Cannabidiol for Orofacial and Upper-Quarter Pain: A Systematic Evaluation of Therapeutic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Search Strategy
2.3. Selection of Studies
- Studies investigating the therapeutic effects of CBD in disorders affecting the upper quarter (head, neck, shoulders, and upper back);
- Clinical, in vivo, and in vitro studies examining the impact of CBD on pain, inflammation, muscle function, or neurological disorders related to the upper quarter;
- Studies where CBD is used as the primary therapeutic agent for treating upper-quarter conditions;
- Research assessing the synergistic effects of CBD in combination with other pharmacological or non-pharmacological treatments for upper-quarter disorders;
- Controlled studies evaluating the effects of CBD compared to placebo, standard treatment, or alternative interventions for upper-quarter disorders;
- Comparative analyses examining the efficacy of CBD versus conventional treatment modalities for upper-quarter disorders;
- Longitudinal studies or those with follow-up periods assessing the sustained therapeutic effects of CBD in upper-quarter management.
- Gray literature sources, case reports, letters to editors, narrative or systematic reviews, books, documents, and other non-journal materials;
- Non-peer-reviewed sources;
- Studies published in languages other than English;
- Duplicate studies or research sharing the same ethical approval number;
- General medical applications unrelated to disorders of the upper quarter;
- Studies without a control or comparison group;
- Research on CBD where it is not applied as a therapeutic intervention for upper-quarter disorders;
- Studies using cannabinoid compounds other than CBD;
- Studies focusing on conditions outside the upper quarter or those that do not specifically assess related disorders;
- In vitro studies that do not replicate physiological conditions relevant to upper-quarter disorders.
2.4. Risk of Bias in Individual Studies and Quality Assessment
| Study | Sequence Generation | Baseline Characteristics | Allocation Concealment | Random Housing | Blinding (Caregivers/ Investigators) | Random Outcome Assessment | Blinding of Outcome Assessor | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias | Overall SYRCLE Judgment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahn et al., 2007 [41] | Unclear risk | Low risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Low risk |
| Brice-Tutt et al., 2025 [42] | Unclear risk | Low risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Burgos et al., 2010 [43] | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Laks et al., 2023 [44] | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Lee et al., 2008 [45] | Unclear risk | Low risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Low risk |
| Wong et al., 2019 [46] | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Zubrzycki et al., 2017 [47] | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall RoB 2 Judgment |
|---|---|---|---|---|---|---|
| Nitecka-Buchta et al., 2019 [48] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Ostenfeld et al., 2011 [49] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Walczynska-Dragon et al., 2024 [50] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
2.5. Data Extraction
3. Results
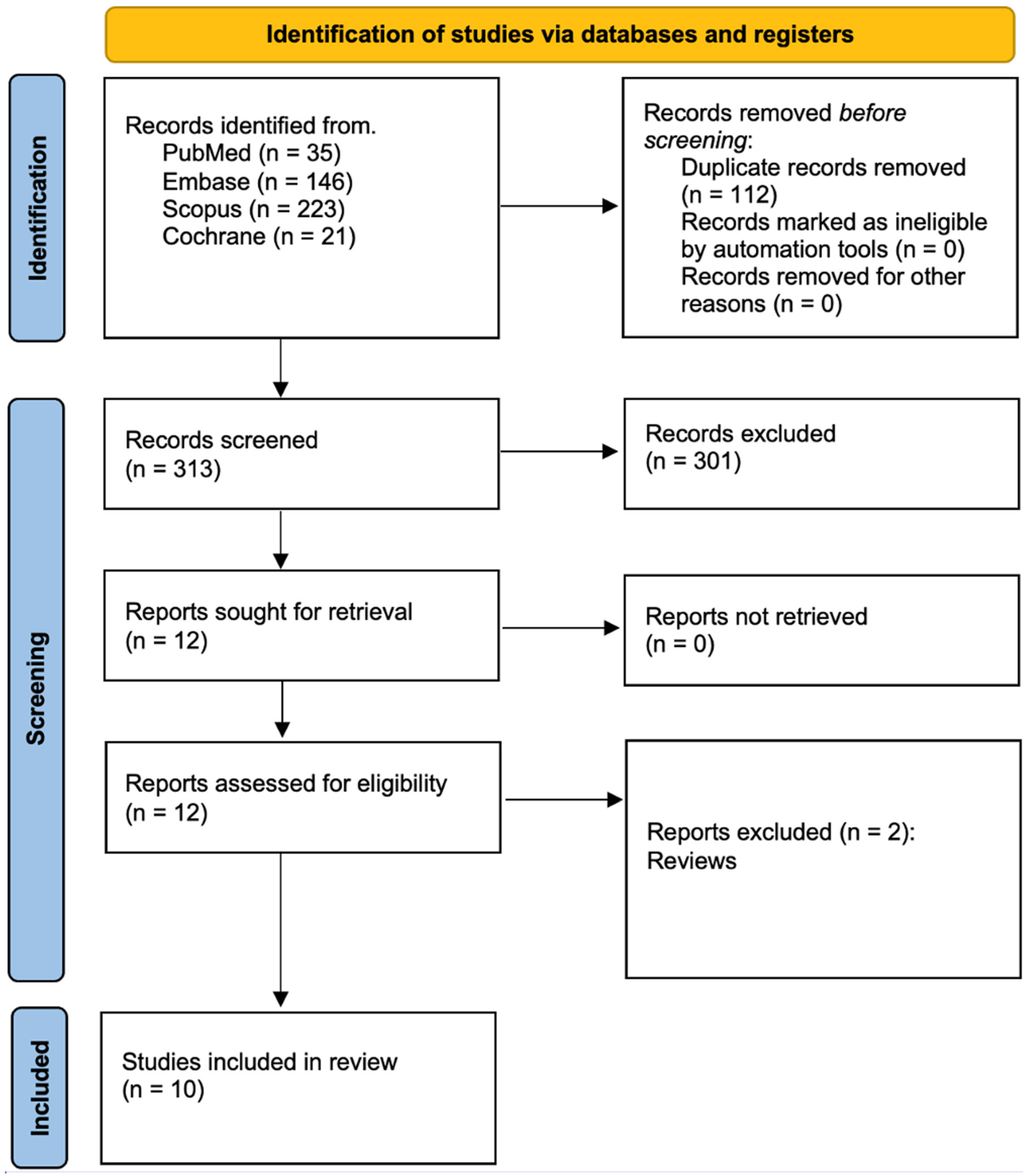
3.1. Study Selection
3.2. Data Presentation
| Author and Year | Country | Study Design |
|---|---|---|
| Ahn et al., 2007 [41] | Republic of Korea, USA | Animal study |
| Brice-Tutt et al., 2025 [42] | USA | Animal study |
| Burgos et al., 2010 [43] | Spain | Animal study |
| Laks et al., 2023 [44] | USA | Animal study |
| Lee et al., 2008 [45] | Republic of Korea, USA | Animal study |
| Wong et al., 2019 [46] | Canada | Animal study |
| Zubrzycki et al., 2017 [47] | Germany, Poland | Animal study |
| Nitecka-Buchta et al., 2019 [48] | Poland | Randomized, double-blind clinical trial |
| Ostenfeld et al., 2011 [49] | UK, Italy, Germany | Randomized, controlled clinical trial |
| Walczynska Dragon et al., 2024 [50] | Poland | Randomized, double-blind clinical trial |
| Study | CBD Administration Route | CBD Dosage/ Concentration | Type of Pain Studied | Study Model (Human/ Animal) | Additional Analgesics Used | Outcome Measures |
|---|---|---|---|---|---|---|
| Ahn et al., 2007 [41] | Intracisternal (direct injection into the cisterna magna) | 10 µg, 30 µg, 80 µg WIN 55,212-2 (synthetic CB1/2 agonist) | Inflammatory temporomandibular joint pain | Animal (Sprague Dawley rats) | COX inhibitors: NS-398 (COX-2 inhibitor), Indomethacin (COX-1/2 inhibitor), Acetaminophen (COX-3 inhibitor), SC-560 (COX-1 inhibitor) | Number and duration of scratching behaviors in response to formalin-induced TMJ pain; Evans’ blue dye extravasation; motor function assessed via rotarod test |
| Brice-Tutt et al., 2025 [42] | Oral (gavage) | 25 mg/kg | Orofacial cutaneous thermal pain | Animal (Sprague Dawley rats) | Oxycodone (1.4 mg/kg) | Lick/contact ratio in an operant orofacial pain assay; number of lick bursts; pain tolerance assessment |
