Surgical Strategies and Challenges in Scheuermann’s Kyphosis: A Comprehensive Review
Abstract
1. Introduction
2. Materials and Methods
3. Epidemiology and Definition
3.1. Prevalence
3.2. Diagnostic Criteria
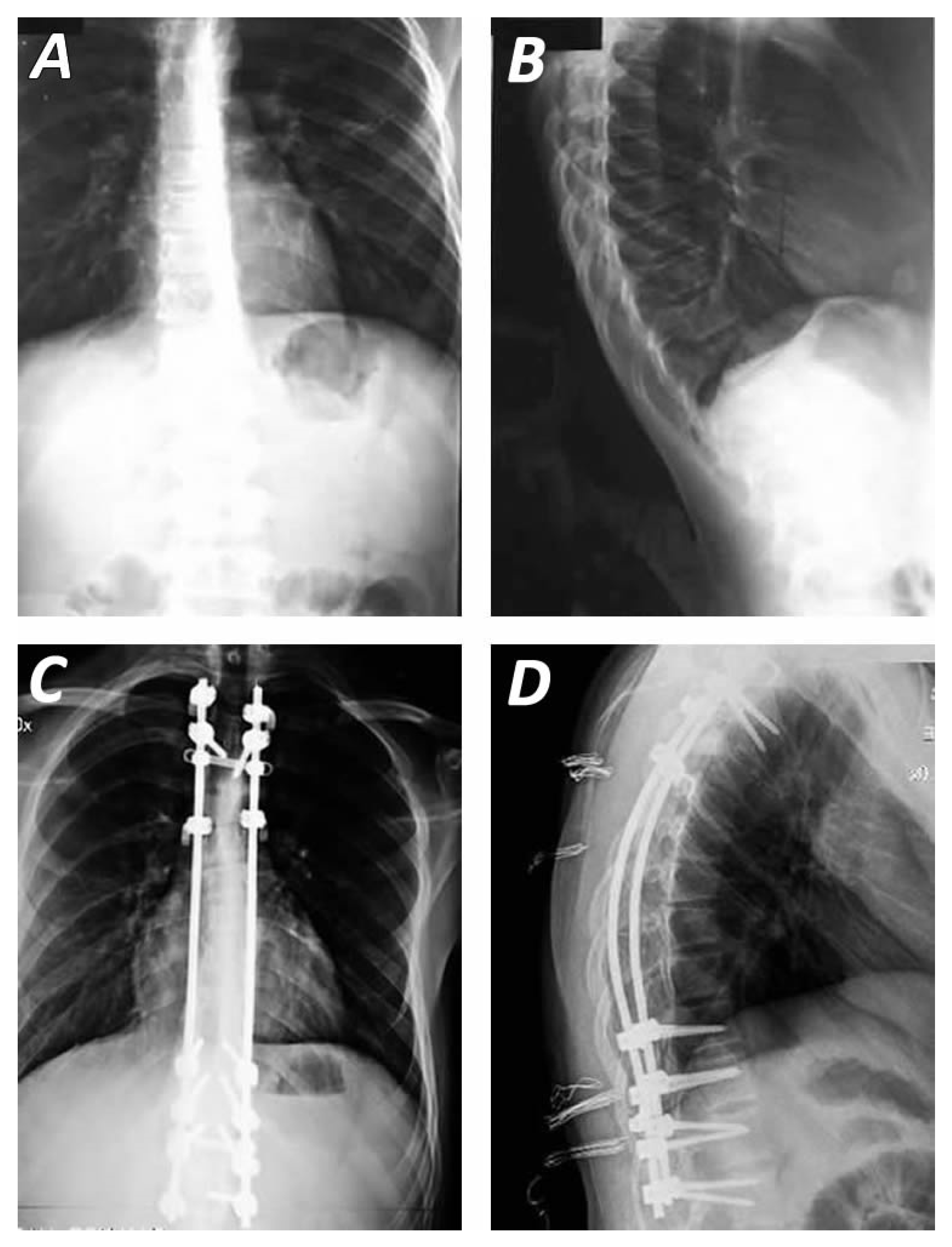
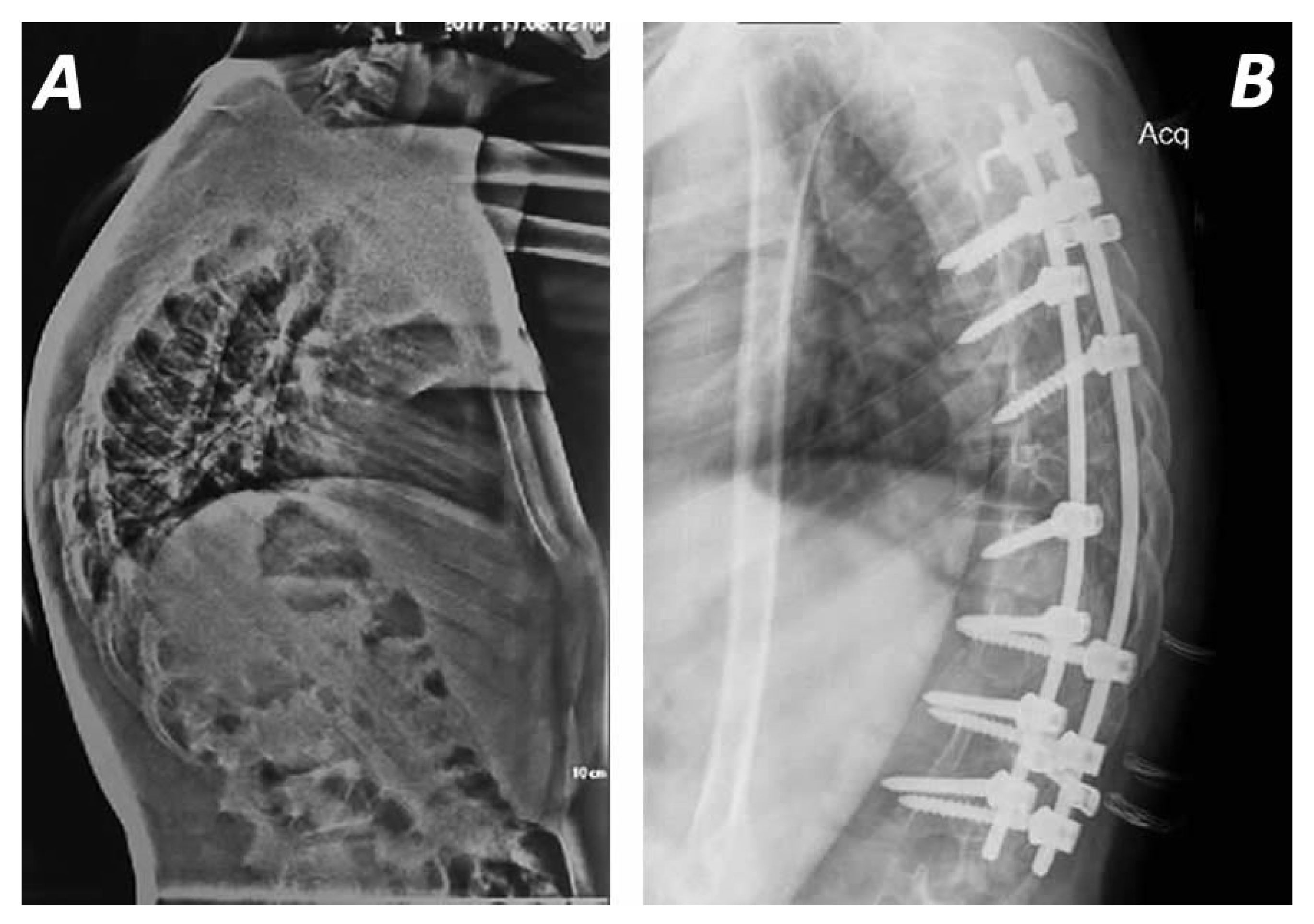
4. Operative Management
4.1. Surgical Approaches
4.2. Surgical Techniques and Instrumentation Methods
5. Outcomes
| Study/Country | Number of Patients (N) | Age Mean and Range (in Years) | Surgical Technique | Mean Preoperative Kyphosis (in Degrees) | Mean Postoperative Kyphosis (in Degrees) | Follow up Mean and Range | Loss of Correction (in Degrees) | Complications and Outcomes (N) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Jensch et al., 2025, Germany [23] | 73 | 27.9 | Posterior-only with pedicle screw-dual rod system | 75.11° | 48.54° | 3 years (2–6) | 0.84° ± 4.99° | Increased SRS-22 functional scores Proximal Junctional Kyphosis (8)—2 revision surgeries Neurological complications (2) Wound healing disorders (2) |
| 2 | Lopez et al., 2024, Spain [24] | 18 | 15.8 | Hybrib bipolar posterior instrumentation with transerse hooks and polyaxial screws Schwab osteotomies (Type I and II) | 73.6° (57–91°) | 57.8° (18–75°) | 3.6 years | N/A | Increased SRS-22 functional scores Increased VAS scores Reduced operation time (269 min), bleeding, instrumantation protrusion (0) and rate of spinal cord injury (0),infections (0) and Proximal or Distal junctional Kyphosis (3) |
| 3 | Baymurat et al., 2024, Turkey [25] | 37 | Posterior approach with pedicle screws 26.58 ± 7.55 Posterior approach with hooks fixation 25.46 ± 9.04 | Posterior approach with pedicle screws (22) Posterior approach with hooks fixation (15) | Posterior approach with pedicle screws 76.55 ± 6.42° Posterior approach with hooks fixation 74.87 ± 7.82° | Posterior approach with pedicle screws 47.59 ± 6.42° Posterior approach with hooks fixation 48.26 ± 5.68° | Posterior approach with pedicle screws 94.73 ± 53.15 months Posterior approach with hooks fixation 103.07 ± 64.48 months | N/A | Increased angles of Proximal and Distal Junctional Kyphosis in Posterior approach with pedicle screws but without statistical diffrences with hooks fixation group SVA and SRS scores and spinopelvic parameters similar between the groups |
| 4 | Aydogan et al., 2024, Turkey [26] | 10 | 13.1 (11–15) | Posterior approach withpedicle screws and tethering cords | 73.6° (72–83°) | 43.8° (41–47°) | 47.6 (months) (36–60) | Further correction to 34.7° (30–35°) | Tether breakage (1) No blood transfusion Increased SRS-22 functional scores Gradual correction of the thoracic kyphosis, reverting vertebral wedging and maintaining the tethered segments‘ mobility |
| 5 | Debnath U et al., 2022, UK [27] | 51 | Posterior-only 18.5 ± 2.2, Anterior–Posterior 21.9 ± 4.8 | 19 Posterior-only, 32 Anterior–posterior | Posterior-only 81.4 ± 3.8°, Anterior–posterior 86.1 ± 6.0° | Posterior-only 45.1 ± 2.6°, Anterior–posterior 47.3 ± 4.8° | 14 years (10–16) | N/A | Proximal Junctional Kyphosis (7) Distal Junctional Kyphosis (5) |
| 6 | Suominen et al., 2022, Finland [28] | 22 | 16.7 (13–19) | Cotrel–Dubousset, Posterior-only | 79° ( 75–90°) | 55° (45–75°) | 24 months | N/A | Deep infection (1), Proximal Junctional Kyphosis (4), |
| 7 | Vital et al., 2021, Portugal [29] | 19 | 18.4 | Cotrel–Dubousset, Posterior-only | 83° | 57° | 72 months (2–12) | N/A | Proximal Junctional Kyphosis (2), Distal Junctional Kyphosis (1), Non-union (1), Infection (1) |
| 8 | Tsirikos A et al., 2021, UK [30] | 88 | 15.9 (12–24.7) | 86 Posterior-only Hybrid, 2 Anterior–posterior Hybrid closing wedge osteotomies (both) | 94.5° | 47.5° | 8.5 years (2–14.9) | N/A | N/A |
| 9 | Riouallon G et al., 2018, France [16] | 131 | Anterior–posterior 23 Posterior-only 10 | Cotrel–Dubousset, 123 Anterior–posterior, 8 Posterior-only | Anterior–posterior 78 ± 13°, Posterior-only 76 ± 23° | Anterior–posterior 59 ± 13°, Posterior-only 53 ± 19° | N/A | N/A | Peptic ulcer (1), Mesenteric artery collapse (2), Infections (7), Acute respiratory distress (2), Pneumonia (2), Pseudarthrosis (4), Proximal Junctional Kyphosis (5), Implant loosening (7) |
