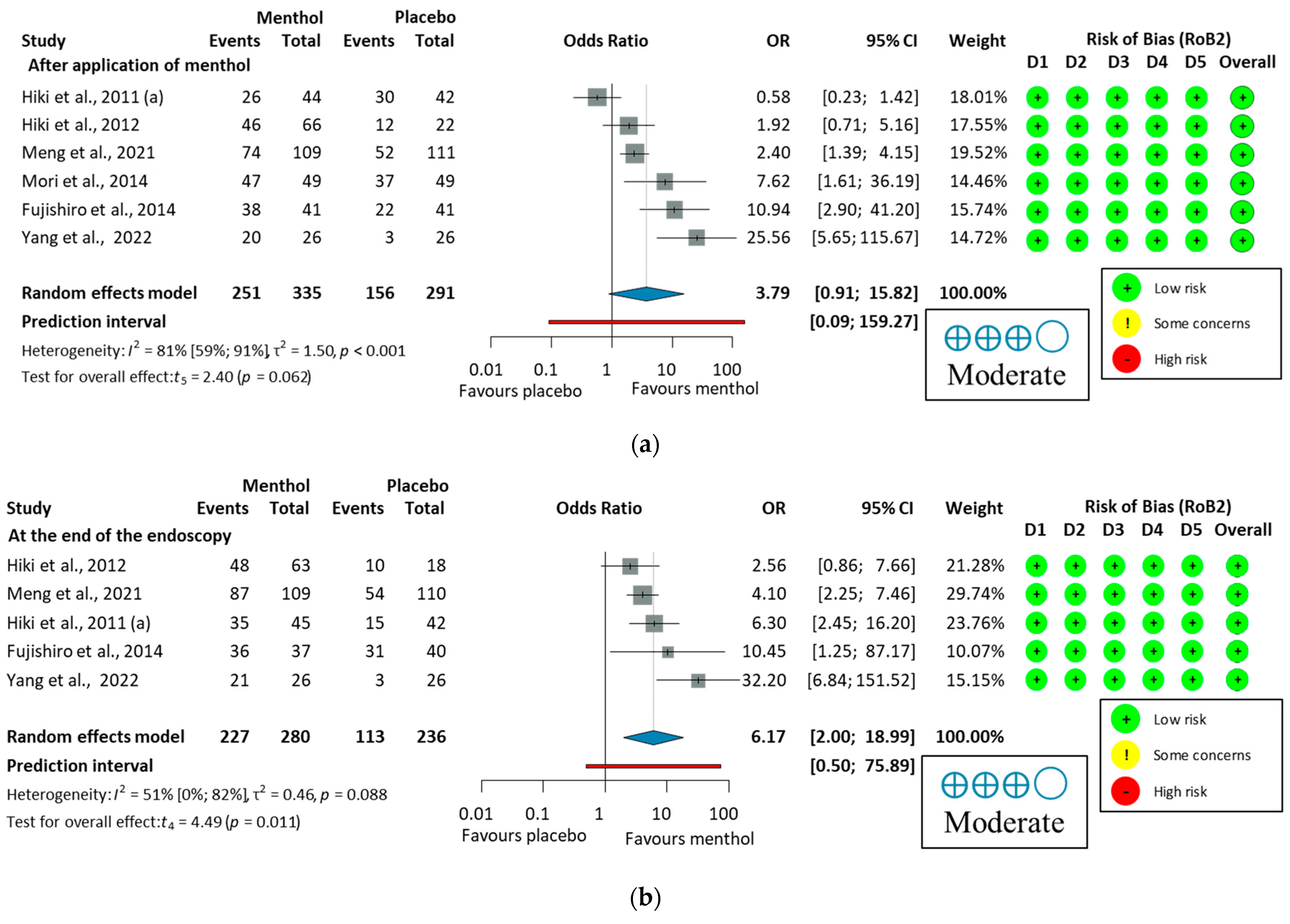
A subgroup analysis was conducted to compare the proportion of peristalsis immediately after applying L-menthol or at the end of the endoscopy. The antiperistaltic effect of L-menthol indicates a trend toward efficacy that did not reach statistical significance immediately after application (p = 0.062), but showed a significant effect at the end of endoscopy (p = 0.011), as shown in Figure 7. These results suggest that L-menthol has a time-dependent antiperistaltic effect, consistent with findings from a pharmacokinetic study [68]. Therefore, presenting combined and subgroup analyses is justified and provides a comprehensive assessment of L-menthol’s antispasmodic efficacy in GI endoscopy. Subgroup analyses also revealed notable differences in peristalsis reduction based on measurement time, with greater antiperistaltic effects observed at the end of the procedure than immediately after application. This supports the efficacy of L-menthol over time. Additionally, Imagawa et al. (2012) [64] reported significantly higher antispasmodic scores in the L-menthol group compared to placebo, with elderly patients (>70 years) benefiting the most.