| Burgos et al., 2010 [43] | Intraperitoneal injection | WIN 55,212-2 (0.5, 1 mg/kg, i.p.) | Orofacial inflammatory pain (formalin-induced) | Animal (rat) | Morphine (2, 5, 10 mg/kg, i.p.), Naloxone (1, 2 mg/kg, i.p.), Indomethacin (2.5, 5 mg/kg, i.p.), Ketamine (25, 50 mg/kg, i.p.) | Nociceptive behavior (face rubbing, head flinching), locomotor activity assessment |
| Laks et al., 2023 [44] | Intraperitoneal (i.p.) | 5 mg/kg | Orofacial pain associated with pulpitis | Animal (Sprague Dawley rats) | None | CBD reduced AIF expression in trigeminal ganglia but did not significantly reduce orofacial sensitivity |
| Lee et al., 2008 [45] | Intracisternal injection | 3, 10, or 30 μg | Temporomandibular joint nociception | Animal (Sprague Dawley rats) | Group II mGluR agonist (APDC) and Group III mGluR agonist (L-AP4) | 30 μg WIN 55,212-2 significantly reduced scratching behavior by 75%; 3 μg WIN 55,212-2 enhanced the antinociceptive effects of APDC and L-AP4 |
| Wong et al., 2019 [46] | Intramuscular injection | 1 mg/mL and 5 mg/mL | Myofascial pain induced by nerve growth factor | Animal (female Sprague Dawley rats) | Cannabinol, Cannabichromene | CBD (5 mg/mL) significantly reduced NGF-induced mechanical sensitization. CBD/CBN combinations provided longer-lasting analgesia than either compound alone |
| Zubrzycki et al., 2017 [47] | Intracerebroventricular (i.c.v.) perfusion | 50 nM and 100 nM | Orofacial pain induced by tooth pulp stimulation | Animal (male Long–Evans rats) | EM-2, URB597 (FAAH inhibitor), JZL195 (FAAH/MAGL dual inhibitor) | CBD significantly reduced pain response in a concentration-dependent manner. The antinociceptive effect was mediated by CB1 and μ receptors and enhanced by FAAH and FAAH/MAGL inhibitors |
| Nitecka-Buchta et al., 2019 [48] | Transdermal application | 20% CBD oil formulation | Myofascial pain in masseter muscles | Human (patients with TMDs) | None | Significant reduction in masseter muscle sEMG activity (11% right, 12.6% left). VAS pain intensity reduced by 70.2% in CBD group compared to 9.81% in placebo group |
| Ostenfeld et al., 2011 [49] | Oral administration | 100 mg and 800 mg | Acute postoperative pain after third molar extraction | Human (Patients undergoing third molar extraction) | Ibuprofen (800 mg pre-op + 400 mg post-op), Co-codamol (rescue analgesic) | GW842166 (100 mg and 800 mg) failed to provide significant analgesia compared to placebo. Ibuprofen was significantly more effective across all endpoints |
| Walczynska Dragon et al., 2024 [50] | Intraoral gel application | 10% and 5% CBD gel | Muscle-related TMDs and sleep bruxism | Human (patients with TMDs and bruxism) | None | 10% CBD gel reduced VAS pain by 57.4% and sEMG activity by 42.1%. 5% CBD gel reduced VAS pain by 40.8%. 10% CBD showed the highest bruxism index reduction (51%). Both CBD formulations were significantly more effective than placebo |
| Reference | Author and Year | Study Groups | Main Study Outcomes |
|---|---|---|---|
| [41] | Ahn et al., 2007 | Formalin Control/Saline Control Groups: Formalin injected into TMJ (5% solution) + intracisternal saline or vehicle (DMSO/saline). Saline injected into TMJ (no formalin) to confirm no nociceptive response WIN 55,212-2 Groups: Formalin injection + low or high dose of WIN 55,212-2 (e.g., 10 μg or 30 μg, intracisternally) WIN 55,212-2 + Cannabinoid Antagonists Formalin injection + WIN 55,212-2 + CB1 antagonist (AM251) Formalin injection + WIN 55,212-2 + CB2 antagonist (AM630) WIN 55,212-2 + Naloxone: Formalin injection + WIN 55,212-2 + opioid receptor antagonist naloxone WIN 55,212-2 + COX Inhibitors: Formalin injection + sub-threshold WIN 55,212-2 (10 μg) + one of the following COX inhibitors: COX Inhibitors Alone: Formalin injection + one of the COX inhibitors (SC-560, NS-398, indomethacin, or acetaminophen) (without WIN 55,212-2) Dose–Response: WIN 55,212-2 ± NS-398: Formalin injection + increasing doses of WIN 55,212-2 (1, 3, 10, 30, or 80 μg). Formalin injection + NS-398 (COX-2 inhibitor) + same doses of WIN 55,212-2 | Central activation of cannabinoid receptor CB1 significantly reduces nociception associated with TMJ inflammation, and blockade of central COX pathways, particularly COX-2 and putative COX-3, enhances this antinociceptive effect. Intracisternal administration of WIN 55,212-2, a non-selective CB1/2 agonist, effectively reduced formalin-induced TMJ pain in rats, with its analgesic effect blocked by CB1 antagonist AM251 but not by CB2 antagonist AM630, indicating that CB1 activation primarily mediates this effect. Furthermore, low doses of COX inhibitors alone did not alter nociception but significantly potentiated the effect of a sub-effective dose of WIN 55,212-2, suggesting that inhibition of central COX pathways augments cannabinoid-induced analgesia. Importantly, the study found no involvement of opioid pathways in this mechanism, as pretreatment with naloxone did not reverse cannabinoid-induced antinociception. These findings suggest that a combined administration of cannabinoids and COX inhibitors could offer a promising therapeutic strategy for managing inflammatory TMJ pain. |