| 10 | Etemadifar M et al., 2015, Iran [31] | 30 | Anterior–posterior 20.9 ± 5.3, Posterior-only 19.3 ± 2.7 | Cotrel–Dubousset, 16 Anterior–posterior, 14 Posterior-only | Anterior–posterior 83.7 ± 8.1°, Posterior-only 81.9 ± 9.4 | Anterior–posterior 41.4 ± 7.7°, Posterior-only 40.1 ± 9.9° | Anterior–posterior 69.6 months (38–95), Posterior-only 45.6 (26–74) | N/A | Proximal Junctional Kyphosis (1) Distal Junctional Kyphosis (1), Pulmonary problems (2), Infection (1), Hook pull out (1) |
| 11 | Heiko Koller E et al., 2014, Germany [22] | 166 | Anterior–posterior 23.6 ± 11.4, Posterior-only 20.7 ± 10.4 | Cotrel–Dubousset, 90 Anterior–posterior, 76 Posterior-only | Anterior–posterior 75.9°± 9.6°, Posterior-only 78.7 ± 10.1° | Anterior–posterior 43.4 ± 12.3°, Posterior-only 47.1 ± 11.7° | N/A | N/A | N/A |
| 12 | Behrbalk E et al., 2014, UK [32] | 21 | 21 ± 7 | Pedicle screws- hd vs. ld, Posterior-only | 75° (61–96°) | Hd 42 ± 7°, Ld 44 ± 9° | 29 months | N/A | Screw penetration into T9 disc space (1), Rod breakage and loss of correction (1), Screw loosening (2)—1 revision, Proximal Junctional Kyphosis (1)—revision, Deep infection (1)— Hardware removal-revision (1) |
| 13 | Temponi EF et al., 2011, Brazil [33] | 28 | Anterior–Posterior 19 (13–35), Posterior-only 27.3 | 19 Anterior–posterior thoracotomy-fusion-pedicles screws, 9 Posterior-only Smith Petersen-pedicle screws construct | Anterior–posterior 77.6°, Posterior-only 72.9° | Anterior–posterior 35.8°, Posterior-only 44.3° | Anterior–posterior 37.5 months (12.6–61.7), Posterior-only 22.8 months (31–31) | N/A | AP superficial infection (1) Screws breakage (1), Deep infection-hardware removal (1), Implant loosening—revision (1), Residual pain (4) PO seroma (1), Implant discomfort-removal (1) |
| 14 | Tsutsui, Shunji et al., 2011, USA [34] | 22 | 15.1 (13–17) | Cotrel–Dubousset, 11 Anterior–posterior, 11 Posterior-only | Anterior–posterior 84.9 ± 10.2°, Posterior-only 82.7 ± 6.4° | Anterior–posterior 48.6 ± 5.7°, Posterior-only 47.9 ± 5.4° | N/A | N/A | N/A |
| 15 | Cho KJ et al., 2009, Korea [35] | 31 | 18.0 ± 5.0 | Cotrel–Dubousset facet osteotomy, 29 anterior–posterior, 2 Posterior-only | 86.6 ± 8.5° | 53.0 ± 10.4° | 44 months ± 1.7 | 2.9° ± 6.5° | Distal Junctional Kyphosis (7), Proximal Junctional Kyphosis (3), Pseudarthrosis (2), Paresis (1), Infections (4) |
| 16 | Koptan WM et al., 2009, Egypt [36] | 33 | Posterior-only 15.9 (13.7–19), Anterior–posterior 16.8 (14.9–21.2) | Cotrel–Dubousset with sublaminar wires, 11 Posterior-only, 19 Anterior–posterior | Posterior-only 85.5° (69–102°), Anterior–posterior 79.8° (65–98°) | Posterior-only 45.1° (40–49°), Anterior–posterior 38.8° (37–45°) | 53 months (24–88) | N/A | Right thigh pain (1), Infections (3) Fracture (1) |
| 17 | Lonner BS et al., 2007, USA [18] | 78 | 16.7 years (9–27) | 42 Anterior–posterior, 36 Posterior-only | 82.6° | 74.4° | 33 months (24–72 years) | N/A | Pain (1), Neurogenic bladder (1), Renal failure (1), Pleural effusion (2), Pneumonic embolism (1), Proximal Junctional Kyphosis (2), Distal Junctional Kyphosis (2), Pseudarthrosis (1), Infections (2) |
| 18 | Geck et al., 2007, USA [37] | 17 | 16.4 (14 to 25) | Cotrel–Dubousset, Posterior-only | 75° (57° to 96°) | 38° (12–50) | 24 months | N/A | Infection (1), Proximal Junctional Kyphosis (1), Distal Junctional Kyphosis (1), |
| 19 | Lee SS et al., 2006, USA [21] | 39 | N/A | hook or hook/screw hybrid construct 18 Posterior-only, 21 Anterior–posterior | Posterior-only 84.4° (70–115°) Anterior–posterior 89.1° (70–104°) | Posterior-only 40.4° (30–57°), Anterior–posterior 58.0° (40–81°) | Posterior-only 31.7 months (range 24–63), Anterior–posterior 67.5 (36–146) | Posterior-only 2.0°, Anterior–posterior 2.7° | Proximal Junctional Kyphosis (2) Hook pullout (1), Distal Junctional Kyphosis (1), Paraplegia (1), Superficial infections (3) |
| 20 | Johnston and Charles et al., 2005, USA [38] | 27 | Posterior-only 6.3 (13.1–19.5), Anterior–posterior 15.6 (14.2–16.9) | Cotrel–Dubousset, 20 Posterior-only, 7 Anterior–posterior | Posterior-only 80.5° (67–97°), Anterior–posterior 79.0° (range 62–93°) | Posterior-only 38.8° (range 22–59°), Anterior–posterior 41.6° (range 34–48°) | 30 months (range 24–56) | 8 (range 12–8°) | N/A |
| 21 | Herrera-Soto et al., 2005, USA [39] | 19 | 17.4 | Cotrel–Dubousset, Anterior and Posterior | 84.8° (69–105°) | 43.7° | 31 months (24–72) | 1.6 | Ulnar dysesthesia (1), Biceps weakness (1), Pull out hooks (2), Pneumothorax (2), Bursitis (1) Bleural effusion (1) None with Junctional Kyphosis |