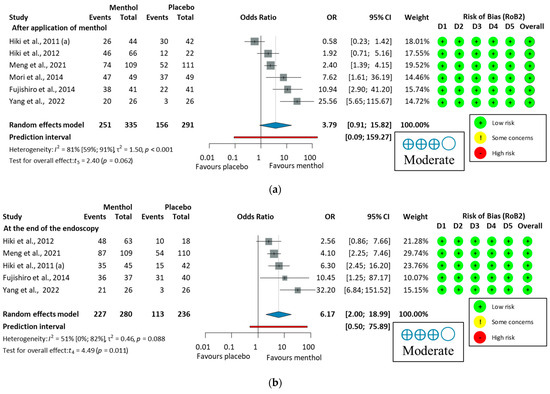
Figure 7.
Forest plot of the proportion of peristalsis at different time points in upper endoscopy, (a), after application of L-menthol and (b), at the end of the endoscopy, showing pooled ORs with 95% CIs for menthol versus placebo. Risk-of-bias assessments are shown as colored circles for each domain and overall. “Moderate” indicates GRADE certainty of evidence. OR = odds ratio, CI = confidence interval, D = domain [57,58,59,60,61,62].
Ease of examination by endoscopists is essential for procedure quality and can reduce operator fatigue [31]. Four upper endoscopy studies (Hiki et al. (2011) [58]; Yang et al. (2022) [62]; Meng et al. (2021) [60]; and Hiki et al. (2012) [59] demonstrated significantly easier examinations with L-menthol use. These findings highlight the role of L-menthol in enhancing procedural efficiency and increasing the number of daily procedures performed.
The reduction in withdrawal time between the L-menthol and the placebo group (MD = 3.24 s) was not significant. This is not concerning, as the U.S. Multi-Society Task Force [83] recommends a withdrawal time of 6–10 min for an effective colonoscopy.
Adverse events were systematically reviewed, with no major, severe, or serious events reported. Commonly reported adverse events included abdominal pain, nausea, diarrhea, and heartburn. Some studies also reported cardiovascular changes, such as electrocardiogram ST-T changes, palpitations, and ventricular premature beats; however, these events were not statistically associated with L-menthol use. The similar proportions of adverse events in the L-menthol and placebo groups in both upper endoscopy and colonoscopy suggest a potentially safe usage profile for L-menthol. Although some studies focused only on serious adverse events, others documented every occurrence. The overall results suggest that L-menthol has a favorable safety profile, as it does not result in more adverse events than placebo. This supports its use as a safe alternative to antispasmodic agents for endoscopic procedures.
Four studies reported adverse drug reactions, with a pooled analysis indicating a significantly lower incidence in the L-menthol group. These findings highlight the equivalent safety profile of L-Menthol compared to placebo.
Previous meta-analyses have examined the antispasmodic effects of L-menthol and peppermint oil during endoscopy. Aziz et al. (2020) [87] focused on colonoscopy and included both oral and topical interventions, while You et al. (2020) [88] evaluated L-menthol for suppressing peristalsis primarily during upper endoscopy, with studies mostly from Japan. In contrast, the present study builds on and extends these works by focusing exclusively on topical application during both colonoscopy and upper endoscopy (EGD), incorporating a larger and more recent evidence base, and providing more detailed subgroup and safety analyses.
Based on current clinical evidence, the topical application (spraying directly onto the mucosa during endoscopic procedures) of L-menthol and peppermint oil seems to be reasonable to achieve an optimal antispasmodic effect. For upper gastrointestinal endoscopy, the established regimen is to spray 20 mL of 0.8% L-menthol solution (160 mg) onto the gastric antrum or body via the endoscope’s working channel before examination. The antiperistaltic effect begins within 30–90 s and is sustained throughout the procedure. Additional doses can be administered if peristalsis recurs during more prolonged examination. For colonoscopy, the regimen is 20 mL of 0.8% L-menthol (160 mg) or 50 mL of peppermint oil solution sprayed or injected onto the colonic mucosa, particularly at the cecum, with additional doses as needed for persistent peristalsis. The antispasmodic effect occurs quickly (within 20–40 s) and lasts at least 15–20 min, covering the withdrawal phase. This approach ensures rapid and sustained suppression of peristalsis, facilitating high-quality mucosal visualization and procedural efficiency, with a favorable safety profile.
4.1. Strengths and Limitations
This study is the first to comprehensively synthesize data on L-menthol’s effects across various endoscopic procedures, covering outcomes such as the ADR, peristalsis suppression, ease of examination, and adverse events. By including studies on both colonoscopy and upper endoscopy, the analysis provides a holistic approach to the utility of L-menthol across various endoscopic procedures. Subgroup analyses and advanced statistical methods strengthen the findings.
Most studies were conducted in Asia, particularly Japan, and some in North America. This geographical concentration may limit the generalizability of our findings to other populations, as differences in genetics, healthcare systems, and endoscopic practice may influence the efficacy and safety of L-menthol. Further studies from diverse geographic regions are needed to confirm these findings and ensure their applicability to broader patient populations.
