Efficacy of Mycophenolate Mofetil in Treating Skin Fibrosis in Systemic Sclerosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria and Search Strategy
- Population: Adult patients (≥18 years) diagnosed with SSc according to the 1980 American College of Rheumatology (ACR) or 2013 ACR/EULAR classification criteria.
- Intervention: Treatment with mycophenolate mofetil (MMF), either as monotherapy or in combination with other agents, provided that the effect of MMF on skin fibrosis could be separately evaluated. Studies were excluded if MMF was co-administered with other immunosuppressants and no stratified outcome data were available.
- Comparator: Studies with or without a comparator group were eligible, including randomized controlled trials (RCTs), prospective or retrospective cohort studies, and case series (≥10 patients).
- Outcomes: Quantitative assessment of skin fibrosis using the modified Rodnan skin score (mRSS) at baseline and follow-up.
- Study design: RCTs, cohort studies, and case series with ≥10 participants.
- Language: English or Japanese peer-reviewed publications.
- Timeframe: Studies published from 1 January 2000 to 1 April 2025.
2.3. Search Strategy
2.4. Data Extraction and Management
- Study characteristics: author, year, country, study design.
- Patient characteristics: sample size, SSc subtype, disease duration.
- Intervention details: MMF dose, treatment duration, concomitant therapies.
- Outcome data: baseline and follow-up mRSS values, mean changes, standard deviations (or confidence intervals).
- Adverse events and treatment discontinuation rates.
2.5. Risk of Bias Assessment
2.6. Outcomes and Data Synthesis
3. Results
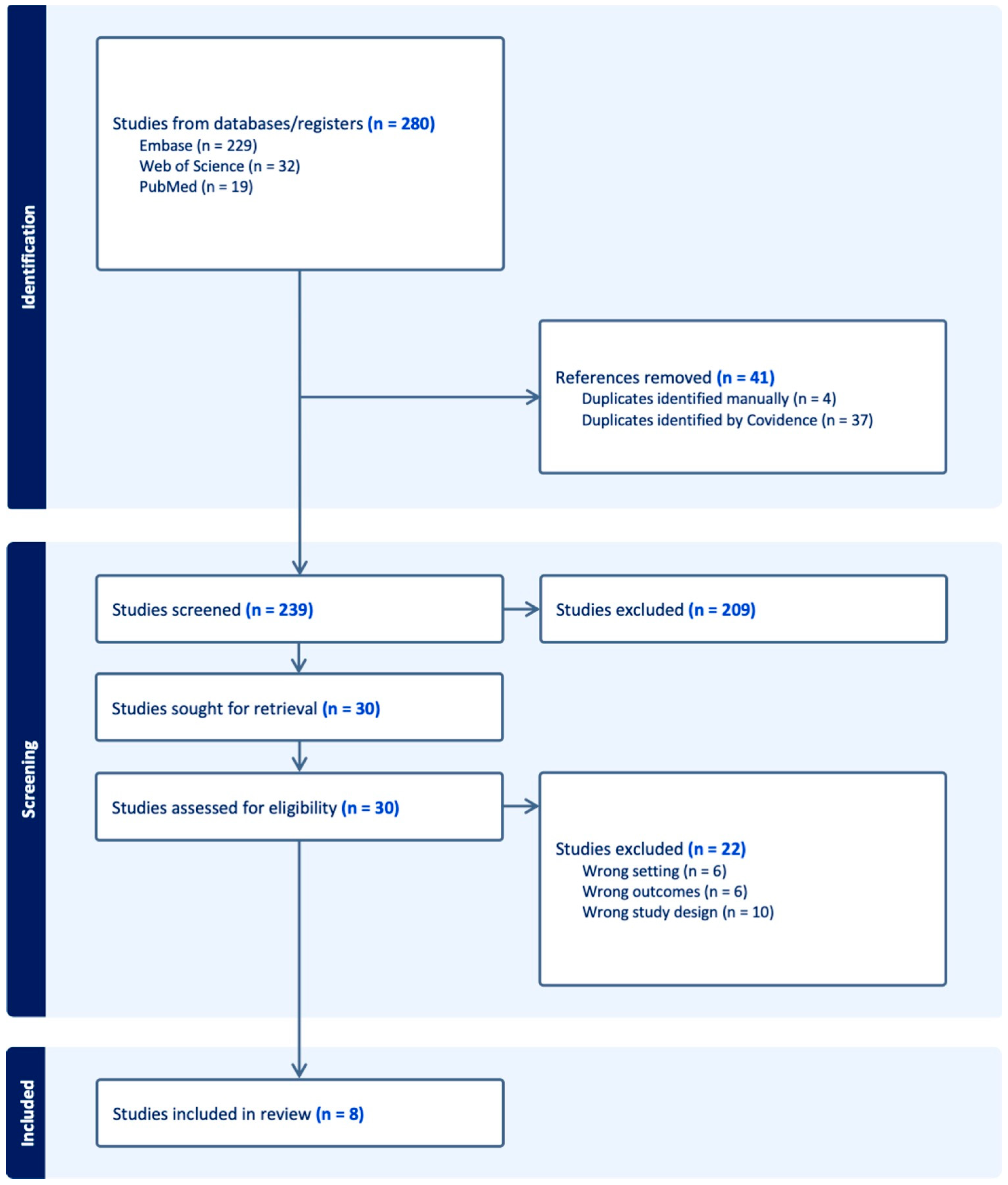
3.1. Study Selection: PRISMA Flow Diagram
3.2. Study Characteristics
3.3. Patient Characteristics
3.4. Intervention Details
3.5. Outcome Data
3.6. Adverse Events and Treatment Discontinuation
3.7. Risk of Bias Within Studies
3.7.1. Randomized Controlled Trials
3.7.2. Post Hoc and Comparative Analyses
3.7.3. Observational Studies
3.7.4. Overall Assessment
3.8. Results of Individual Studies and Synthesis
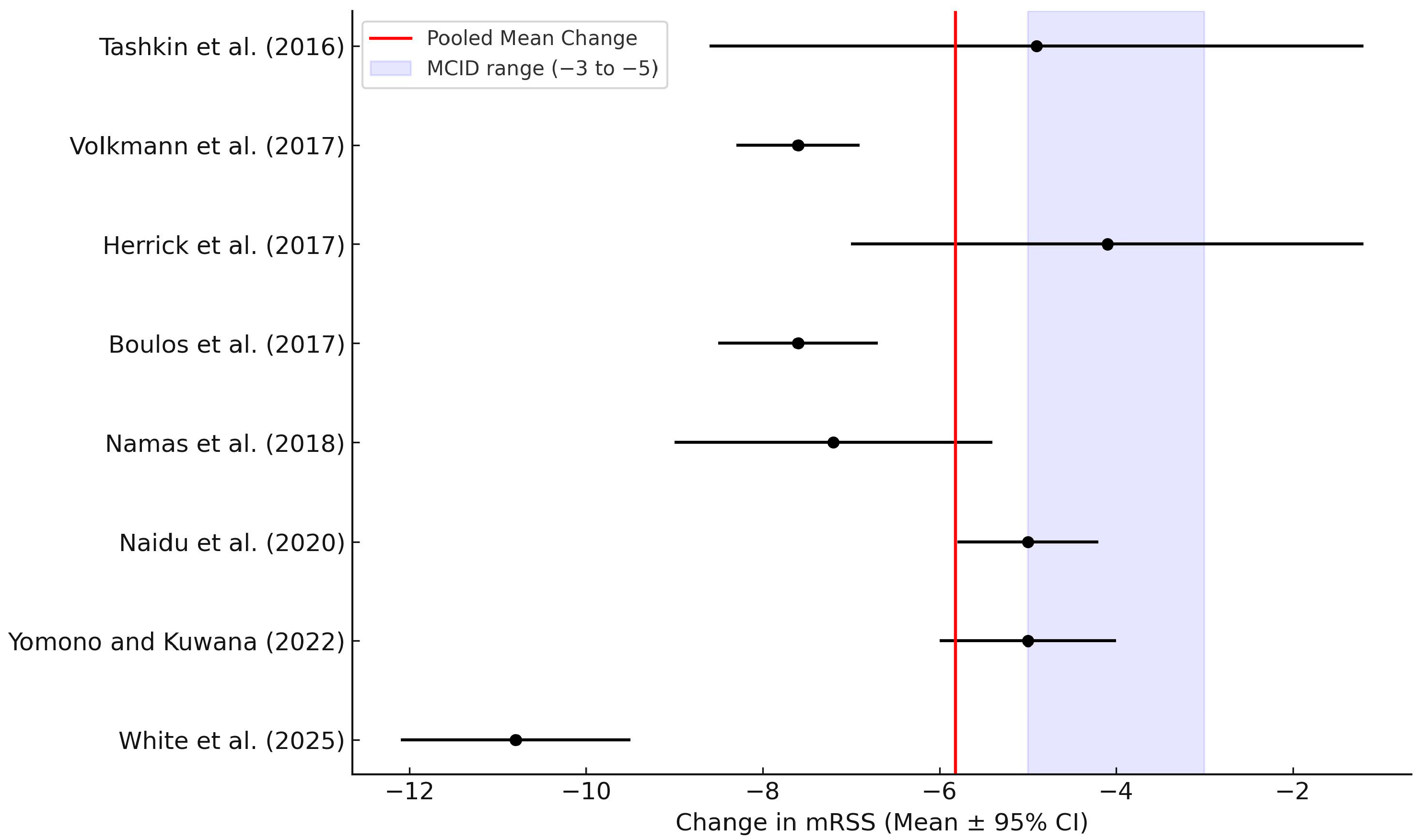
3.9. Meta-Analysis
4. Discussion
4.1. Summary of Main Findings
