Bone Grafting with Albumin-Impregnated Bone Allograft After Odontogenic Cyst Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Production of the Bone Grafting Material
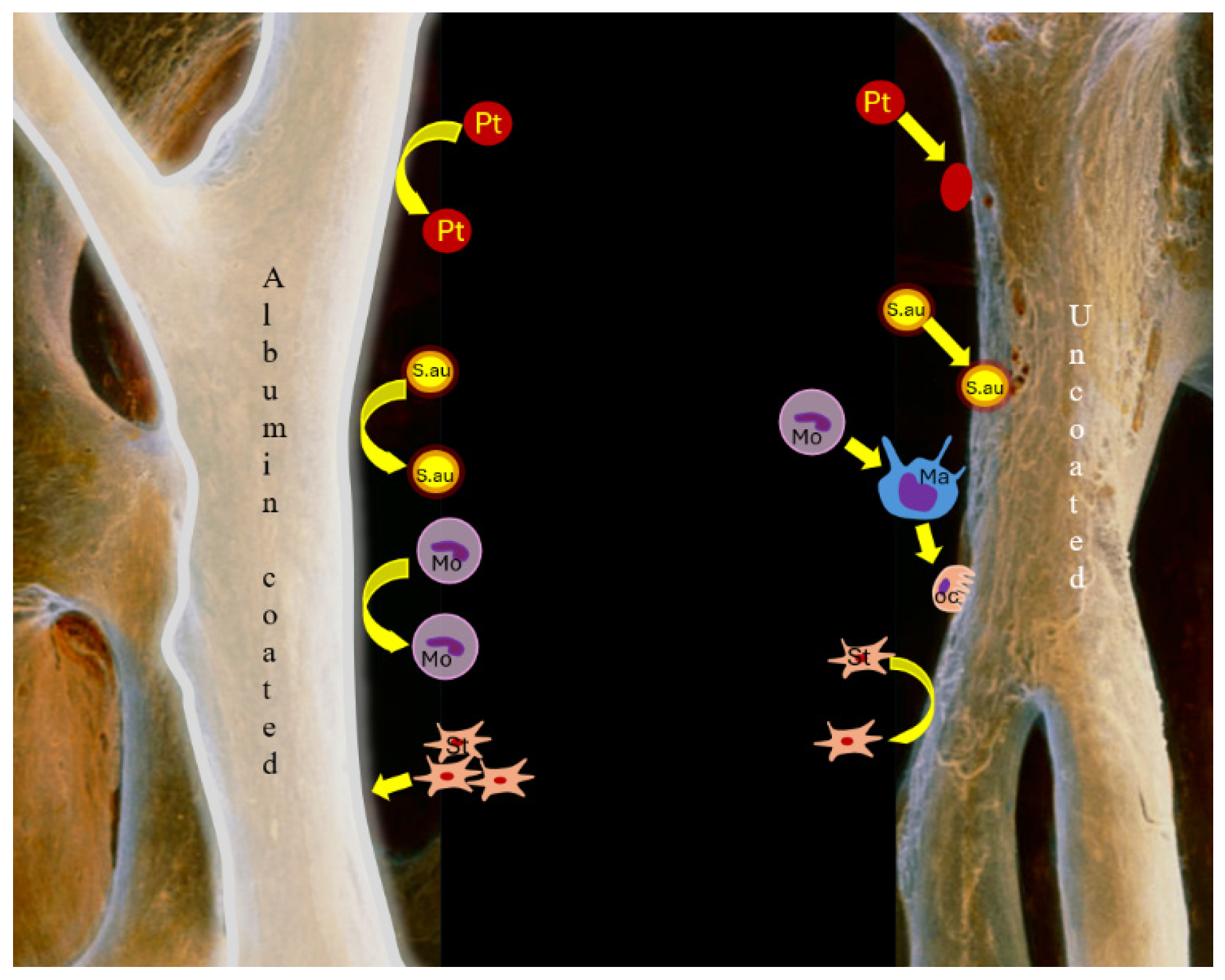