However, high heterogeneity was observed for both the adenoma detection rate (ADR, I2 = 51%) and proportion of no peristalsis (PNP, I2 = 94%) in colonoscopy, limiting the validity of our results. For the ADR, heterogeneity likely arises from differences in patient age and comorbidity profiles, inconsistent sedation protocols, and variability in endoscopist experience—all known to affect detection rates. For PNP, the much higher heterogeneity reflects broader variations in patient age, sedation practices, and subjective differences in endoscopist assessments of peristalsis (details in Table S4). Approximately one-third of included studies [30,53,54,61,64,65] had inadequate blinding (e.g., single-blind or open-label designs), which may have introduced performance and detection bias, particularly for subjective outcomes such as ease of examination or peristalsis assessment. While some trials [57,63] employed independent committees and reported moderate-to-good inter-observer agreement, most studies did not validate peristalsis grading scales or assess inter-rater reliability. The scale for the ease of examination outcome has not been formally validated, and subjective assessments by unblinded endoscopists may introduce measurement bias. Furthermore, while procedures were performed by experienced endoscopists in many trials, heterogeneity in operator skill and training may have influenced outcomes. Future studies should prioritize standardized training for outcome assessors and report inter-observer agreement metrics to enhance reproducibility. Adverse events were only assessed during or shortly after the procedure; analysis of any delayed complications or mucosal injury was not possible due to a lack of data. The relatively small sample size of some included studies limits the generalizability and robustness of the conclusions. Furthermore, it was not possible to perform subgroup analyses by age or gender, as the included studies did not provide stratified outcome data [89]. Future studies should assess whether L-menthol’s effects differ across demographic groups, such as older adults or between genders, to clarify potential differences in efficacy or safety. Four studies were sponsored by a pharmaceutical company [57,58,59,63], while others were funded by academic or hospital sources [52,53,56,71,73]. Two studies reported no funding [51,55], the remaining studies did not report their funding sources [30,54,60,61,62,64,65,72]. Details are summarized in Table S2. The cost effectiveness of L-menthol use was not studied due to a lack of available data; it should be addressed in future research. It is also important to note that despite our broad inclusion criteria, we could not perform meta-analyses for ERCP and EUS due to a lack of available studies. This limitation highlights the need for further research into L-menthol’s efficacy in these endoscopic procedures.
4.2. Implications for Practice and Research
Translating scientific findings into clinical practice is essential to improve patient care and achieve better healthcare outcomes [90,91]. Using L-menthol in routine clinical practice may enhance examination quality, increase procedural efficiency, and reduce operator fatigue. Developing guidelines for standardizing L-menthol use in endoscopic protocols will be crucial to maximizing its clinical benefits. Future research should focus on standardizing methodologies, including dosing regimens and outcome measurement. Larger, multicenter trials are needed to confirm the efficacy of L-menthol and determine its optimal clinical application. With these considerations, L-menthol may play a pivotal role in improving the quality and efficiency of gastrointestinal endoscopic procedures.
5. Conclusions
L-Menthol may have beneficial effects in gastrointestinal endoscopy, particularly in reducing peristalsis and potentially facilitating easier examinations without an observed increase in adverse events. Although its impact on the ADR remains inconclusive, probably due to variability in study designs and procedural factors, its possible antiperistaltic benefits and improved ease of examination highlight its potential for application in both colonoscopy and upper endoscopy. In addition, L-menthol offers a promising alternative antispasmodic option for patients who cannot receive pharmaceutical agents due to the risks of adverse effects or comorbidities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14124296/s1, Figure S1. Leave-One-Out Sensitivity Analysis on the adenoma detection rate; Figure S2. Forest plot of proportion of no and mild peristalsis (PNMP) in colonoscopy and upper endoscopy; Figure S3. Leave-One-Out Sensitivity Analysis on the proportion of no peristalsis; Figure S4. Leave-One-Out Sensitivity Analysis on the ease of examination for the operator; Figure S5. Forest plot of withdrawal time in colonoscopy; Figure S6. Forest plot of adverse drug reactions; Figure S7. Risk of bias assessment of the analyzed studies (RoB2); Table S1. Versions of the search key in different databases; Table S2. Sponsor/Funding Source of the included studies; Table S3. Summary for quality of evidence, GRADE assessment; Table S4. Additional baseline characteristics of the RCTs with colonoscopy and upper endoscopy patients investigated in the systematic review and meta-analysis; Table S5. PRISMA 2020 Checklist.