4.2. Comparison with the Previous Literature
4.3. Clinical Implications
4.4. Limitations
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SSc | Systemic sclerosis |
| MMF | Mycophenolate mofetil |
| mRSS | Modified Rodnan skin score |
| ILD | Interstitial lung disease |
| dcSSc | Diffuse cutaneous systemic sclerosis |
| RCT | Randomized controlled trial |
| AE | Adverse event |
| MCID | Minimal clinically important difference |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| CI | Confidence interval |
| SD | Standard deviation |
| CYC | Cyclophosphamide |
| ACR | American College of Rheumatology |
| EULAR | European League Against Rheumatism |
| ROBINS-I | Risk Of Bias In Non-randomized Studies of Interventions |
References
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic sclerosis. Lancet 2023, 401, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Lazzaroni, M.-G.; Piantoni, S.; Angeli, F.; Bertocchi, S.; Franceschini, F.; Airò, P. A Narrative Review of Pathogenetic and Histopathologic Aspects, Epidemiology, Classification Systems, and Disease Outcome Measures in Systemic Sclerosis. Clin. Rev. Allergy Immunol. 2023, 64, 358–377. [Google Scholar] [CrossRef]
- Vacchi, C.; Sebastiani, M.; Cassone, G.; Cerri, S.; Della Casa, G.; Salvarani, C.; Manfredi, A. Therapeutic Options for the Treatment of Interstitial Lung Disease Related to Connective Tissue Diseases. A Narrat. Rev. J. Clin. Med. 2020, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Son, H.-H.; Moon, S.-J. Pathogenesis of systemic sclerosis: An integrative review of recent advances. J. Rheum Dis. 2025, 32, 89–104. [Google Scholar] [CrossRef]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef]
- Pope, J.E.; Denton, C.P.; Johnson, S.R.; Fernandez-Codina, A.; Hudson, M.; Nevskaya, T. State-of-the-art evidence in the treatment of systemic sclerosis. Nat. Rev. Rheumatol. 2023, 19, 212–226. [Google Scholar] [CrossRef]