| [42] | Brice-Tutt et al., 2025 | Vehicle (Control): Rats received an oral gavage of the Cremophor–ethanol–water vehicle solution (no active drug). CBD Alone: Rats received cannabidiol 25 mg/kg by oral gavage. OXY Alone: Rats received oxycodone 1.4 mg/kg by oral gavage. CBD + OXY Combination: Rats received cannabidiol (25 mg/kg) and oxycodone (1.4 mg/kg) co-administered in a single oral gavage. | CBD, when co-administered with OXY, enhances opioid-induced analgesia in an orofacial thermal pain model using rats. This study is notable for its use of an oral administration route, aligning more closely with clinical applications. The results demonstrated that the combination of CBD and OXY provided greater pain relief than OXY alone, suggesting a potential role for CBD in reducing opioid dosages required for effective pain management. Additionally, the study observed that CBD alone exhibited analgesic-like properties, reinforcing prior preclinical findings. However, while preclinical data largely support the analgesic and opioid-sparing effects of CBD, clinical evidence remains inconsistent, highlighting the need for further research to confirm these benefits in human populations. |
| [43] | Burgos et al., 2010 | Saline Control: Rats received saline (i.p.) prior to formalin injection. Cannabinoid Vehicle: Rats received the vehicle solution for WIN 55,212-2 (i.p.) prior to formalin. CB1 Antagonist Alone: Rats received SR141716A (CB1 antagonist) (i.p.) prior to formalin. CB2 Antagonist Alone: Rats received SR144528 (CB2 antagonist) (i.p.) prior to formalin. WIN 55,212-2: Rats received the cannabinoid agonist WIN 55,212-2 (0.5 or 1 mg/kg i.p.) prior to formalin. WIN 55,212-2 + CB1 or CB2 Antagonist: Rats received SR141716A or SR144528 (i.p.) 30 min before WIN 55,212-2, then formalin. Morphine: Rats received morphine (2, 5, or 10 mg/kg i.p.) prior to formalin. Morphine + Naloxone: Rats received naloxone (1 or 2 mg/kg i.p.) before morphine (5 or 10 mg/kg i.p.), then formalin. Indomethacin: Rats received indomethacin (2.5 or 5 mg/kg i.p.) prior to formalin. Ketamine: Rats received ketamine (25 or 50 mg/kg i.p.) prior to formalin. | WIN 55,212-2 significantly reduced nociceptive behavioral responses in inflammatory models of orofacial pain, including the TMJ formalin test, at doses of 0.5 and 1 mg/kg. The study found that this antinociceptive effect was mediated primarily by the CB1 receptor, as its action was blocked by the CB1-selective antagonist SR141716A but not by the CB2 antagonist SR144528. Additionally, WIN was as effective as morphine (10 mg/kg) and more effective than indomethacin and ketamine in attenuating inflammatory pain. Importantly, WIN-induced analgesia did not result in significant motor impairment, suggesting a strong therapeutic potential for cannabinoid-based treatments in managing upper-quarter inflammatory pain conditions. |
| [44] | Laks et al., 2023 | Sham + Vehicle Underwent the sham procedure (no tooth pulp exposure) Treated systemically with a vehicle control solution Drilled (Pulp-Exposed) + Vehicle Underwent coronal pulpotomy in the left mandibular first molar (to create pulpitis). Treated systemically with the same vehicle control solutionDrilled (Pulp-Exposed) + CBDUnderwent coronal pulpotomy in the left mandibular first molar Treated systemically with 5 mg/kg of CBDDrilled (Pulp-Exposed) + β-Caryophyllene Underwent coronal pulpotomy in the left mandibular first molar Treated systemically with 30 mg/kg of β-CP | In this rat model of pulpitis, CBD at 5 mg/kg did not robustly reduce orofacial mechanical allodynia compared with vehicle treatment, but it did significantly decrease the expression of the microglial/macrophage marker AIF-1 in the ipsilateral trigeminal ganglion, suggesting a measurable anti-inflammatory effect. Although this reduction in AIF-1 did not translate into substantial behavioral analgesia—particularly when compared with β-caryophyllene, which more strongly attenuated both allodynia and inflammatory markers—the findings indicate that CBD can modulate aspects of neuroinflammation linked to dental orofacial pain, and therefore it warrants further investigation for its therapeutic potential in upper-quarter (orofacial) disorders. |
| [45] | Lee et al., 2008 | Vehicle Control: Received the vehicle (e.g., saline or cyclodextrin solution) rather than active drugs Cannabinoid Agonist (WIN 55,212-2): Various doses (3, 10, or 30 μg) administered alone to assess dose-dependent effects on formalin-induced nociception Group II mGluR Agonist (APDC): Administered alone (multiple doses) to test antinociceptive effects against TMJ formalin injection Group III mGluR Agonist (L-AP4): Administered alone (multiple doses) similarly to assess formalin-induced nociception Combined Sub-Analgesic Cannabinoid + mGluR Agonist: Sub-threshold dose of WIN 55,212-2 (3 μg) given along with a sub-analgesic dose of APDC or L-AP4 to examine additive or synergistic antinociceptive effectsmGluR Antagonists: LY341495 (Group II antagonist) or CPPG (Group III antagonist) given before APDC or L-AP4, respectively, to confirm receptor-specific involvement | Activating central cannabinoid receptors markedly reduced inflammation-induced nociception in the TMJ and further revealed that sub-analgesic (i.e., low, non-impairing) doses of a cannabinoid agonist significantly enhanced the pain-relieving effects of metabotropic glutamate receptor (mGluR) agonists from groups II and III. Specifically, when the cannabinoid WIN 55,212-2 was administered together with mGluR agonists APDC (group II) or L-AP4 (group III) in a rodent model of TMJ pain, it both lowered the effective dose needed for relief and potentiated overall antinociceptive outcomes, without producing notable motor dysfunction. These findings underscore the therapeutic promise of combining cannabinoids with mGluR-based interventions for managing inflammatory pain in the TMJ—an approach that might extend to other upper-quarter disorders—by achieving strong analgesic effects at lower cannabinoid doses, thereby minimizing unwanted side effects. |