| 22 | Hosman AJ et al., 2002, Netherlands [40] | 33 | 25.8 ± 7.8 | H frame, 16 Posterior-only, 17 anterior–posterior | 78.7 ± 8.9° | 51.7 ± 10.3° | 49 months ± 24 | 1.4° ± 3.9° | Infections (3), Metallosis (4), Loss of correction (1), Rod brake (1), Proximal Junctional Kyphosis (1) |
| 23 | T de Jong et al., 2001, Hungary [41] | 8 | 19 (13–27) | Cotrel–Dubousset, Posterior-only | 86° (71–99°) | 44° (32–58°) | 60 months | 4.6° (1–12°) | Superficial infection (1), Proximal loosening (1), Proximal Junctional Kyphosis (1) |
| 24 | Lim M et al., 2001, USA [42] | 23 | 19 | ISOLA 14, 9 Cotrel–Dubousset, 20 anterior–posterior, 3 Posterior-only | 83° (63–104°) | 46° (32–67°) | 38 months (10–123) | N/A | Pleural effusions (7), Pneumothoraxes (2), Healing elongation (1), Arms pain (1), Loss of fixation (3) |
| 25 | Lowe et al., 1994, USA [43] | 32 | 25.8 (14.3–57.6) | Cotrel–Dubousset, 28 Anterior–posterior 4 Posterior-only | 85° (75–105°) | 43° (26–65°) | 42 months (24–74) | 4° (0–19°) | Proximal Junctional Kyphosis (10), Distal Junctional Kyphosis (9), Cervical pain (4), Back pain (28) |
| 26 | Sturm P et al., 1992, Canada [44] | 39 | 19 (12–37) | Harrington rod, Posterior-only | 71.5° | 37.7° | 71.8 (23–144) | 6 | Intraoperative hook failure (4), Superficial wound infection (2), Retained piece of drain (1), Deep infection (1), Hook pullout (1), Broken rods (3) |
| 27 | Otsuka NY et al., 1990, Canada [45] | 10 | N/A | Harrington rod, Posterior-only | 71.4° | 39.3° | 26.6 | 7.8 | N/A |
| 28 | Herndon WA et al., 1981, USA [46] | 13 | 19 (14–30) | Harrington rod Anterior–posterior | 78° | 38° | 29 months (12–66) | 7.8 | Death (1), Laminae fracture (1), Deep Venous Thrombosis (1), Prominent hardware-revision (1) |
| 29 | Taylor TC et al., 1979, USA [47] | 27 | N/A | Harrington rod Posterior-only | 72° | 46.1° | 27.6 | 5.7 | N/A |
| 30 | Bradford DS et al., 1975, USA [48] | 22 | N/A | Harrington rod, Posterior-only | 72° | 47° | 35 months | 21 in 16 (72%) pts | N/A |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scheuermann, H. Kyphosis dorsalis juvenilis. Ugeskr. Laeger 1920, 82, 385. [Google Scholar]
- Braun, S.; Brenneis, M.; Schonnagel, L.; Caffard, T.; Diaremes, P. Surgical Treatment of Spinal Deformities in Pediatric Orthopedic Patients. Life 2023, 13, 1341. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.H. Scheuermann’s Juvenile Kyphosis: Clinical Appearances, Radiography, Aetiology, and Prognosis; Munksgaard: Copenhagen, Denmark, 1964. [Google Scholar]
- Souza, G.V.; Oki, L.Y.; Portelinha, A.M.; Pontes, M.D.S.; Herrero, C.F.P.D.S. Study of the Prevalence of Atypical Scheuermann’s Kyphosis Using Computed Tomography Scans. Rev. Bras. Ortop. 2024, 59, e854–e860. [Google Scholar]
- Papagelopoulos, P.J.; Mavrogenis, A.F.; Savvidou, O.D.; Mitsiokapa, E.A.; Themistocleous, G.S.; Soucacos, P.N. Current concepts in Scheuermann’s kyphosis. Orthopedics 2008, 31, 52–58. [Google Scholar]
- Damborg, F.; Engell, V.; Andersen, M.; Kyvik, K.O.; Thomsen, K. Prevalence, concordance, and heritability of Scheuermann kyphosis based on a study of twins. J. Bone Jt. Surg. 2006, 88, 2133–2136. [Google Scholar]
- Scoles, P.V.; Latimer, B.M.; DigIovanni, B.F.; Vargo, E.; Bauza, S.; Jellema, L.M. Vertebral alterations in Scheuermann’s kyphosis. Spine 1991, 16, 509–515. [Google Scholar] [CrossRef]
- Hart, E.S.; Merlin, G.; Harisiades, J.; Grottkau, B.E. Scheuermann’s thoracic kyphosis in the adolescent patient. Orthop. Nurs. 2010, 29, 365–371. [Google Scholar] [CrossRef]
- Peleg, S.; Kallevag, R.P.; Dar, G.; Steinberg, N.; Lenzner, Z.; May, H. The effect of Scheuermann’s kyphosis on rib cage morphology: A skeletal study. Ann Anat. 2025, 257, 152348. [Google Scholar] [CrossRef]
- Coneys, U.; Tabard-Fougère, A.; Gavira, N.; Dayer, R. Validating rasterstereography to evaluate thoracic kyphosis in patients with Scheuermann’s disease. Eur. Spine J. 2025, 34, 831–836. [Google Scholar] [CrossRef]
- Weiss, H.R.; Turnbull, D.; Bohr, S. Brace treatment for patients with Scheuermann’s disease—A review of the literature and first experiences with a new brace design. Scoliosis 2009, 4, 22. [Google Scholar] [CrossRef]