Author Contributions
Conceptualization, D.G., A.T.-M., A.S.W., U.N.D.T., P.H., B.E., A.V. and D.C.; Data curation, D.G., A.T.-M. and U.N.D.T.; Formal analysis, P.F.; Methodology, P.F., P.H., B.E., A.V. and D.C.; Project administration, D.G., A.S.W. and D.C.; Supervision, P.H.; Validation, P.F.; Visualization, D.G. and P.F.; Writing—original draft, D.G., B.E., A.V. and D.C.; Writing—review and editing, A.S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research, Development, and Innovation Fund of Hungary (NKFIH) (project code TKP2021-EGA-32), funded through the Ministry of Innovation and Technology as part of the ITM NKFIA funding scheme. The research was also funded by the Semmelweis Fund for Science and Innovation (STIA-OTKA-2022), and NKFIH OTKA (grant no. FK-146487). DG was funded by the Semmelweis 250+ Excellence Fellowship and the Semmelweis Predoctoral Scholarship (EFOP-3.6.3-VEKOP-16-2017-00009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Gantsetseg Garmaa for her insightful comments during the project’s early phase. We sincerely thank Mikolt Bakony for her invaluable statistical support and guidance. We thank the National Research, Development and Innovation Office, Hungary, and the Semmelweis Fund for Science and Innovation for their support. During the preparation of this manuscript/study, the authors used [ChatGPT-3.5] for the purposes of improving text clarity and language editing. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADR | adenoma detection rate |
| APC | adenoma per colonoscopy |
| AS | antispasmodic scores |
| C | control |
| CI | confidence interval |
| CR | contraction ratio |
| CRC | colorectal cancer |
| EGC | early gastric cancer |
| EGD | esophagogastroduodenoscopy |
| EIE | ease of the intragastric examination |
| EMR | endoscopic mucosal resection |
| ESD | endoscopic submucosal dissection |
| GI | gastrointestinal |
| GPPM | gastric peristalsis per minute |
| GRADE | Grading of Recommendations, Assessment, Development and Evaluations |
| I | intervention |
| IBD | inflammatory bowel disease |
| LOO | leave-one-out |
| MD | mean difference |
| OR | odds ratio |
| PNMP | proportion of no or mild peristalsis |
| PNP | proportion of no peristalsis |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| PUD | peptic ulcer disease |
| RCT | randomized controlled trial |
| sec | second |
| TADR | total adverse drug reaction |
| TAE | total adverse event |
| WT | withdrawal time |
References
- Steele, S.R.; Johnson, E.K.; Champagne, B.; Davis, B.; Lee, S.; Rivadeneira, D.; Ross, H.; Hayden, D.A.; Maykel, J.A. Endoscopy and polyps-diagnostic and therapeutic advances in management. World J. Gastroenterol. 2013, 19, 4277–4288. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.C.; Mittal, P.K.; Sullivan, P.S.; Rutherford, R.; Staley, C.A.; Cardona, K.; Hawk, N.N.; Dixon, W.T.; Kitajima, H.D.; Kang, J.; et al. Colorectal Cancer Initial Diagnosis: Screening Colonoscopy, Diagnostic Colonoscopy, or Emergent Surgery, and Tumor Stage and Size at Initial Presentation. Clin. Color. Cancer. 2016, 15, 67–73. [Google Scholar] [CrossRef] [PubMed]
- American Society for Gastrointestinal Endoscopy Standards of Practice Committee; Shergill, A.K.; Lightdale, J.R.; Bruining, D.H.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Evans, J.A.; et al. The role of endoscopy in inflammatory bowel disease. Gastrointest. Endosc. 2015, 81, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- ASGE Standards of Practice Committee; Banerjee, S.; Cash, B.D.; Dominitz, J.A.; Baron, T.H.; Anderson, M.A.; Ben-Menachem, T.; Fisher, L.; Fukami, N.; Harrison, M.E.; et al. The role of endoscopy in the management of patients with peptic ulcer disease. Gastrointest. Endosc. 2010, 71, 663–668. [Google Scholar]
- ASGE Standards of Practice Committee; Coelho-Prabhu, N.; Forbes, N.; Thosani, N.C.; Storm, A.C.; Pawa, S.; Kohli, D.R.; Fujii-Lau, L.L.; Elhanafi, S.; Calderwood, A.H.; et al. Adverse events associated with EGD and EGD-related techniques. Gastrointest. Endosc. 2022, 96, 389–401.e1. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Jensen, E.T.; Kim, H.P.; Egberg, M.D.; Lund, J.L.; Moon, A.M.; Pate, V.; Barnes, E.L.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022, 162, 621–644. [Google Scholar] [CrossRef]
- Park, W.G.; Shaheen, N.J.; Cohen, J.; Pike, I.M.; Adler, D.G.; Inadomi, J.M.; Laine, L.A.; Lieb, J.G., 2nd; Rizk, M.K.; Sawhney, M.S.; et al. Quality indicators for EGD. Gastrointest. Endosc. 2015, 81, 17–30. [Google Scholar] [CrossRef]
- Rex, D.K.; Schoenfeld, P.S.; Cohen, J.; Pike, I.M.; Adler, D.G.; Fennerty, M.B.; Lieb, J.G., 2nd; Park, W.G.; Rizk, M.K.; Sawhney, M.S.; et al. Quality indicators for colonoscopy. Gastrointest. Endosc. 2015, 81, 31–53. [Google Scholar] [CrossRef]
- Corte, C.J.; Leong, R.W. Improving the utility of colonoscopy: Recent advances in practice. J. Gastroenterol. Hepatol. 2016, 31, 32–44. [Google Scholar] [CrossRef]
- Bazerbachi, F.; Panganamamula, K.; Nieto, J.M.; Murad, M.H.; Keswani, R.N.; Shaukat, A.; Day, L.W. Interventions to improve the performance of upper GI endoscopy quality indicators. Gastrointest. Endosc. 2022, 96, 184–188.e4. [Google Scholar] [CrossRef]