- Takada, T.; Aoki, A.; Shima, K.; Kikuchi, T. Advancements in the treatment of interstitial lung disease in systemic sclerosis with the approval of mycophenolate mofetil. Respir. Investig. 2024, 62, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Roth, M.D.; Clements, P.J.; Furst, D.E.; Khanna, D.; Kleerup, E.C.; Goldin, J.; Arriola, E.; Volkmann, E.R.; Kafaja, S.; et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): A randomised controlled, double-blind, parallel group trial. Lancet Respir. Med. 2016, 4, 708–719. [Google Scholar] [CrossRef]
- Luo, X.; Deng, Q.; Xue, Y.; Zhang, T.; Wu, Z.; Peng, H.; Xuan, L.; Pan, G. Anti-Fibrosis Effects of Magnesium Lithospermate B in Experimental Pulmonary Fibrosis: By Inhibiting TGF-βRI/Smad Signaling. Molecules 2021, 26, 1715. [Google Scholar] [CrossRef]
- Shihab, F.S.; Bennett, W.M.; Yi, H.; Choi, S.O.; Andoh, T.F. Mycophenolate mofetil ameliorates arteriolopathy and decreases transforming growth factor-beta1 in chronic cyclosporine nephrotoxicity. Am. J. Transplant. 2003, 3, 1550–1559. [Google Scholar] [CrossRef]
- Morris, E.A.; Parvizi, R.; Orzechowski, N.M.; Whitfield, M.L.; Pioli, P.A. Mycophenolate mofetil directly modulates myeloid viability and pro-fibrotic activation of human macrophages. Rheumatology 2025, 64, 3125–3133. [Google Scholar] [CrossRef]
- Van den Bosch, L.; Luppi, F.; Ferrara, G.; Mura, M. Immunomodulatory treatment of interstitial lung disease. Ther. Adv. Respir. Dis. 2022, 16, 17534666221117002. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Tashkin, D.P.; Li, N.; Roth, M.D.; Khanna, D.; Hoffmann-Vold, A.-M.; Goldin, J.; Clements, P.J.; Furst, D.E.; Elashoff, R.M.; et al. Mycophenolate Mofetil Versus Placebo for Systemic Sclerosis-Related Interstitial Lung Disease: An Analysis of Scleroderma Lung Studies I and II. Arthritis Rheumatol. 2017, 69, 1451–1460. [Google Scholar] [CrossRef]
- Herrick, A.L.; Pan, X.; Peytrignet, S.; Lunt, M.; Hesselstrand, R.; Mouthon, L.; Silman, A.; Brown, E.; Czirják, L.; Distler, J.H.W.; et al. Treatment outcome in early diffuse cutaneous systemic sclerosis: The European Scleroderma Observational Study (ESOS). Ann. Rheum Dis. 2017, 76, 1207–1218. [Google Scholar] [CrossRef]