- Sebaaly, A.; Farjallah, S.; Kharrat, K.; Kreichati, G.; Daher, M. Scheuermann’s kyphosis: Update on pathophysiology and surgical treatment. EFORT Open Rev. 2022, 7, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Arlet, V.; Schlenzka, D. Scheuermann’s kyphosis: Surgical management. Eur. Spine J. 2005, 14, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Cobden, A.; Albayrak, A.; Camurcu, Y.; Sofu, H.; Tacal, T.; Kaygusuz, M.A. Posterior-Only Approach with Pedicle Screws for the Correction of Scheuermann’s Kyphosis. Asian Spine J. 2017, 11, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Sailhan, F.; Revel, M. Scheuermann’s disease: An update. Jt. Bone Spine 2014, 81, 209–214. [Google Scholar] [CrossRef]
- French Scoliosis Study Group; Riouallon, G.; Morin, C.; Charles, Y.-P.; Roussouly, P.; Kreichati, G.; Obeid, I.; Wolff, S. Posterior-only versus combined anterior/posterior fusion in Scheuermann disease: A large retrospective study. Eur. Spine J. 2018, 27, 2322–2330. [Google Scholar] [CrossRef]
- Yun, C.; Shen, C.L. Anterior release for Scheuermann’s disease: A systematic literature review and meta-analysis. Eur. Spine J. 2017, 26, 921–927. [Google Scholar] [CrossRef]
- Lonner, B.S.; Newton, P.; Betz, R.; Scharf, C.; O’Brien, M.; Sponseller, P.; Lenke, L.; Crawford, A.; Lowe, T.; Letko, L.; et al. Operative management of Scheuermann’s kyphosis in 78 patients: Radiographic outcomes, complications, and technique. Spine 2007, 32, 2644–2652. [Google Scholar] [CrossRef]
- Li, Q. Surgical Procedures Used for Correction of Scheuermann’s Kyphosis: A Meta-Analysis. Pain. Res. Manag. 2021, 2021, 2142964. [Google Scholar] [CrossRef]
- Huq, S.; Ehresman, J.; Cottrill, E.; Ahmed, A.K.; Pennington, Z.; Westbroek, E.M.; Sciubba, D.M. Treatment approaches for Scheuermann kyphosis: A systematic review of historic and current management. J. Neurosurg. Spine 2020, 32, 235–247. [Google Scholar] [CrossRef]
- Lee, S.S.; Lenke, L.G.; Kuklo, T.R.; Valenté, L.; Bridwell, K.H.; Sides, B.; Blanke, K.M. Comparison of Scheuermann kyphosis correction by posterior-only thoracic pedicle screw fixation versus combined anterior/posterior fusion. Spine 2006, 31, 2316–2321. [Google Scholar] [CrossRef]
- Koller, H.; Lenke, L.G.; Meier, O.; Zenner, J.; Umschlaeger, M.; Hempfing, A.; Hitzl, W.; Bridwell, K.H.; Koester, L.A. Comparison of Anteroposterior to Posterior-Only Correction of Scheuermann’s Kyphosis: A Matched-Pair Radiographic Analysis of 92 Patients. Spine Deform. 2015, 3, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Jensch, M.L.; Platz, U.; Quante, M.; Köszegvary, M.; Thomsen, B.; Gliemroth, J.; Berlin, C.; Halm, H. Posterior instrumented correction and fusion of Scheuermann’s results in physiological reconstruction of sagittal alignment and excellent overall clinical outcome- clinical trail of 73 patients. BMC Musculoskelet. Disord. 2025, 26, 90. [Google Scholar] [CrossRef] [PubMed]
- Solans Lopez, M.C.; Hernández Mateo, J.M.; Barrios Ayuso, A.; Igualada Blázquez, C.; Quevedo Narciso, T.; García Martín, A.; Riquelme García, O.G.; Esparragoza Cabrera, L.A. Bipolar hybrid posterior instrumentation tecnique for the correction of Scheuermann’s kyphosis. Spine Deform. 2024, 12, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Baymurat, A.C.; Yapar, A.; Tokgoz, M.A.; Daldal, I.; Akcan, Y.O.; Senkoylu, A. Using Proximal Hooks as a Soft-Landing Strategy to Prevent Proximal Junctional Kyphosis in the Surgical Treatment of Scheuermann’s Kyphosis. Turk. Neurosurg. 2024, 34, 505–513. [Google Scholar]
- Aydogan, M.; Pehlivanoglu, T.; Erdag, Y.; Akturk, U.D.; Akar, A. Flexible posterior vertebral tethering for the management of Scheuermann’s kyphosis: Correction by using growth modulation-clinical and radiographic outcomes of the first 10 patients with at least 3 years of follow-up. Eur. Spine J. 2024, 33, 2677–2687. [Google Scholar] [CrossRef]
- Debnath, U.K.; Quraishi, N.A.; McCarthy, M.J.H.; McConnell, J.R.; Mehdian, S.M.H.; Shetaiwi, A.; Grevitt, M.P.; Webb, J.K. Long-term outcome after surgical treatment of Scheuermann’s Kyphosis (SK). Spine Deform. 2022, 10, 387–397. [Google Scholar] [CrossRef]