- Gutzeit, A.; Binkert, C.A.; Koh, D.M.; Hergan, K.; von Weymarn, C.; Graf, N.; Patak, M.A.; Roos, J.E.; Horstmann, M.; Kos, S.; et al. Evaluation of the anti-peristaltic effect of glucagon and hyoscine on the small bowel: Comparison of intravenous and intramuscular drug administration. Eur. Radiol. 2012, 22, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Sanagapalli, S.; Agnihotri, K.; Leong, R.; Corte, C.J. Antispasmodic drugs in colonoscopy: A review of their pharmacology, safety and efficacy in improving polyp detection and related outcomes. Ther. Adv. Gastroenterol. 2017, 10, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Dyde, R.; Chapman, A.H.; Gale, R.; Mackintosh, A.; Tolan, D.J. Precautions to be taken by radiologists and radiographers when prescribing hyoscine-N-butylbromide. Clin. Radiol. 2008, 63, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R. Routine use of hyoscine N butylbromide (Buscopan) in double contrast barium enema examinations. Clin. Radiol. 1982, 33, 273–276. [Google Scholar] [CrossRef]
- Chakraborty, K.; Chakravarti, A.R.; Bhattacharjee, S. Bioactive components of peppermint (Mentha piperita L.), their pharmacological and ameliorative potential and ethnomedicinal benefits: A review. J. Pharmacogn. Phytochem. 2022, 11, 109–114. [Google Scholar] [CrossRef]
- Scarpellini, E.; Broeders, B.; Schol, J.; Santori, P.; Addarii, M.; Boccuto, L.; Carbone, F.; Abenavoli, L.; Tack, J. The Use of Peppermint Oil in Gastroenterology. Curr. Pharm. Des. 2023, 29, 576–583. [Google Scholar] [CrossRef]
- Ingrosso, M.R.; Ianiro, G.; Nee, J.; Lembo, A.J.; Moayyedi, P.; Black, C.J.; Ford, A.C. Systematic review and meta-analysis: Efficacy of peppermint oil in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2022, 56, 932–941. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef]
- Grigoleit, H.G.; Grigoleit, P. Pharmacology and preclinical pharmacokinetics of peppermint oil. Phytomedicine 2005, 12, 612–616. [Google Scholar] [CrossRef]
- Amato, A.; Liotta, R.; Mulè, F. Effects of menthol on circular smooth muscle of human colon: Analysis of the mechanism of action. Eur. J. Pharmacol. 2014, 740, 295–301. [Google Scholar] [CrossRef]
- Hawthorn, M.; Ferrante, J.; Luchowski, E.; Rutledge, A.; Wei, X.Y.; Triggle, D.J. The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Aliment. Pharmacol. Ther. 1988, 2, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Hills, J.M.; Aaronson, P.I. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology 1991, 101, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Mahawongkajit, P.; Kanlerd, A. A prospective randomized controlled trial comparing simethicone, N-acetylcysteine, sodium bicarbonate and peppermint for visualization in upper gastrointestinal endoscopy. Surg. Endosc. 2021, 35, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, A.; Namikawa, K.; Tokai, Y.; Yoshimizu, S.; Horiuchi, Y.; Yoshio, T.; Hirasawa, T.; Tsuchida, T.; Itoh, F.; Fujisaki, J. Effect of spraying l-menthol on peristalsis resumption during endoscopic submucosal dissection of gastric tumors. JGH Open 2021, 5, 653–657. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024). Cochrane, 2024. Available online: www.training.cochrane.org/handbook (accessed on 24 October 2024).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Grainger, M.J.; Gray, C.T. Citationchaser: A tool for transparent and efficient forward and backward citation chasing in systematic searching. Res. Synth. Methods 2022, 13, 533–545. [Google Scholar] [CrossRef]
- Rex, D.K.; Petrini, J.L.; Baron, T.H.; Chak, A.; Cohen, J.; Deal, S.E.; Hoffman, B.; Jacobson, B.C.; Mergener, K.; Petersen, B.T.; et al. Quality indicators for colonoscopy. Am. J. Gastroenterol. 2006, 101, 873–885. [Google Scholar] [CrossRef]
- Asao, T.; Mochiki, E.; Suzuki, H.; Nakamura, J.; Hirayama, I.; Morinaga, N.; Shoji, H.; Shitara, Y.; Kuwano, H. An easy method for the intraluminal administration of peppermint oil before colonoscopy and its effectiveness in reducing colonic spasm. Gastrointest. Endosc. 2001, 53, 172–177. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, J.; Chen, Y.; Sun, H.; Li, B.; Zhang, Q.; Sun, K.; Wang, Z.; Qian, X.; Zhan, T.; et al. Negative Effects of Endoscopists' Fatigue on Colonoscopy Quality on 34,022 Screening Colonoscopies. J. Gastrointestin Liver Dis. 2021, 30, 358–365. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.B.J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 16 January 2025).
- GRADEpro GDT. GRADEpro Guideline Development Tool. McMaster University and Evidence Prime, 2023. Available online: https://www.gradepro.org/ (accessed on 18 January 2025).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D.D. 2019 Dmetar: Companion R Package For The Guide ‘Doing Meta-Analysis in R’. R Package Version 0.1.0, 2019. Available online: http://dmetar.protectlab.org/ (accessed on 9 December 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 12 November 2024).