| [46] | Wong et al., 2019 | Vehicle only (4% Tween 80 in saline) CBD at 1 mg/mL CBD at 5 mg/mL CBN at 1 mg/mL CBC at 1 mg/mL CBD + CBN (1:1) (1 mg/mL CBD + 1 mg/mL CBN) CBD + CBN (5:1) (5 mg/mL CBD + 1 mg/mL CBN) | In this rat model of NGF-induced masseter muscle pain, higher-dose cannabidiol (CBD at 5 mg/mL), as well as CBD alone and CBD/CBN combinations, effectively reversed mechanical sensitization without causing motor impairment or other central adverse effects. While CBC alone was not effective, combining CBD and CBN produced longer-lasting analgesia compared to either compound alone. These results suggest that non-psychoactive cannabinoids, particularly CBD and CBN, can act peripherally at the muscle to reduce pain in the upper quarter (e.g., as in temporomandibular disorders or fibromyalgia) and may therefore hold therapeutic potential for chronic muscle pain disorders with minimal central side effects. |
| [47] | Zubrzycki et al., 2017 | Control- aCSF (vehicle), AEA AEA alone AEA + AM251 (CB₁ antagonist)/β-FNA (μ-antagonist) AEA + URB597 (FAAH inhibitor) AEA + JZL184 (MAGL inhibitor) AEA + JZL195 (FAAH/MAGL dual inhibitor) 2-AG 2-AG alone 2-AG + JZL184 (±AM251) EM-2 EM-2 alone EM-2 + β-FNA/AM251 EM-2 + URB597 EM-2 + AEA | In this study of orofacial pain using a rat model, the authors found that enhancing the endocannabinoid system—particularly by elevating levels of AEA through the inhibition of its degradative enzyme FAAH—produced significant antinociceptive effects. Although CBD was not directly tested, these results underscore that therapies targeting endocannabinoid signaling can suppress trigeminal nociceptive transmission in the brainstem, reduce pain responses, and work synergistically with opioid pathways. By extension, interventions that bolster or mimic the activity of endocannabinoids (including the use of phytocannabinoids like CBD) may offer promising, centrally mediated analgesic benefits for upper-quarter disorders such as orofacial pain syndromes, without some of the limitations often seen with traditional cannabinoid receptor agonists. |
| [48] | Nitecka-Buchta et al., 2019 | Group 1 (Experimental/CBD Group): Received a topical formulation containing CBD Group 2 (Control/Placebo Group): Received a placebo topical formulation (no CBD) | Topical application of CBD significantly reduced both sEMG activity and perceived pain in masseter muscles of patients with myofascial pain, a common TMD. After two weeks of applying a CBD-based formulation, masseter muscle activity decreased by over 11% on average, and patients reported a 70% reduction in pain intensity, with no adverse effects. In comparison, those receiving a placebo formulation did not show statistically meaningful changes. These findings highlight CBD’s potential as a locally applied therapy for muscle relaxation, pain relief, and improvement in masticatory function, supporting broader investigations into CBD’s benefits for upper-quarter musculoskeletal disorders. |
| [49] | Ostenfeld et al., 2011 | GW842166 (100 mg) + Placebo Received a single preoperative oral dose of GW842166 100 mg Received placebo at 4 h postoperatively GW842166 (800 mg) + Placebo Received a single preoperative oral dose of GW842166 800 mg Received placebo at 4 h postoperatively Ibuprofen Received an 800 mg ibuprofen dose preoperatively Received an additional 400 mg ibuprofen dose at 4 h postoperatively Placebo Received a placebo dose preoperatively Received a second placebo dose at 4 h postoperatively | In this randomized, double-blind, placebo-controlled study evaluating the analgesic efficacy of a selective cannabinoid receptor-2 agonist (GW842166) compared with ibuprofen or placebo in the context of acute pain after third molar extraction, the primary finding was that single oral doses of GW842166 (100 mg or 800 mg) failed to demonstrate clinically meaningful analgesia and showed little separation from the placebo across multiple pain outcomes, whereas ibuprofen produced significantly greater pain relief; moreover, no beneficial adjunctive effect emerged when GW842166 was coadministered with rescue analgesia, though the treatment was well tolerated from a safety standpoint, suggesting that under the conditions tested, the selective CB2 agonist did not yield therapeutic benefits in this acute postoperative pain model. |