- Suominen, E.N.; Saarinen, A.J.; Syvänen, J.; Diarbakerli, E.; Helenius, L.; Gerdhem, P.; Helenius, I. Health-related quality of life outcomes in adolescent Scheuermann’s kyphosis patients treated with posterior spinal fusion: A comparison with age- and sex-matched controls. J. Child. Orthop. 2022, 16, 290–296. [Google Scholar] [CrossRef]
- Vital, L.; Nunes, B.; Santos, S.A.; Veludo, V.; Serdoura, F.; Pinho, A. Sagittal Plane Alignment and Functional Outcomes Following Surgery for Scheuermann Kyphosis. Rev. Bras. Ortop. 2021, 56, 446–452. [Google Scholar]
- Tsirikos, A.I.; Jain, A.K. Scheuermann’s kyphosis; current controversies. J. Bone Jt. Surg. Br. 2011, 93, 857–864. [Google Scholar] [CrossRef]
- Etemadifar, M.; Ebrahimzadeh, A.; Hadi, A.; Feizi, M. Comparison of Scheuermann’s kyphosis correction by combined anterior-posterior fusion versus posterior-only procedure. Eur. Spine J. 2016, 25, 2580–2586. [Google Scholar] [CrossRef]
- Behrbalk, E.; Uri, O.; Parks, R.M.; Grevitt, M.P.; Rickert, M.; Boszczyk, B.M. Posterior-only correction of Scheuermann kyphosis using pedicle screws: Economical optimization through screw density reduction. Eur. Spine J. 2014, 23, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Temponi, E.F.; de Macedo, R.D.; Pedrosa, L.O.; Fontes, B.P. Scheuermann’s Kyphosis: Comparison between the Posterior Approach Associated with Smith-Petersen Osteotomy and Combined Anterior-Posterior Fusion. Rev. Bras. Ortop. 2011, 46, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, S.; Pawelek, J.B.; Bastrom, T.P.; Shah, S.A.; Newton, P.O. Do discs “open” anteriorly with posterior-only correction of Scheuermann’s kyphosis? Spine 2011, 36, E1086–E1092. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J.; Lenke, L.G.; Bridwell, K.H.; Kamiya, M.; Sides, B. Selection of the optimal distal fusion level in posterior instrumentation and fusion for thoracic hyperkyphosis: The sagittal stable vertebra concept. Spine 2009, 34, 765–770. [Google Scholar] [CrossRef]
- Koptan, W.M.; Elmiligui, Y.H.; Elsebaie, H.B. All pedicle screw instrumentation for Scheuermann’s kyphosis correction: Is it worth it? Spine J. 2009, 9, 296–302. [Google Scholar] [CrossRef]
- Geck, M.J.; Macagno, A.; Ponte, A.; Shufflebarger, H.L. The Ponte procedure: Posterior only treatment of Scheuermann’s kyphosis using segmental posterior shortening and pedicle screw instrumentation. J. Spinal Disord. Tech. 2007, 20, 586–593. [Google Scholar] [CrossRef]
- Johnston, C.E.; 2nd Elerson, E.; Dagher, G. Correction of adolescent hyperkyphosis with posterior-only threaded rod compression instrumentation: Is anterior spinal fusion still necessary? Spine 2005, 30, 1528–1534. [Google Scholar] [CrossRef]
- Herrera-Soto, J.A.; Parikh, S.N.; Al-Sayyad, M.J.; Crawford, A.H. Experience with combined video-assisted thoracoscopic surgery (VATS) anterior spinal release and posterior spinal fusion in Scheuermann’s kyphosis. Spine 2005, 30, 2176–2181. [Google Scholar] [CrossRef]
- Hosman, A.J.; Langeloo, D.D.; de Kleuver, M.; Anderson, P.G.; Veth, R.P.; Slot, G.H. Analysis of the sagittal plane after surgical management for Scheuermann’s disease: A view on overcorrection and the use of an anterior release. Spine 2002, 27, 167–175. [Google Scholar] [CrossRef]
- de Jonge, T.; Illes, T.; Bellyei, A. Surgical correction of Scheuermann’s kyphosis. Int. Orthop. 2001, 25, 70–73. [Google Scholar] [CrossRef]
- Lim, M.; Green, D.W.; Billinghurst, J.E.; Huang, R.C.; Rawlins, B.A.; Widmann, R.F.; Burke, S.W.; Boachie-Adjei, O. Scheuermann kyphosis: Safe and effective surgical treatment using multisegmental instrumentation. Spine 2004, 29, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.G.; Kasten, M.D. An analysis of sagittal curves and balance after Cotrel-Dubousset instrumentation for kyphosis secondary to Scheuermann’s disease. A review of 32 patients. Spine 1994, 19, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Sturm, P.F.; Dobson, J.C.; Armstrong, G.W. The surgical management of Scheuermann’s disease. Spine 1993, 18, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, N.Y.; Hall, J.E.; Mah, J.Y. Posterior fusion for Scheuermann’s kyphosis. Clin. Orthop. Relat. Res. 1990, 251, 134–139. [Google Scholar] [CrossRef]
- Herndon, W.A.; Emans, J.B.; Micheli, L.J.; Hall, J.E. Combined anterior and posterior fusion for Scheuermann’s kyphosis. Spine 1981, 6, 125–130. [Google Scholar] [CrossRef]