2.3. Molecular Background of Albumin Coating
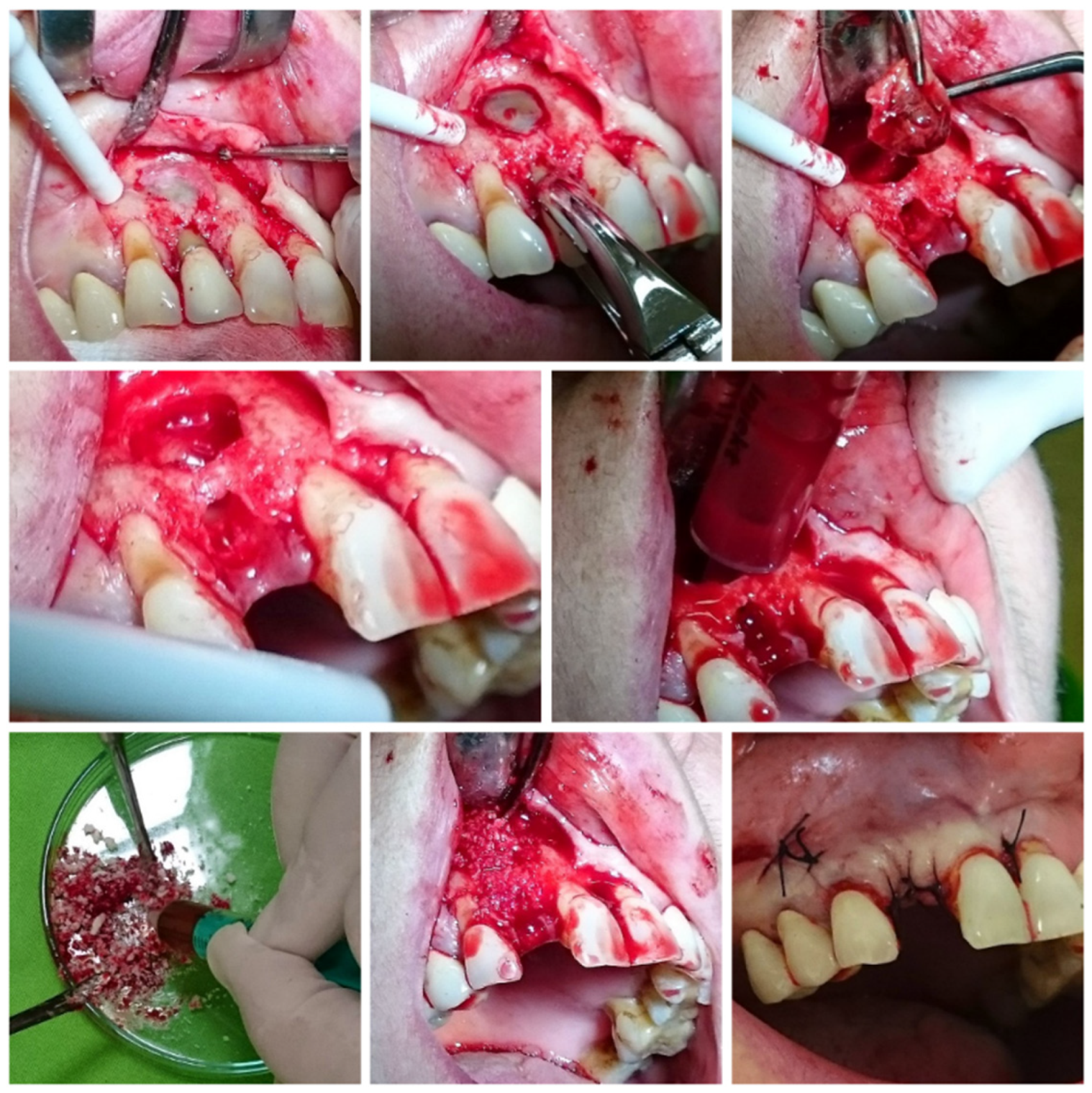
2.4. Surgical Protocol