| [50] | Walczynska-Dragon et al., 2024 | Group 1a—Received a 10% CBD (cannabidiol) intraoral formulation Group 1b—Received a 5% CBD intraoral formulation Group 2 (Control)—Received a placebo intraoral formulation (no CBD) | The intraoral use of CBD formulations can significantly reduce pain, muscle tension, and sleep bruxism intensity in patients with TMDs, highlighting the therapeutic value of CBD for upper-quarter musculoskeletal conditions. In this randomized, double-blind clinical trial, patients receiving 10% CBD experienced the most substantial outcomes, with a 57.4% decrease in pain, a 42.1% reduction in muscle activity, and a 51% drop in sleep bruxism episodes, surpassing the improvements seen with the 5% CBD group. In contrast, the placebo group showed no meaningful changes in these measures. Overall, the findings indicate that higher-concentration CBD formulations exert a superior myorelaxant and analgesic effect, underscoring CBD’s promise as a non-invasive therapeutic option for disorders of the upper quarter involving muscular tension and pain. |
4. Discussion
4.1. Results in the Context of Other Evidence
4.2. Limitations of the Evidence
4.3. Limitations of the Review Process
4.4. Implications for Practice, Policy, and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Bruni, N.; Pepa, C.D.; Oliaro-Bosso, S.; Pessione, E.; Dosio, F.; Ranaldi, M. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef]
- Stella, B.; Baratta, F.; Della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid formulations and delivery systems: Current and future options to treat pain. Drugs 2021, 81, 1513–1557. [Google Scholar] [CrossRef]
- McCarberg, B.H.; Barkin, R.L. The future of cannabinoids as analgesic agents: A pharmacologic, pharmacokinetic, and pharmacodynamic overview. Am. J. Ther. 2007, 14, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Green, G.B.; Razdan, R.K.; Bruni, G.; Dalton, W.S. The Cannabinoids: Pharmacological Evaluation of Synthetic and Naturally Occurring Compounds. In The Cannabinoids: Chemical, Pharmacologic, and Therapeutic Aspects; Academic Press: New York, NY, USA, 2018; pp. 89–104. [Google Scholar]
- Amato, L.; Minozzi, S.; Mitrova, Z.; Parmelli, E.; Saulle, R.; Cruciani, F.; Vecchi, S.; Davoli, M. Systematic review of safeness and therapeutic efficacy of cannabis in patients with multiple sclerosis, neuropathic pain, and oncology patients treated with chemotherapy. Epidemiol. Prev. 2017, 41, 279–293. [Google Scholar] [PubMed]
- Hill, K.P. Medical Marijuana for Treatment of Chronic Pain and Other Medical and Psychiatric Problems. JAMA 2015, 313, 2474–2483. [Google Scholar] [CrossRef]
- Shah, N.; Asuncion, R.M.D.; Hameed, S. Muscle Contraction Tension Headache. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562274/ (accessed on 11 December 2024).
- Linaker, C.H.; Walker-Bone, K. Shoulder disorders and occupation. Best. Pract. Res. Clin. Rheumatol. 2015, 29, 405–423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dionne, R.A. Pharmacologic treatments for temporomandibular disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 83, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Truelove, E. Role of oral medicine in the teaching of temporomandibular disorders and orofacial pain. J. Orofac. Pain 2002, 16, 185–190. [Google Scholar] [PubMed]
- Dworkin, R.H.; O’Connor, A.B.; Backonja, M.; Farrar, J.T.; Finnerup, N.B.; Jensen, T.S.; Kalso, E.A.; Loeser, J.D.; Miaskowski, C.; Nurmikko, T.J.; et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain 2007, 132, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Cherny, N.I. The treatment of neuropathic pain: From hubris to humility. Pain 2007, 132, 225–226. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.B.; Dworkin, R.H. Treatment of neuropathic pain: An overview of recent guidelines. Am. J. Med. 2009, 122 (Suppl. S10), S22–S32. [Google Scholar] [CrossRef] [PubMed]
- Okeson, J.P. (Ed.) Management of Temporomandibular Disorders and Occlusion, 8th ed.; Elsevier: St. Louis, MO, USA, 2020. [Google Scholar]
- Elikkottil, J.; Gupta, P.; Gupta, K. The analgesic potential of cannabinoids. J. Opioid. Manag. 2009, 5, 341–357, Erratum in J. Opioid. Manag. 2010, 6, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casey, S.L.; Atwal, N.; Vaughan, C.W. Cannabis constituent synergy in a mouse neuropathic pain model. Pain 2017, 158, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, A.J.; Navarrete, F.; Saldaña, R.; García-Gutiérrez, M.S.; Montalbán, B.; Navarro, D.; Gómez-Guijarro, F.M.; Gasparyan, A.; Murcia-Sánchez, E.; Torregrosa, A.B.; et al. Cannabinoid analgesia in postoperative pain management: From molecular mechanisms to clinical reality. Int. J. Mol. Sci. 2024, 25, 6268. [Google Scholar] [CrossRef]
- Farag, S.; Kayser, O. The Cannabis Plant: Botanical Aspects. In Handbook of Cannabis and Related Pathol-ogies: Biology, Pharmacology, Diagnosis, and Treatment; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–12. [Google Scholar]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis sativa L. Bioactive Com-pounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neu-rological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenberg, E.C.; Patra, P.H.; Whalley, B.J. Therapeutic effects of cannabinoids in animal models of seizures, epi-lepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav. 2017, 70 Pt B, 319–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greenwald, M.K.; Stitzer, M.L. Antinociceptive, Subjective, and Behavioral Effects of Opioid Analgesics in Humans. Drug Alcohol. Depend. 2002, 66, 111–124. [Google Scholar]