- Taylor, T.C.; Wenger, D.R.; Stephen, J.; Gillespie, R.; Bobechko, W.P. Surgical management of thoracic kyphosis in adolescents. J. Bone Jt. Surg. Am. 1979, 61, 496–503. [Google Scholar] [CrossRef]
- Bradford, D.S.; Moe, J.H.; Montalvo, F.J.; Winter, R.B. Scheuermann’s kyphosis. Results of surgical treatment by posterior spine arthrodesis in twenty-two patients. J. Bone Jt. Surg. Am. 1975, 57, 439–448. [Google Scholar] [CrossRef]
- Sardar, Z.M.; Ames, R.J.; Lenke, L. Scheuermann’s Kyphosis: Diagnosis, Management, and Selecting Fusion Levels. J. Am. Acad. Orthop. Surg. 2019, 27, e462–e472. [Google Scholar] [CrossRef]
- Makurthou, A.A.; Oei, L.; El Saddy, S.; Breda, S.J.; Castaño-Betancourt, M.C.; Hofman, A.; van Meurs, J.B.; Uitterlinden, A.G.; Rivadeneira, F.; Oei, E.H. Scheuermann disease: Evaluation of radiological criteria and population prevalence. Spine 2013, 38, 1690–1694. [Google Scholar] [CrossRef]
- Wassmann, K. Kyphosis juvenilis Scheuermann--an occupational disorder. Acta Orthop. Scand. 1951, 21, 65–74. [Google Scholar] [CrossRef]
- Armbrecht, G.; Felsenberg, D.; Ganswindt, M.; Lunt, M.; Kaptoge, S.; Abendroth, K.; Dias, A.A.; Bhalla, A.; Andia, J.C.; Dequeker, J.; et al. Vertebral Scheuermann’s disease in Europe: Prevalence, geographic variation and radiological correlates in men and women aged 50 and over. Osteoporos. Int. 2015, 26, 2509–2519. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, J.; Narvaez, F.; Besa, P.; Meissner-Haecker, A.; Rios, C.; Piza, C. Scheuermann’s disease in patients 15–40 years old: A study to determine its prevalence and its relationship with age and sex using chest radiographs as screening tool. J. Orthop. Sci. 2019, 24, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Wenger, D.R.; Frick, S.L. Scheuermann kyphosis. Spine 1999, 24, 2630–2639. [Google Scholar] [CrossRef] [PubMed]
- Sachs, B.; Bradford, D.; Winter, R.; Lonstein, J.; Moe, J.; Willson, S. Scheuermann kyphosis. Follow-up of Milwaukee-brace treatment. J. Bone Jt. Surg. Am. 1987, 69, 50–57. [Google Scholar] [CrossRef]
- Gokce, E.; Beyhan, M. Radiological imaging findings of scheuermann disease. World J. Radiol. 2016, 8, 895–901. [Google Scholar] [CrossRef]
- Paajanen, H.; Alanen, A.; Erkintalo, M.; Salminen, J.J.; Katevuo, K. Disc degeneration in Scheuermann disease. Skelet. Radiol. 1989, 18, 523–526. [Google Scholar] [CrossRef]
- Lonner, B.S.; Toombs, C.S.; Mechlin, M.; Ciavarra, G.; Shah, S.A.; Samdani, A.F.; Sponseller, P.; Shufflebarger, H.L.; Betz, R.R.; Yaszay, B.; et al. MRI Screening in Operative Scheuermann Kyphosis: Is it Necessary? Spine Deform. 2017, 5, 124–133. [Google Scholar] [CrossRef]
- Ristolainen, L.; Kettunen, J.A.; Heliovaara, M.; Kujala, U.M.; Heinonen, A.; Schlenzka, D. Untreated Scheuermann’s disease: A 37-year follow-up study. Eur. Spine J. 2012, 21, 819–824. [Google Scholar] [CrossRef]
- Murray, P.M.; Weinstein, S.L.; Spratt, K.F. The natural history and long-term follow-up of Scheuermann kyphosis. J. Bone Jt. Surg. Am. 1993, 75, 236–248. [Google Scholar] [CrossRef]
- Garrido, E.; Roberts, S.B.; Duckworth, A.; Fournier, J. Long-term follow-up of untreated Scheuermann’s kyphosis. Spine Deform. 2021, 9, 1633–1639. [Google Scholar] [CrossRef]
- Ristolainen, L.; Kettunen, J.A.; Kujala, U.M.; Heinonen, A.; Schlenzka, D. Progression of untreated mild thoracic Scheuermann’s kyphosis—Radiographic and functional assessment after mean follow-up of 46 years. J. Orthop. Sci. 2017, 22, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Papagelopoulos, P.J.; Klassen, R.A.; Peterson, H.A.; Dekutoski, M.B. Surgical treatment of Scheuermann’s disease with segmental compression instrumentation. Clin. Orthop. Relat. Res. 2001, 386, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Tome-Bermejo, F.; Tsirikos, A.I. Current concepts on Scheuermann kyphosis: Clinical presentation, diagnosis and controversies around treatment. Rev. Esp. Cir. Ortop. Traumatol. 2012, 56, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.G. Double L-rod instrumentation in the treatment of severe kyphosis secondary to Scheuermann’s disease. Spine 1987, 12, 336–341. [Google Scholar] [CrossRef]
- Polly, D.W., Jr.; Ledonio, C.G.T.; Diamond, B.; Labelle, H.; Sucato, D.J.; Hresko, M.T.; Emans, J.B.; Vitale, M.G.; Erickson, M.A.; Larson, A.N.; et al. What Are the Indications for Spinal Fusion Surgery in Scheuermann Kyphosis? J. Pediatr. Orthop. 2019, 39, 217–221. [Google Scholar] [CrossRef]