- Boulos, D.; Ngian, G.; Rajadurai, A.; Elford, K.; Stevens, W.; Proudman, S.; Owen, C.; Roddy, J.; Nikpour, M.; Youssef, P.; et al. Long-term efficacy and tolerability of mycophenolate mofetil therapy in diffuse scleroderma skin disease. Int. J. Rheum Dis. 2017, 20, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Namas, R.; Tashkin, D.P.; Furst, D.E.; Wilhalme, H.; Tseng, C.; Roth, M.D.; Kafaja, S.; Volkmann, E.; Clements, P.J.; Khanna, D.; et al. Efficacy of Mycophenolate Mofetil and Oral Cyclophosphamide on Skin Thickness: Post Hoc Analyses from Two Randomized Placebo-Controlled Trials. Arthritis Care Res. 2018, 70, 439–444. [Google Scholar] [CrossRef]
- Naidu, G.S.R.S.N.K.; Sharma, S.K.; Adarsh, M.B.; Dhir, V.; Sinha, A.; Dhooria, S.; Jain, S. Effect of mycophenolate mofetil (MMF) on systemic sclerosis-related interstitial lung disease with mildly impaired lung function: A double-blind, placebo-controlled, randomized trial. Rheumatol. Int. 2020, 40, 207–216. [Google Scholar] [CrossRef]
- Yomono, K.; Kuwana, M. Outcomes in patients with systemic sclerosis undergoing early vs delayed intervention with potential disease-modifying therapies. Rheumatology 2022, 61, 3677–3685. [Google Scholar] [CrossRef]
- White, B.; Furst, D.E.; Frech, T.M.; Kuwana, M.; Hummers, L.; Stevens, W.; Kafaja, S.; Lee, E.B.; Distler, O.; Khanna, D.; et al. Comparative Efficacy of Immunosuppressive Therapies in the Treatment of Diffuse Cutaneous Systemic Sclerosis. ACR Open Rheumatol. 2025, 7, e70004. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Vold, A.-M.; Allanore, Y.; Bendstrup, E.; Bruni, C.; Distler, O.; Maher, T.M.; Wijsenbeek, M.; Kreuter, M. The need for a holistic approach for SSc-ILD-achievements and ambiguity in a devastating disease. Respir. Res. 2020, 21, 197. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Can, M.; Kocakaya, D.; Karakurt, S.; Yavuz, S. Two-year experience with mycophenolate mofetil in patients with scleroderma lung disease: A case series. Int. J. Rheum. Dis. 2014, 17, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Mihai, C.; Dobrota, R.; Assassi, S.; Mayes, M.D.; Distler, O. Enrichment Strategy for Systemic Sclerosis Clinical Trials Targeting Skin Fibrosis: A Prospective, Multiethnic Cohort Study. ACR Open Rheumatol. 2020, 2, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P. Challenges in systemic sclerosis trial design. Semin. Arthritis Rheum. 2019, 49, S3–S7. [Google Scholar] [CrossRef]