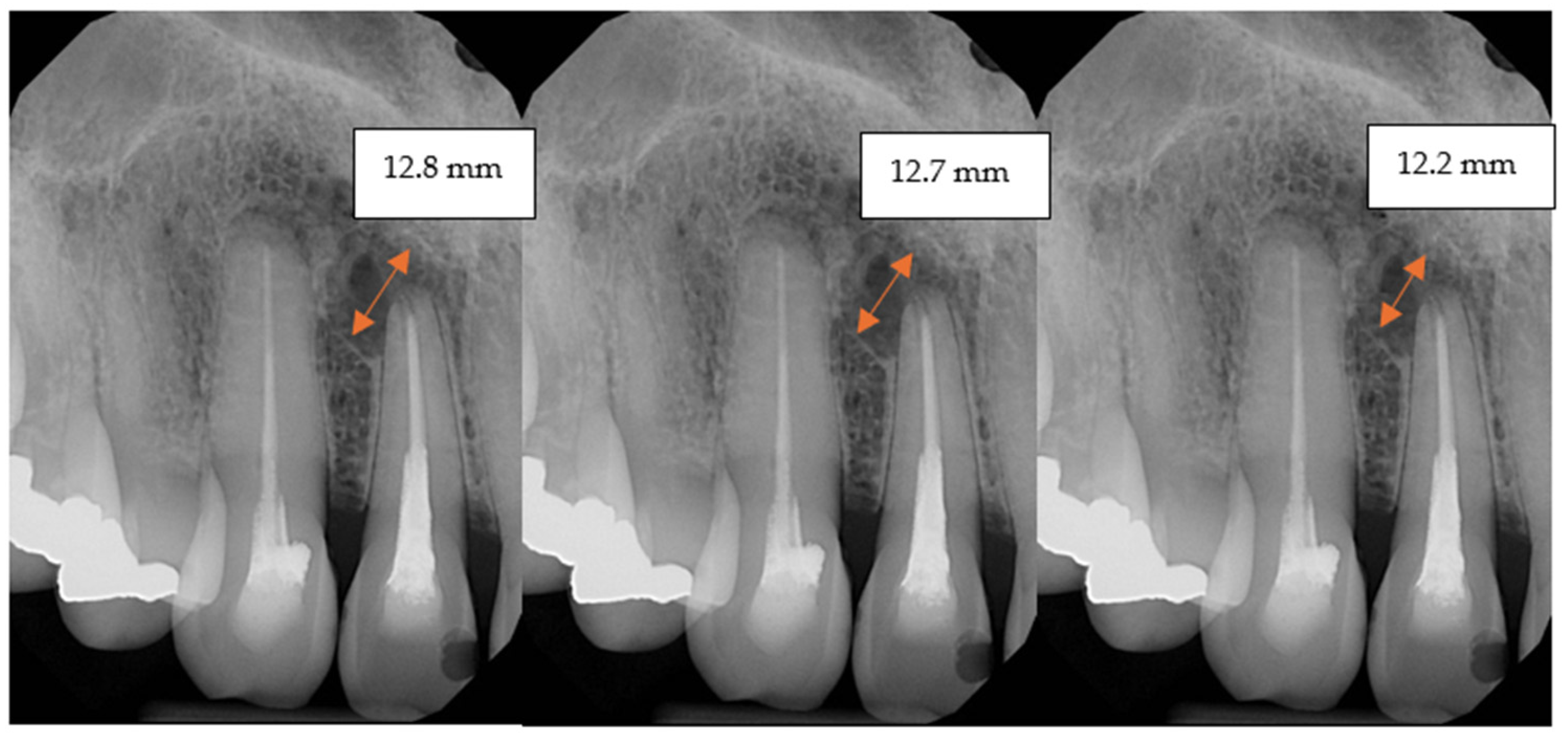
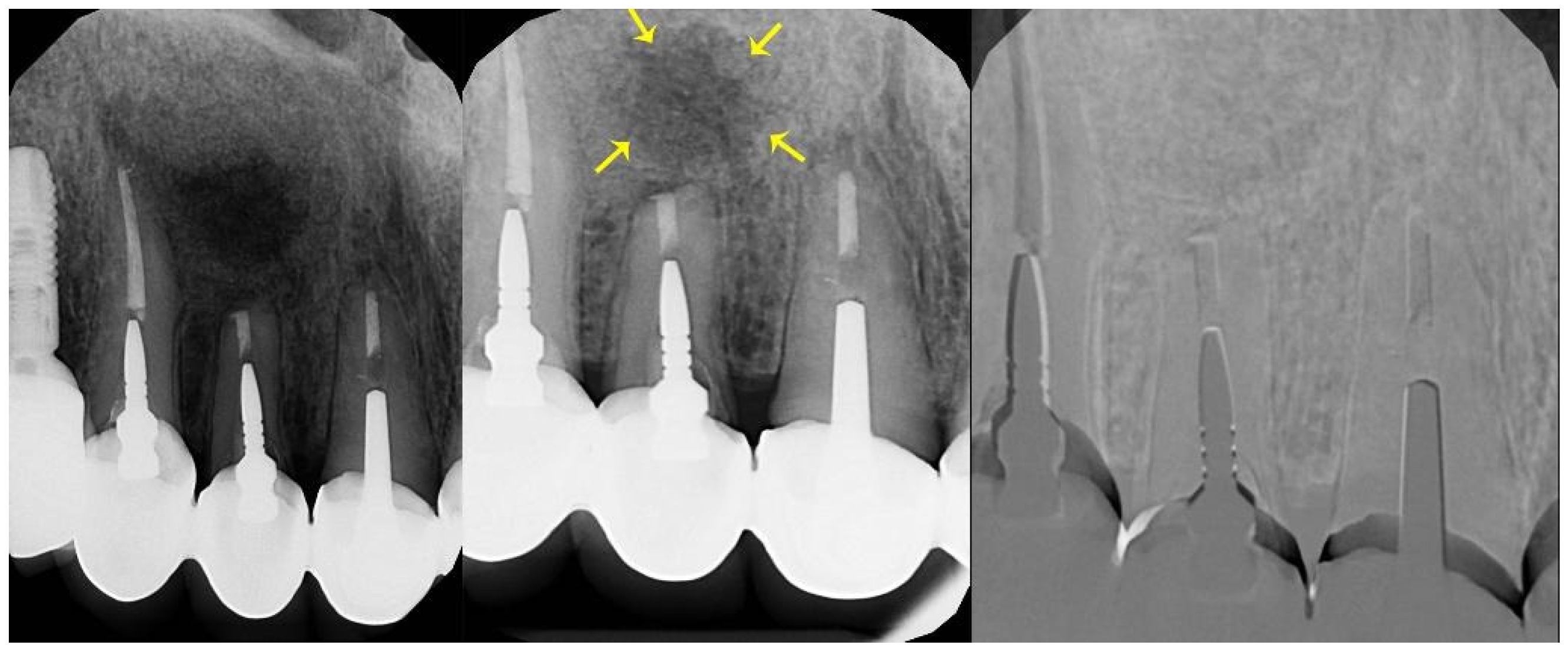
2.5. Follow-Up