- Haselhuhn, J.J.; Odland, K.; Soriano, P.B.O.; Jones, K.E.; Polly, D.W., Jr. A Novel Surgical Indication for Scheuermann’s Kyphosis. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2024, 8, e23. [Google Scholar] [CrossRef]
- Amadi, I.J.; Kabangu, J.K.; Bhargav, A.G.; Camarata, P.J. The Legacy of Harrington’s Rod and the Evolution of Long-Segment Constructs in Spine Surgery. J. Clin. Med. 2024, 13, 5556. [Google Scholar] [CrossRef]
- de Loubresse, C.G.; Vialle, R.; Wolff, S. Cyphoses Pathologiques. EMC-Appar. Locomoteur 2005, 2, 294–334. [Google Scholar] [CrossRef]
- McDonnell, J.M.; Ahern, D.P.; Lui, D.F.; Yu, H.; Lehovsky, J.; Noordeen, H.; Molloy, S.; Butler, J.S.; Gibson, A. Two-stage anterior and posterior fusion versus one-stage posterior fusion in patients with Scheuermann’s kyphosis. Bone Jt. J. 2020, 102-B, 1368–1374. [Google Scholar] [CrossRef]
- Ponte, A.; Orlando, G.; Siccardi, G.L. The True Ponte Osteotomy: By the One Who Developed It. Spine Deform. 2018, 6, 2–11. [Google Scholar] [CrossRef]
- Nasto, L.A.; Mousavi Nasab, S.H.; Sieczak, A.; Cattolico, A.; Ulisse, P.; Pola, E. Ponte osteotomies for treatment of spinal deformities: They are not all made equal. Eur. Spine J. 2024, 33, 2787–2793. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.J.; Bylski-Austrow, D.I.; Shelton, F.S.; Crawford, A.H.; Kolata, R.J.; Baum, D.S. Endoscopic discectomy increases thoracic spine flexibility as effectively as open discectomy. A mechanical study in a porcine model. Spine 1998, 23, 9–15; discussion 15–16. [Google Scholar] [CrossRef] [PubMed]
- Coscia, M.F.; Bradford, D.S.; Ogilvie, J.W. Scheuermann’s kyphosis: Results in 19 cases treated by spinal arthrodesis and L-rodi nstrumentation. Orthop. Trans. 1988, 12, 255–260. [Google Scholar]
- Dubousset, J. Past, present, and future in pediatric spinal surgery. Ann. Transl. Med. 2020, 8, 36. [Google Scholar] [CrossRef]
- Luque, E.R. The anatomic basis and development of segmental spinal instrumentation. Spine 1982, 7, 256–259. [Google Scholar] [CrossRef]
- Luque, E.R. Segmental spinal instrumentation for correction of scoliosis. Clin. Orthop. Relat. Res. 1982, 163, 192–198. [Google Scholar] [CrossRef]
- Bridwell, K.H. Cotrel-Dubousset instrumentation. Orthop. Nurs. 1988, 7, 11–16. [Google Scholar] [CrossRef]
- Bradford, D.S.; Ahmed, K.B.; Moe, J.H.; Winter, R.B.; Lonstein, J.E. The surgical management of patients with Scheuermann’s disease: A review of twenty-four cases managed by combined anterior and posterior spine fusion. J. Bone Jt. Surg. Am. 1980, 62, 705–712. [Google Scholar] [CrossRef]
- Reinhardt, P.; Bassett, G.S. Short segmental kyphosis following fusion for Scheuermann’s disease. J. Spinal Disord. 1990, 3, 162–168. [Google Scholar] [CrossRef]
- Speck, G.R.; Chopin, D.C. The surgical treatment of Scheuermann’s kyphosis. J. Bone Jt. Surg. Br. 1986, 68, 189–193. [Google Scholar] [CrossRef]
- Bradford, D.S.; Moe, J.H. Scheuermann’s juvenile kyphosis. A histologic study. Clin. Orthop. Relat. Res. 1975, 110, 45–53. [Google Scholar] [CrossRef]
- Birnbaum, K.; Siebert, C.H.; Hinkelmann, J.; Prescher, A.; Niethard, F.U. Correction of kyphotic deformity before and after transection of the anterior longitudinal ligament--a cadaver study. Arch. Orthop. Trauma. Surg. 2001, 121, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Tsirikos, A.I.; Carter, T.H. The surgical treatment of severe Scheuermann’s kyphosis. Bone Jt. J. 2021, 103-B, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Coe, J.D.; Smith, J.S.; Berven, S.; Arlet, V.; Donaldson, W.; Hanson, D.; Mudiyam, R.; Perra, J.; Owen, J.; Marks, M.C.; et al. Complications of spinal fusion for scheuermann kyphosis: A report of the scoliosis research society morbidity and mortality committee. Spine 2010, 35, 99–103. [Google Scholar] [CrossRef]
- Graham, E.J.; Lenke, L.G.; Lowe, T.G.; Betz, R.R.; Bridwell, K.H.; Kong, Y.; Blanke, K. Prospective pulmonary function evaluation following open thoracotomy for anterior spinal fusion in adolescent idiopathic scoliosis. Spine 2000, 25, 2319–2325. [Google Scholar] [CrossRef]