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Thompson, S.G.; Turner, R.M.; Warn, D.E. Multilevel models for meta-analysis, and their application to absolute risk differences. Stat. Methods Med. Res. 2001, 10, 375–392. [Google Scholar] [CrossRef]
- Robins, J.; Greenland, S.; Breslow, N.E. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am. J. Epidemiol. 1986, 124, 719–723. [Google Scholar] [CrossRef]
- Knapp, G.; Hartung, J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003, 22, 2693–2710. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef]
- Cooper, H.; Hedges, L.V.; Valentine, J.C. The Handbook of Research Synthesis and Meta-Analysis, 2nd ed.; Russell Sage Foundation: New York, NY, USA, 2009. [Google Scholar]
- Sweeting, M.J.; Sutton, A.J.; Lambert, P.C. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat. Med. 2004, 23, 1351–1375. [Google Scholar] [CrossRef]
- Paule, R.C.; Mandel, J. Consensus Values and Weighting Factors. J. Res. Natl. Bur. Stand. 1982, 87, 377–385. [Google Scholar] [CrossRef]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Meta-Analysis with R; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W.; Cheung, M.W. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Nakamura, T.; Fujino, M. Endoscopic Observation on Gastric Peristalsis and Pyloric Movement. Gastrointest. Endosc. 1975, 17, 236–242. [Google Scholar]
- Dhillon, A.S.; Alshankiti, S.; Khorasani-zadeh, A.; Sultanian, R.; Sandha, G.S.; Kohansal, A.R.; Montano-Loza, A.J.; Zepeda-Gomez, S. A247 L-menthol during colonoscopy for adenoma detection in an intermediate risk patient population: A double-blind, randomized controlled trial. J. Can. Assoc. Gastroenterol. 2018, 1, 360–361. [Google Scholar] [CrossRef]
- University Hospitals Cleveland Medical Center L-Menthol Injection as a Novel Technique During Colonoscopy (MINT-C) NCT02588248, 2015. Available online: https://clinicaltrials.gov/study/NCT02588248 (accessed on 18 June 2023).
- Inoue, K.; Dohi, O.; Gen, Y.; Jo, M.; Mazaki, T.; Tokita, K.; Yoshida, N.; Okayama, T.; Kamada, K.; Katada, K.; et al. L-menthol improves adenoma detection rate during colonoscopy: A randomized trial. Endoscopy 2014, 46, 196–202. [Google Scholar] [CrossRef]
- Inoue, K.; Okuda, T.; Oka, K.; Sugino, S.; Endo, Y.; Ota, T.; Minagawa, Y.; Yasue, C.; Tsuji, T.; Katayama, T.; et al. Effects of L-Menthol and Carbon Dioxide on the Adenoma Detection Rate during Colonoscopy: L-Menthol and Carbon Dioxide on Colonoscopy. Digestion 2020, 101, 323–331. [Google Scholar] [CrossRef]
- Sattayalertyanyong, O.; Sathirawich, P.; Maipang, K.; Chukaewrungroj, P.; Limsrivilai, J.; Kaosombatwattana, U. Efficacy and safety of intraluminal peppermint oil during colonoscopy on colonic peristalsis and adenoma detection rate: A randomized, double-blinded, placebo-controlled trial. Gastrointest. Endosc. 2023, 97, AB696–AB697. [Google Scholar] [CrossRef]
- Shah, I.; Baffy, N.J.; Horsley-Silva, J.L.; Langlais, B.T.; Ruff, K.C. Peppermint Oil to Improve Visualization in Screening Colonoscopy: A Randomized Controlled Clinical Trial. Gastroenterol. Res. 2019, 12, 141–147. [Google Scholar] [CrossRef]
- Fujishiro, M.; Kaminishi, M.; Hiki, N.; Oda, I.; Fujisaki, J.; Uedo, N.; Kaise, M.; Tanabe, S.; Iguchi, M.; Matsuhashi, N.; et al. Efficacy of spraying l-menthol solution during endoscopic treatment of early gastric cancer: A phase III, multicenter, randomized, double-blind, placebo-controlled study. J. Gastroenterol. 2014, 49, 446–454. [Google Scholar] [CrossRef]
- Hiki, N.; Kaminishi, M.; Yasuda, K.; Uedo, N.; Honjo, H.; Matsuhashi, N.; Hiratsuka, T.; Sekine, C.; Nomura, S.; Yahagi, N.; et al. Antiperistaltic effect and safety of L-menthol sprayed on the gastric mucosa for upper GI endoscopy: A phase III, multicenter, randomized, double-blind, placebo-controlled study. Gastrointest. Endosc. 2011, 73, 932–941. [Google Scholar] [CrossRef]