- Herrick, A.L.; Assassi, S.; Denton, C.P. Skin involvement in early diffuse cutaneous systemic sclerosis: An unmet clinical need. Nat. Rev. Rheumatol. 2022, 18, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, F.A.; Mansoor, M.; Jimenez, S.A. Treatment of Rapidly Progressive Systemic Sclerosis: Current and Futures Perspectives. Expert Opin. Orphan Drugs 2016, 4, 31–47. [Google Scholar] [CrossRef]
- Allanore, Y.; Matucci-Cerinic, M.; Distler, O. Treatment of systemic sclerosis: Is there any hope for the future? RMD Open 2016, 2, e000260. [Google Scholar] [CrossRef]
- Boleto, G.; Avouac, J.; Allanore, Y. The role of antifibrotic therapies in the treatment of systemic sclerosis–associated interstitial lung disease. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X211066686. [Google Scholar] [CrossRef]
- Black, C.M.; Stephens, C.O.; Maddison, P.J.; Belch, J.J.F. Regressive systemic sclerosis. Ann. Rheum. Dis. 1986, 45, 384–388. [Google Scholar] [CrossRef]
| Author (Year) | Design | Sample Size | SSc Subtype | MMF Dose | Treatment Duration | Baseline mRSS | Mean Disease Duration | Mean mRSS Change | Reported AE/Discontinuation |
|---|---|---|---|---|---|---|---|---|---|
| Tashkin et al. (2016) [8] | RCT | 63 | dcSSc | 2–3 g/day | 24 months | 23.1 | 4.1 years | −4.9 | Fewer AEs than CYC; good tolerability |
| Volkmann et al. (2017) [13] | Comparative analysis (SLS I vs. II) | 69 | dcSSc | 2–3 g/day | 24 months | 24.6 | 3.8 years | −4.9 (adjusted) | Not reported |
| Herrick et al. (2017) [14] | Prospective observational | 118 | Early dcSSc | Not specified | 12–24 months | 22.3 | <1 year | −4.1 | Not reported |
| Boulos et al. (2017) [15] | Prospective cohort | 42 | dcSSc | Up to 3 g/day | 60 months | 25.7 | 5.6 years | −7.6 (at 24 mo) | Well tolerated; 5% discontinued |
| Namas et al. (2018) [16] | Post hoc RCT analysis | 58 | dcSSc | 2–3 g/day | 24 months | 27.4 | 2.9 years | −7.2 | Not reported |
| Naidu et al. (2020) [17] | RCT | 20 | SSc-ILD | 2 g/day | 6 months | 20.0 | 1.5 years | −5 (median) | Well tolerated; no serious AE |
| Yomono and Kuwana (2022) [18] | Retrospective cohort | 25 | dcSSc | Not clearly defined | 12–24 months | 26.1 | 3.2 years | Approx. −5 | Not detailed |