- Yuan, N.; Hu, G.; Bridwell, K.H.; Koester, L.A.; Lenke, L.G. How to determine the optimal proximal fusion level for Scheuermann kyphosis. Eur. Spine J. 2024, 33, 1021–1027. [Google Scholar] [CrossRef]
- Bernhardt, M.; Bridwell, K.H. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine 1989, 14, 717–721. [Google Scholar] [CrossRef]
- Denis, F.; Sun, E.C.; Winter, R.B. Incidence and risk factors for proximal and distal five-year follow-up. Spine 2009, 34, E729–E734. [Google Scholar] [CrossRef]
- Gomez, J.A.; Kubat, O.; Tovar Castro, M.A.; Hanstein, R.; Flynn, T.; Lafage, V.; Hurry, J.K.; Soroceanu, A.; Schwab, F.; Skaggs, D.L.; et al. The Effect of Spinopelvic Parameters on the Development of Proximal Junctional Kyphosis in Early Onset: Mean 4.5-Year Follow-up. J. Pediatr. Orthop. 2020, 40, 261–266. [Google Scholar] [CrossRef]
- Nasto, L.A.; Perez-Romera, A.B.; Shalabi, S.T.; Quraishi, N.A.; Mehdian, H. Correlation between preoperative spinopelvic alignment and risk of proximal junctional kyphosis after posterior-only surgical correction of Scheuermann kyphosis. Spine J. 2016, 16 (Suppl. S4), S26–S33. [Google Scholar] [CrossRef]
- Tyrakowski, M.; Mardjetko, S.; Siemionow, K. Radiographic spinopelvic parameters in skeletally mature patients with Scheuermann disease. Spine 2014, 39, E1080–E1085. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Qiu, Y.; Xu, L.; Liu, Z.; Wang, Z.; Sha, S.; Zhu, Z. Sagittal spinopelvic alignment in adolescents associated with Scheuermann’s kyphosis: A comparison with normal population. Eur. Spine J. 2014, 23, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Ponchelet, L.; Khalife, M.; Finoco, M.; Duray, C.; Guigui, P.; Ferrero, E. Influence of pelvic tilt correction on PJK occurrence after adult spinal deformity surgery. Eur. Spine J. 2024, 33, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Gupta, S.; Farooqi, A.S.; Yin, T.; Soroceanu, A.; Schwab, F.J.; Lafage, V.; Kelly, M.P.; Kebaish, K.; Hostin, R.; et al. Predictive role of global spinopelvic alignment and upper instrumented vertebra level in symptomatic proximal junctional kyphosis in adult spinal deformity. J. Neurosurg. Spine 2023, 39, 774–784. [Google Scholar] [CrossRef]
- Luzzi, A.; Sardar, Z.; Cerpa, M.; Ferrer, X.; Coury, J.; Crockatt, W.; Ha, A.; Roye, B.; Vitale, M.; Lenke, L.; et al. Risk of distal junctional kyphosis in scheuermann’s kyphosis is decreased by selecting the LIV as two vertebrae distal to the first lordotic disc. Spine Deform. 2022, 10, 1437–1442. [Google Scholar] [CrossRef]
- Yan, C.; Li, Y.; Yu, Z. Prevalence and Consequences of the Proximal Junctional Kyphosis After Spinal Deformity Surgery: A Meta-Analysis. Medicine 2016, 95, e3471. [Google Scholar] [CrossRef]
- Hassan, R.U.; Abbas, N.; Ko, J. Toward Customizable Smart Gels: A Comprehensive Review of Innovative Printing Techniques and Applications. Gels 2025, 11, 32. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaspiris, A.; Spyrou, I.; Panagopoulos, F.; Marougklianis, V.; Pelantis, P.; Vavourakis, M.; Sakellariou, E.; Lianou, I.; Ntourantonis, D.; Repantis, T.; et al. Surgical Strategies and Challenges in Scheuermann’s Kyphosis: A Comprehensive Review. J. Clin. Med. 2025, 14, 4276. https://doi.org/10.3390/jcm14124276
Kaspiris A, Spyrou I, Panagopoulos F, Marougklianis V, Pelantis P, Vavourakis M, Sakellariou E, Lianou I, Ntourantonis D, Repantis T, et al. Surgical Strategies and Challenges in Scheuermann’s Kyphosis: A Comprehensive Review. Journal of Clinical Medicine. 2025; 14(12):4276. https://doi.org/10.3390/jcm14124276
Chicago/Turabian StyleKaspiris, Angelos, Ioannis Spyrou, Fotios Panagopoulos, Vasileios Marougklianis, Periklis Pelantis, Michail Vavourakis, Evangelos Sakellariou, Ioanna Lianou, Dimitrios Ntourantonis, Thomas Repantis, and et al. 2025. "Surgical Strategies and Challenges in Scheuermann’s Kyphosis: A Comprehensive Review" Journal of Clinical Medicine 14, no. 12: 4276. https://doi.org/10.3390/jcm14124276
APA StyleKaspiris, A., Spyrou, I., Panagopoulos, F., Marougklianis, V., Pelantis, P., Vavourakis, M., Sakellariou, E., Lianou, I., Ntourantonis, D., Repantis, T., Vasiliadis, E. S., & Pneumaticos, S. G. (2025). Surgical Strategies and Challenges in Scheuermann’s Kyphosis: A Comprehensive Review. Journal of Clinical Medicine, 14(12), 4276. https://doi.org/10.3390/jcm14124276