- Hiki, N.; Kaminishi, M.; Yasuda, K.; Uedo, N.; Kobari, M.; Sakai, T.; Hiratsuka, T.; Ohno, K.; Honjo, H.; Nomura, S.; et al. Multicenter phase II randomized study evaluating dose-response of antiperistaltic effect of L-menthol sprayed onto the gastric mucosa for upper gastrointestinal endoscopy. Dig. Endosc. 2012, 24, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Li, W.; Zhi, F.; Li, Z.; Xue, Z.; He, S.; Chen, W.; Chen, Y.; Xing, X.; Yao, C.; et al. Antiperistaltic effect and safety of l-menthol oral solution on gastric mucosa for upper gastrointestinal endoscopy in Chinese patients: Phase III, multicenter, randomized, double-blind, placebo-controlled study. Dig. Endosc. 2021, 33, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Hachiya, H.; Yumura, T.; Ito, S.; Hayashi, S.; Nozaki, M.; Yoshida, A.; Ohashi, N. l-Menthol sprayed on gastric mucosa causes edematous change. Endosc. Int. Open. 2014, 2, E51–E57. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Chen, P.H.; Hou, M.C.; Peng, L.N.; Lin, M.H.; Chen, L.K.; Huang, Y.H. Antiperistaltic effect and safety of L-menthol for esophagogastroduodenoscopy in the elderly with contraindication to hyoscine-N-butylbromide. Sci. Rep. 2022, 12, 10418. [Google Scholar] [CrossRef]
- Hiki, N.; Kaminishi, M.; Hasunuma, T.; Nakamura, M.; Nomura, S.; Yahagi, N.; Tajiri, H.; Suzuki, H. A phase I study evaluating tolerability, pharmacokinetics, and preliminary efficacy of L-menthol in upper gastrointestinal endoscopy. Clin. Pharmacol. Ther. 2011, 90, 221–228. [Google Scholar] [CrossRef]
- Imagawa, A.; Hata, H.; Nakatsu, M.; Yoshida, Y.; Takeuchi, K.; Inokuchi, T.; Imada, T.; Kohno, Y.; Takahara, M.; Matsumoto, K.; et al. Peppermint oil solution is useful as an antispasmodic drug for esophagogastroduodenoscopy, especially for elderly patients. Dig. Dis. Sci. 2012, 57, 2379–2384. [Google Scholar] [CrossRef]
- Yoshida, N.; Naito, Y.; Hirose, R.; Ogiso, K.; Inada, Y.; Fernandopulle, N.; Kamada, K.; Katada, K.; Uchiyama, K.; Handa, O.; et al. Prevention of colonic spasm using L-menthol in colonoscopic examination. Int. J. Color. Dis. 2014, 29, 579–583. [Google Scholar] [CrossRef]
- Anishchenko, M.; Schapovalyanz, S.; Nazmeev, M.; Budzinskiy, S.; Rogov, A.Z.; Ruslan, Z.; Fedorov, E. Antiperistalsis effect of l-menthol sprayed into duodenum during ERCP: Initial results of prospective two-center randomized placebo-controlled study. Gastrointest. Endosc. 2023, 97, AB611. [Google Scholar] [CrossRef]
- Park, S.; Chun, H.J.; Kim, E.S.; Park, S.C.; Jung, E.S.; Lee, S.D.; Jang, J.S.; Kwon, Y.D.; Keum, B.; Seo, Y.S.; et al. Peppermint Oil Solution Is An Effective Antispasmodics On Esophagogastroduodenoscopy. Gastrointest. Endosc. 2009, 69, AB209. [Google Scholar] [CrossRef]
- Mabuchi, M.; Adachi, T.; Kajiyama, H.; Kuniyoshi, N.; Onda, T.; Matsumoto, K.; Tsunashima, H.; Sekine, K.; Tsujikawa, T.; Kajiyama, Y.; et al. Antispasmodic effect of l-menthol during endoscopic retrograde cholangiopancreatography. J. Gastroenterol. Hepatol. 2017, 32, 272. [Google Scholar]
- Yamamoto, N.; Nakai, Y.; Sasahira, N.; Hirano, K.; Tsujino, T.; Isayama, H.; Komatsu, Y.; Tada, M.; Yoshida, H.; Kawabe, T.; et al. Efficacy of peppermint oil as an antispasmodic during endoscopic retrograde cholangiopancreatography. J. Gastroenterol. Hepatol. 2006, 21, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Liu, H.; Li, W.; Shen, J. Spray of peppermint oil on papilla shortens the cannulation time of endoscopic retrograde cholangiopancreatography (ERCP): A randomized study. Int. J. Clin. Exp. Med. 2019, 12, 2813–2818. [Google Scholar]
- Shavakhi, A.; Ardestani, S.K.; Taki, M.; Goli, M.; Keshteli, A.H. Premedication with peppermint oil capsules in colonoscopy: A double blind placebo-controlled randomized trial study. Acta Gastroenterol. Belg. 2012, 75, 349–353. [Google Scholar] [PubMed]
- Al Moussawi, H.; Al Khatib, M.; El Ahmar, M.; Al Masri, H.; Leddy, A.; Akel, T.; Khalil, A. The effect of premedication with peppermint oil capsules (Colpermin) prior to colonoscopy: A double blind randomized placebo-controlled trial. Arab. J. Gastroenterol. 2017, 18, 220–223. [Google Scholar] [CrossRef]
- Han, J.Y.; Moosvi, Z.; Duh, E.; Park, S.; Albers, G.C.; Samarasena, J.B.; Karnes, W. Oral IBGard™ Before Colonoscopy: A Single-Center Double-Blinded, Randomized, Placebo-Controlled Trial. Dig. Dis. Sci. 2021, 66, 1611–1619. [Google Scholar] [CrossRef]
- IBgard®. Available online: https://www.nestlemedicalhub.com/products/ibgard (accessed on 28 November 2024).