- Pacher, P.; Kunos, G. Modulating the endocannabinoid system in human health and disease—Successes and failures. FEBS J. 2013, 280, 1918–1943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khosropoor, S.; Alavi, M.S.; Etemad, L.; Roohbakhsh, A. Cannabidiol Goes Nuclear: The Role of PPARγ. Phytomedicine 2023, 114, 154771. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A Narrative Review of Molecular Mechanism and Therapeutic Effect of Cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef]
- Newmeyer, M.N.; Swortwood, M.J.; Barnes, A.J.; Abulseoud, O.A.; Scheidweiler, K.B.; Huestis, M.A. Free and Glucuronide Whole Blood Cannabinoids’ Pharmacokinetics after Controlled Smoked, Vaporized, and Oral Cannabis Administration in Frequent and Occasional Cannabis Users: Identification of Recent Cannabis Intake. Clin. Chem. 2016, 62, 1579–1592. [Google Scholar] [CrossRef]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef] [PubMed]
- Ujváry, I.; Hanuš, L. Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis Cannabinoid Res. 2016, 1, 90–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Voicu, V.; Brehar, F.M.; Toader, C.; Covache-Busuioc, R.A.; Corlatescu, A.D.; Bordeianu, A.; Costin, H.P.; Bratu, B.G.; Glavan, L.A.; Ciurea, A.V. Cannabinoids in medicine: Therapeutic applications and emerging opportunities in neurodegenerative diseases and cancer therapy. Biomolecules 2023, 13, 1388. [Google Scholar] [CrossRef]
- Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol. Ther. 2012, 133, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McDonagh, M.S.; Morasco, B.J.; Wagner, J.; Ahmed, A.Y.; Fu, R.; Kansagara, D.; Chou, R. Cannabis-based products for chronic pain: A systematic review. Ann. Intern. Med. 2022, 175, 1143–1153. [Google Scholar] [CrossRef]
- Mostafaei Dehnavi, M.; Ebadi, A.; Peirovi, A.; Taylor, G.; Salami, S.A. THC and CBD Fingerprinting of an Elite Cannabis Collection from Iran: Quantifying Diversity to Underpin Future Cannabis Breeding. Plants 2022, 11, 129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balant, M.; Vitales, D.; Wang, Z.; Barina, Z.; Fu, L.; Gao, T.; Garnatje, T.; Gras, A.; Hayat, M.Q.; Oganesian, M.; et al. Integrating target capture with whole genome sequencing of recent and natural history collections to explain the phylogeography of wild-growing and cultivated Cannabis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Salami, S.A.; Martinelli, F.; Giovino, A.; Bachari, A.; Arad, N.; Mantri, N. It Is Our Turn to Get Cannabis High: Put Cannabinoids in Food and Health Baskets. Molecules 2020, 25, 4036. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G.; McKenzie, J.E.; Veroniki, A.A. Chapter 10: Analysing data and undertaking meta-analyses [last updated November 2024]. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2024; Available online: https://training.cochrane.org/handbook (accessed on 1 June 2025).
- Ahn, D.K.; Choi, H.S.; Yeo, S.P.; Woo, Y.W.; Lee, M.K.; Yang, G.Y.; Jeon, H.J.; Park, J.S.; Mokha, S.S. Blockade of central cyclooxygenase (COX) pathways enhances the cannabinoid-induced antinociceptive effects on inflammatory temporomandibular joint (TMJ) nociception. Pain 2007, 132, 23–32. [Google Scholar] [CrossRef]
- Brice-Tutt, A.C.; Murphy, N.P.; Setlow, B.; Senetra, A.S.; Malphurs, W.; Caudle, R.M.; Bruijnzeel, A.W.; Febo, M.; Sharma, A.; Neubert, J.K. Cannabidiol interactions with oxycodone analgesia in an operant orofacial cutaneous thermal pain assay following oral administration in rats. Pharmacol. Biochem. Behav. 2025, 250, 173968. [Google Scholar] [CrossRef]
- Burgos, E.; Pascual, D.; Martín, M.I.; Goicoechea, C. Antinociceptive effect of the cannabinoid agonist, WIN 55,212-2, in the orofacial and temporomandibular formalin tests. Eur. J. Pain 2010, 14, 40–48. [Google Scholar] [CrossRef]
- Laks, E.Y.; Li, H.; Ward, S.J. Non-psychoactive cannabinoid modulation of nociception and inflammation associated with a rat model of pulpitis. Biomolecules 2023, 13, 846. [Google Scholar] [CrossRef]
- Lee, M.K.; Choi, B.Y.; Yang, G.Y.; Jeon, H.J.; Kyung, H.M.; Kwon, O.W.; Park, H.S.; Bae, Y.C.; Mokha, S.S.; Ahn, D.K. Low doses of cannabinoids enhance the antinociceptive effects of intracisternally-administered mGluRs groups II and III agonists in formalin-induced TMJ nociception in rats. Pain 2008, 139, 367–375. [Google Scholar] [CrossRef]
- Wong, H.; Cairns, B.E. Cannabidiol, cannabinol and their combinations act as peripheral analgesics in a rat model of myofascial pain. Arch. Oral Biol. 2019, 104, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zubrzycki, M.; Janecka, A.; Liebold, A.; Ziegler, M.; Zubrzycka, M. Effects of centrally administered endocannabinoids and opioids on orofacial pain perception in rats. Br. J. Pharmacol. 2017, 174, 3780–3789. [Google Scholar] [CrossRef]
- Nitecka-Buchta, A.; Nowak-Wachol, A.; Wachol, K.; Walczyńska-Dragon, K.; Olczyk, P.; Batoryna, O.; Kempa, W.; Baron, S. Myorelaxant effect of transdermal cannabidiol application in patients with TMD: A randomized, double-blind trial. J. Clin. Med. 2019, 8, 1886. [Google Scholar] [CrossRef] [PubMed]