| White et al. (2025) [19] | Post hoc analysis | 98 | Early dcSSc | Not specified | 12 months | 28.4 | <2 years | −10.8 | Low AE incidence; no unexpected safety signals |
| Study | Design | n | mRSS Change (Mean) | SD | Duration (Months) | Comments |
|---|---|---|---|---|---|---|
| Tashkin et al. (2016) [8] | RCT | 63 | −4.9 | 8.6 | 24 | SLS-II trial |
| Volkmann et al. (2017) [13] | Prospective cohort | 42 | −7.6 | 8.3 | 24 | Int J Rheum Dis |
| Herrick et al. (2017) [14] | Prospective observational | 118 | −4.1 | 7 | 12 | ESOS; SD estimated from CI |
| Boulos et al. (2017) [15] | Comparative analysis (SLS I vs. II) | 69 | −7.6 | 8.5 | 24 | Matched placebo comparison |
| Namas et al. (2018) [16] | RCT comparator | 52 | −3 | 8 | 12 | Placebo arm; used for comparative reference |
| Namas et al. (2018) [16] | Post hoc RCT | 58 | −7.2 | 9 | 24 | SLS I and II analysis; SD approximated |
| Naidu et al. (2020) [17] | RCT | 20 | −5 | 5.8 | 6 | Median reported; SD estimated |
| Yomono and Kuwana (2022) [18] | Retrospective cohort | 25 | −5 | 6 | 12 | Graph data extraction |
| White et al. (2025) [19] | Post hoc RCT | 98 | −10.8 | 9.5 | 12 | Early dcSSc subgroup; post hoc analysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohta, R.; Horinishi, Y.; Sano, C.; Ichinose, K. Efficacy of Mycophenolate Mofetil in Treating Skin Fibrosis in Systemic Sclerosis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4187. https://doi.org/10.3390/jcm14124187
Ohta R, Horinishi Y, Sano C, Ichinose K. Efficacy of Mycophenolate Mofetil in Treating Skin Fibrosis in Systemic Sclerosis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(12):4187. https://doi.org/10.3390/jcm14124187
Chicago/Turabian StyleOhta, Ryuichi, Yuta Horinishi, Chiaki Sano, and Kunihiro Ichinose. 2025. "Efficacy of Mycophenolate Mofetil in Treating Skin Fibrosis in Systemic Sclerosis: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 12: 4187. https://doi.org/10.3390/jcm14124187
APA StyleOhta, R., Horinishi, Y., Sano, C., & Ichinose, K. (2025). Efficacy of Mycophenolate Mofetil in Treating Skin Fibrosis in Systemic Sclerosis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(12), 4187. https://doi.org/10.3390/jcm14124187