- Colpermin™. Available online: https://www.tillotts.com/products/colpermin/ (accessed on 28 November 2024).
- Merat, S.; Khalili, S.; Mostajabi, P.; Ghorbani, A.; Ansari, R.; Malekzadeh, R. The effect of enteric-coated, delayed-release peppermint oil on irritable bowel syndrome. Dig. Dis. Sci. 2010, 55, 1385–1390. [Google Scholar] [CrossRef]
- Lucak, S.; Chang, L.; Halpert, A.; Harris, L.A. Current and emergent pharmacologic treatments for irritable bowel syndrome with diarrhea: Evidence-based treatment in practice. Ther. Adv. Gastroenterol. 2017, 10, 253–275. [Google Scholar] [CrossRef]
- Sugino, S.; Inoue, K.; Kobayashi, R.; Hirose, R.; Doi, T.; Harusato, A.; Dohi, O.; Yoshida, N.; Uchiyama, K.; Ishikawa, T.; et al. Association Between the Cool Temperature-dependent Suppression of Colonic Peristalsis and Transient Receptor Potential Melastatin 8 Activation in Both a Randomized Clinical Trial and an Animal Model. J. Neurogastroenterol. Motil. 2022, 28, 693–705. [Google Scholar] [CrossRef]
- Nemoto, D.; Suzuki, S.; Mori, H.; Katsuki, S.; Iwaki, T.; Aizawa, M.; Takeuchi, Y.; Uraoka, T.; Matsuda, T.; Fujita, T.; et al. Inhibitory effect of lidocaine on colonic spasm during colonoscopy: A multicenter double-blind, randomized controlled trial. Dig. Endosc. 2018, 31, 173–179. [Google Scholar] [CrossRef]
- Nemoto, D.; Utano, K.; Isohata, N.; Endo, S.; Kumamoto, K.; Koshimizu, T.A.; Lefor, A.; Togashi, K. Topical lidocaine inhibits spasm during colonoscopy: A double-blind, randomized controlled trial (with video). Endosc. Int. Open. 2017, 5, E402–E407. [Google Scholar] [CrossRef]
- Gubbiotti, A.; Spadaccini, M.; Badalamenti, M.; Hassan, C.; Repici, A. Key factors for improving adenoma detection rate. Expert. Rev. Gastroenterol. Hepatol. 2022, 16, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Rondonotti, E.; Andrealli, A.; Amato, A.; Paggi, S.; Conti, C.B.; Spinzi, G.; Radaelli, F. Technical interventions to increase adenoma detection rate in colonoscopy. Expert. Rev. Gastroenterol. Hepatol. 2016, 10, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Bond, J.H.; Winawer, S.; Levin, T.R.; Burt, R.W.; Johnson, D.A.; Kirk, L.M.; Litlin, S.; Lieberman, D.A.; Waye, J.D.; et al. Quality in the technical performance of colonoscopy the continuous quality improvement process for colonoscopy: Recommendations of the, U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2002, 97, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, R.; Zulfiqar, L.; Gangwani, M.K.; Aziz, M. Adenoma detection rate vs. adenoma per colonoscopy as quality indicators for colon cancer screening. Transl. Gastroenterol. Hepatol. 2023, 8, 24. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.J.; Hyun, J.H.; Han, K.S.; Kim, B.C.; Hong, C.W.; Lee, S.J.; Sohn, D.K. Correlation Between Bowel Preparation and the Adenoma Detection Rate in Screening Colonoscopy. Ann. Coloproctol. 2017, 33, 93–98. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, S.; Huang, W.; Zhang, Y.; Liu, Y.; Yu, X.; Shen, L. The Role and Function of TRPM8 in the Digestive System. Biomolecules 2024, 14, 877. [Google Scholar] [CrossRef]
- Aziz, M.; Sharma, S.; Ghazaleh, S.; Fatima, R.; Acharya, A.; Ghanim, M.; Sheikh, T.; Lee-Smith, W.; Hamdani, S.U.; Nawras, A. The anti-spasmodic effect of peppermint oil during colonoscopy: A systematic review and meta-analysis. Minerva Gastroenterol. Dietol. 2020, 66, 164–171. [Google Scholar] [CrossRef]
- You, Q.; Li, L.; Chen, H.; Lin, C.; Chen, X.; Liu, Y. L-Menthol for Gastrointestinal Endoscopy: A Systematic Review and Meta-Analysis. Clin. Transl. Gastroenterol. 2020, 11, e00252. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; Wiley: Chichester, UK, 2009; Chapter 20. [Google Scholar]
- Hegyi, P.; Petersen, O.H.; Holgate, S.; Erőss, B.; Garami, A.; Szakács, Z.; Dobszai, D.; Balaskó, M.; Kemény, L.; Peng, S.; et al. Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits. J. Clin. Med. 2020, 9, e1532. [Google Scholar] [CrossRef]
- Hegyi, P.; Erőss, B.; Izbéki, F.; Párniczky, A.; Szentesi, A. Accelerating the translational medicine cycle: The Academia Europaea pilot. Nat. Med. 2021, 27, 1317–1319. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).