- Ostenfeld, T.; Price, J.; Albanese, M.; Bullman, J.; Guillard, F.; Meyer, I.; Leeson, R.; Costantin, C.; Ziviani, L.; Nocini, P.F.; et al. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin. J. Pain 2011, 27, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Walczyńska-Dragon, K.; Kurek-Górecka, A.; Niemczyk, W.; Nowak, Z.; Baron, S.; Olczyk, P.; Nitecka-Buchta, A.; Kempa, W.M. Canna-bidiol intervention for muscular tension, pain, and sleep bruxism intensity: A randomized, double-blind clinical trial. J. Clin. Med. 2024, 13, 1417. [Google Scholar] [CrossRef]
- Votrubec, C.; Tran, P.; Lei, A.; Brunet, Z.; Bean, L.; Olsen, B.W.; Sharma, D. Cannabinoid therapeutics in orofacial pain management: A systematic review. Aust. Dent. J. 2022, 67, 314–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Côté, M.; Trudel, M.; Wang, C.; Fortin, A. Improving Quality of Life With Nabilone During Radiotherapy Treatments for Head and Neck Cancers: A Randomized Double-Blind Placebo-Controlled Trial. Ann. Otol. Rhinol. Laryngol. 2016, 125, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Raft, D.; Gregg, J.; Ghia, J.; Harris, L. Effects of intravenous tetrahydrocannabinol on experimental and surgical pain; Psychological correlates of the analgesic response. Clin. Pharmacol. Ther. 1977, 21, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Andreae, M.H.; Carter, G.M.; Shaparin, N.; Suslov, K.; Ellis, R.J.; Ware, M.A.; Abrams, D.I.; Prasad, H.; Wilsey, B.; Indyk, D.; et al. Inhaled Cannabis for Chronic Neuropathic Pain: A Meta-analysis of Individual Patient Data. J. Pain 2015, 16, 1221–1232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mücke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Häuser, W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2018, 3, CD012182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aviram, J.; Samuelly-Leichtag, G. Efficacy of Cannabis-Based Medicines for Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Physician 2017, 20, E755–E796. [Google Scholar] [CrossRef] [PubMed]
- Jeddi, H.M.; Busse, J.W.; Sadeghirad, B.; Levine, M.; Zoratti, M.J.; Wang, L.; Noori, A.; Couban, R.J.; Tarride, J.E. Cannabis for medical use versus opioids for chronic non-cancer pain: A systematic review and network meta-analysis of randomised clinical trials. BMJ Open 2024, 14, e068182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deshpande, A.; Mailis-Gagnon, A.; Zoheiry, N.; Lakha, S.F. Efficacy and adverse effects of medical marijuana for chronic noncancer pain: Systematic review of randomized controlled trials. Can. Fam. Physician 2015, 61, e372–e381. [Google Scholar] [PubMed] [PubMed Central]
- Boyd, A.; Bleakley, C.; Hurley, D.A.; Gill, C.; Hannon-Fletcher, M.; Bell, P.; McDonough, S. Herbal medicinal products or preparations for neuropathic pain. Cochrane Database Syst Rev. 2019, 4, CD010528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nitecka-Buchta, A.; Walczynska-Dragon, K.; Kempa, W.M.; Baron, S. Platelet-Rich Plasma Intramuscular Injections—Antinociceptive Therapy in Myofascial Pain Within Masseter Muscles in Temporomandibular Disorders Patients: A Pilot Study. Front. Neurol. 2019, 10, 250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lynch, M.E.; Ware, M.A. Cannabinoids for the treatment of chronic non-cancer pain: An updated systematic review of randomized controlled trials. J. Neuroimmune Pharmacol. 2015, 10, 293–301. [Google Scholar] [CrossRef]
- Ware, M.A.; Doyle, C.R.; Woods, R.; Lynch, M.E.; Clark, A.J. Cannabis use for chronic non-cancer pain: Results of a prospective survey. Pain 2003, 102, 211–216. [Google Scholar] [CrossRef]
- Turcotte, D.; Doupe, M.; Torabi, M.; Gomori, A.; Ethans, K.; Esfahani, F. Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: A randomized controlled trial. Pain Med. 2014, 16, 149–159. [Google Scholar] [CrossRef]
- Toth, C.; Mawani, S.; Brady, S.; Chan, C.; Liu, C.; Mehina, E.; Garven, A. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain 2012, 153, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Pini, L.A.; Guerzoni, S.; Cainazzo, M.M.; Ferrari, A.; Sarchielli, P.; Tiraferri, I.; Ciccarese, M.; Zappaterra, M. Nabilone for the treatment of medication overuse headache: Results of a preliminary double-blind, active-controlled, randomized trial. J. Headache Pain 2012, 13, 677–684. [Google Scholar] [CrossRef]
- Moulin, D.; Clark, A.J.; Speechly, M.; Morley-Forster, P. Chronic pain in Canada: Prevalence, treatment, impact and the role of opioid analgesia. Pain Res. Manag. 2002, 7, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.E.; Campbell, F. Cannabinoids for treatment of chronic non-cancer pain: A systematic review of randomized controlled trials. Br. J. Clin. Pharmacol. 2011, 72, 735–744. [Google Scholar] [CrossRef]
- Kahan, M.; Srivastava, A.; Spithoff, S.; Bromley, L. Prescribing smoked cannabis for chronic noncancer pain: Preliminary recommendations. Can. Fam. Physician 2014, 60, 1083–1090. [Google Scholar] [PubMed]
- Häuser, W.; Welsch, P.; Radbruch, L.; Fisher, E.; Bell, R.F.; Moore, R.A. Cannabis-based medicines and medical cannabis for adults with cancer pain. Cochrane Database Syst. Rev. 2023, 6, CD014915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hauser, W.; Fitzcharles, M.A. The Perils of Overestimating the Efficacy of Cannabis-Based Medicines for Chronic Pain Management. Pain Physician 2018, 21, E79–E80. [Google Scholar] [CrossRef] [PubMed]
- Petzke, F.; Enax-Krumova, E.K.; Häuser, W. Wirksamkeit, Verträglichkeit und Sicherheit von Cannabinoiden bei neuropathischen Schmerzsyndromen: Eine systematische Übersichtsarbeit von randomisierten, kontrollierten Studien [Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: A systematic review of randomized controlled studies]. Der Schmerz 2016, 30, 62–88, Erratum in Der Schmerz 2017, 31, 619. [Google Scholar] [CrossRef] [PubMed]
- Aviram, J.; Samuelly-Leichtag, G. The Perils of Overestimating the Efficacy of Cannabis-Based Medicines for Chronic Pain Management. Pain Physician 2018, 21, E81–E83. [Google Scholar] [CrossRef] [PubMed]
- Petzke, F.; Tölle, T.; Fitzcharles, M.A.; Häuser, W. Cannabis-Based Medicines and Medical Cannabis for Chronic Neuropathic Pain. CNS Drugs 2022, 36, 31–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Häuser, W.; Petzke, F.; Fitzcharles, M.A. Efficacy, tolerability and safety of cannabis-based medicines for chronic pain management—An overview of systematic reviews. Eur. J. Pain 2018, 22, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.M.; Finley, C.R.; Ton, J.; Perry, D.; Ramji, J.; Crawford, K.; Lindblad, A.J.; Korownyk, C.; Kolber, M.R. Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity, and harms. Can. Fam. Physician 2018, 64, e78–e94. [Google Scholar] [PubMed] [PubMed Central]
- Grant, K.S.; Conover, E.; Chambers, C.D. Update on the developmental consequences of cannabis use during pregnancy and lactation. Birth Defects Res. 2020, 112, 1126–1138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vinette, B.; Côté, J.; El-Akhras, A.; Mrad, H.; Chicoine, G.; Bilodeau, K. Routes of administration, reasons for use, and approved indications of medical cannabis in oncology: A scoping review. BMC Cancer 2022, 22, 319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, S.S.; Wilens, T.E. Medical Cannabinoids in Children and Adolescents: A Systematic Review. Pediatrics 2017, 140, e20171818. [Google Scholar] [CrossRef] [PubMed]
| Source | Search Term | Number of Results |
|---|---|---|
| PubMed | ((Cannabidiol [MeSH Terms] OR CBD[TIAB] OR “cannabidiol”[TIAB] OR “medical cannabis”[TIAB] OR “Medical Marijuana”[MeSH Terms] OR “cannabinoids”[MeSH Terms] OR “cannabinoid”[TIAB]) AND (Temporomandibular Joint Disorders[MeSH Terms] OR TMD[TIAB] OR “temporomandibular disorder”[TIAB] OR “TMJ disorder”[TIAB] OR “orofacial pain”[TIAB] OR “Facial Pain”[MeSH Terms] OR “jaw pain”[TIAB] OR “myofascial pain”[TIAB] OR “Myofascial Pain Syndromes”[MeSH Terms])) | 35 |
| Embase | (‘cannabidiol’/exp OR ‘cbd’:ti,ab OR ‘cannabidiol’:ti,ab OR ‘medical cannabis’:ti,ab OR ‘medical marijuana’/exp OR ‘medical marijuana’:ti,ab OR ‘cannabinoids’/exp OR ‘cannabinoid’:ti,ab) AND (‘temporomandibular joint disorder’/exp OR ‘tmd’:ti,ab OR ‘temporomandibular disorder’:ti,ab OR ‘tmj disorder’:ti,ab OR ‘orofacial pain’:ti,ab OR ‘facial pain’/exp OR ‘facial pain’:ti,ab OR ‘jaw pain’:ti,ab OR ‘myofascial pain’:ti,ab OR ‘myofascial pain syndromes’/exp OR ‘myofascial pain syndromes’:ti,ab) | 146 |
| Scopus | (TITLE-ABS-KEY(cannabidiol) OR TITLE-ABS-KEY(CBD) OR TITLE-ABS-KEY(“medical cannabis”) OR TITLE-ABS-KEY(“medical marijuana”) OR TITLE-ABS-KEY(cannabinoids) OR TITLE-ABS-KEY(cannabinoid)) AND (TITLE-ABS-KEY(“Temporomandibular Joint Disorders”) OR TITLE-ABS-KEY(TMD) OR TITLE-ABS-KEY(“temporomandibular disorder”) OR TITLE-ABS-KEY(“TMJ disorder”) OR TITLE-ABS-KEY(“orofacial pain”) OR TITLE-ABS-KEY(“Facial Pain”) OR TITLE-ABS-KEY(“jaw pain”) OR TITLE-ABS-KEY(“myofascial pain”) OR TITLE-ABS-KEY(“Myofascial Pain Syndromes”)) | 223 |
| Cochrane | ((MH “Cannabidiol” OR “Cannabidiol”) OR (MH “CBD” OR “CBD”) OR (MH “Medical Cannabis” OR “Medical Cannabis”) OR (MH “Medical Marijuana” OR “Medical Marijuana”) OR (MH “Cannabinoids” OR “Cannabinoids”) OR (MH “Cannabinoid” OR “Cannabinoid”)) AND ((MH “Temporomandibular Joint Disorders” OR “Temporomandibular Joint Disorders”) OR (MH “TMD” OR “TMD”) OR (MH “Temporomandibular Disorder” OR “Temporomandibular Disorder”) OR (MH “TMJ Disorder” OR “TMJ Disorder”) OR (MH “Orofacial Pain” OR “Orofacial Pain”) OR (MH “Facial Pain” OR “Facial Pain”) OR (MH “Jaw Pain” OR “Jaw Pain”) OR (MH “Myofascial Pain” OR “Myofascial Pain”) OR (MH “Myofascial Pain Syndromes” OR “Myofascial Pain Syndromes”)) | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walczyńska-Dragon, K.; Fiegler-Rudol, J.; Nitecka-Buchta, A.; Baron, S. Cannabidiol for Orofacial and Upper-Quarter Pain: A Systematic Evaluation of Therapeutic Potential. J. Clin. Med. 2025, 14, 4186. https://doi.org/10.3390/jcm14124186
Walczyńska-Dragon K, Fiegler-Rudol J, Nitecka-Buchta A, Baron S. Cannabidiol for Orofacial and Upper-Quarter Pain: A Systematic Evaluation of Therapeutic Potential. Journal of Clinical Medicine. 2025; 14(12):4186. https://doi.org/10.3390/jcm14124186
Chicago/Turabian StyleWalczyńska-Dragon, Karolina, Jakub Fiegler-Rudol, Aleksandra Nitecka-Buchta, and Stefan Baron. 2025. "Cannabidiol for Orofacial and Upper-Quarter Pain: A Systematic Evaluation of Therapeutic Potential" Journal of Clinical Medicine 14, no. 12: 4186. https://doi.org/10.3390/jcm14124186
APA StyleWalczyńska-Dragon, K., Fiegler-Rudol, J., Nitecka-Buchta, A., & Baron, S. (2025). Cannabidiol for Orofacial and Upper-Quarter Pain: A Systematic Evaluation of Therapeutic Potential. Journal of Clinical Medicine, 14(12), 4186. https://doi.org/10.3390/jcm14124186