2.6. Statistical Analysis
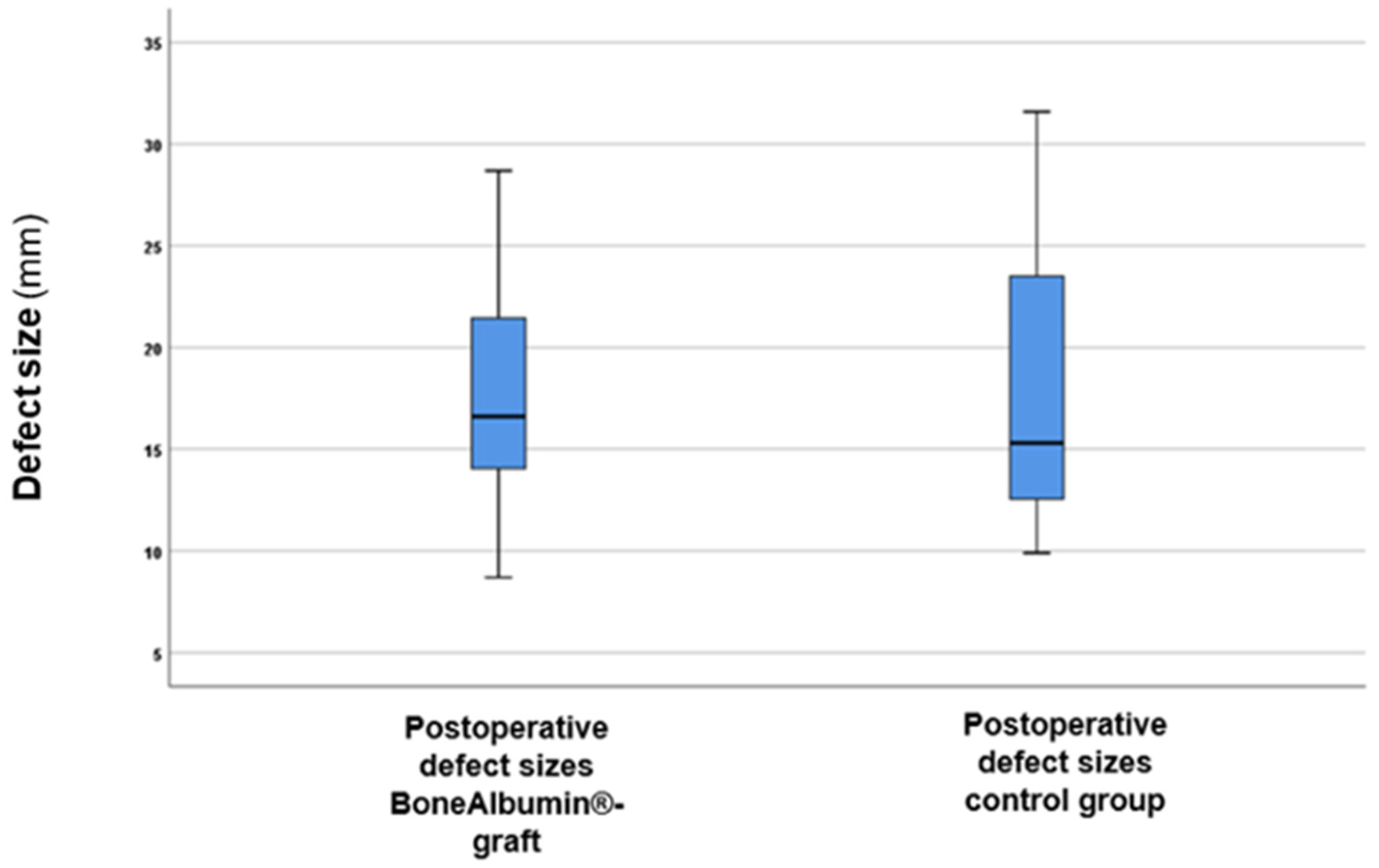
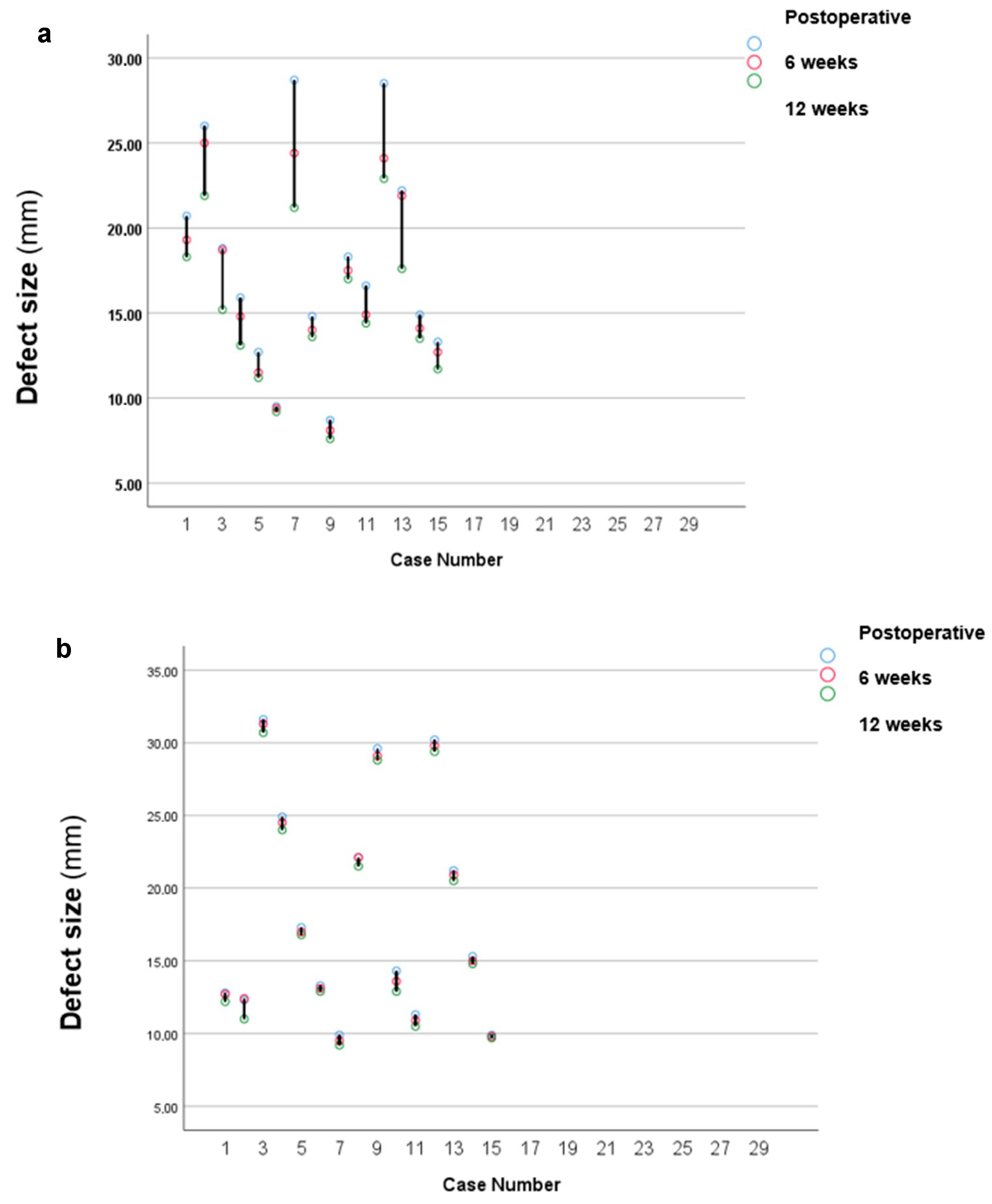
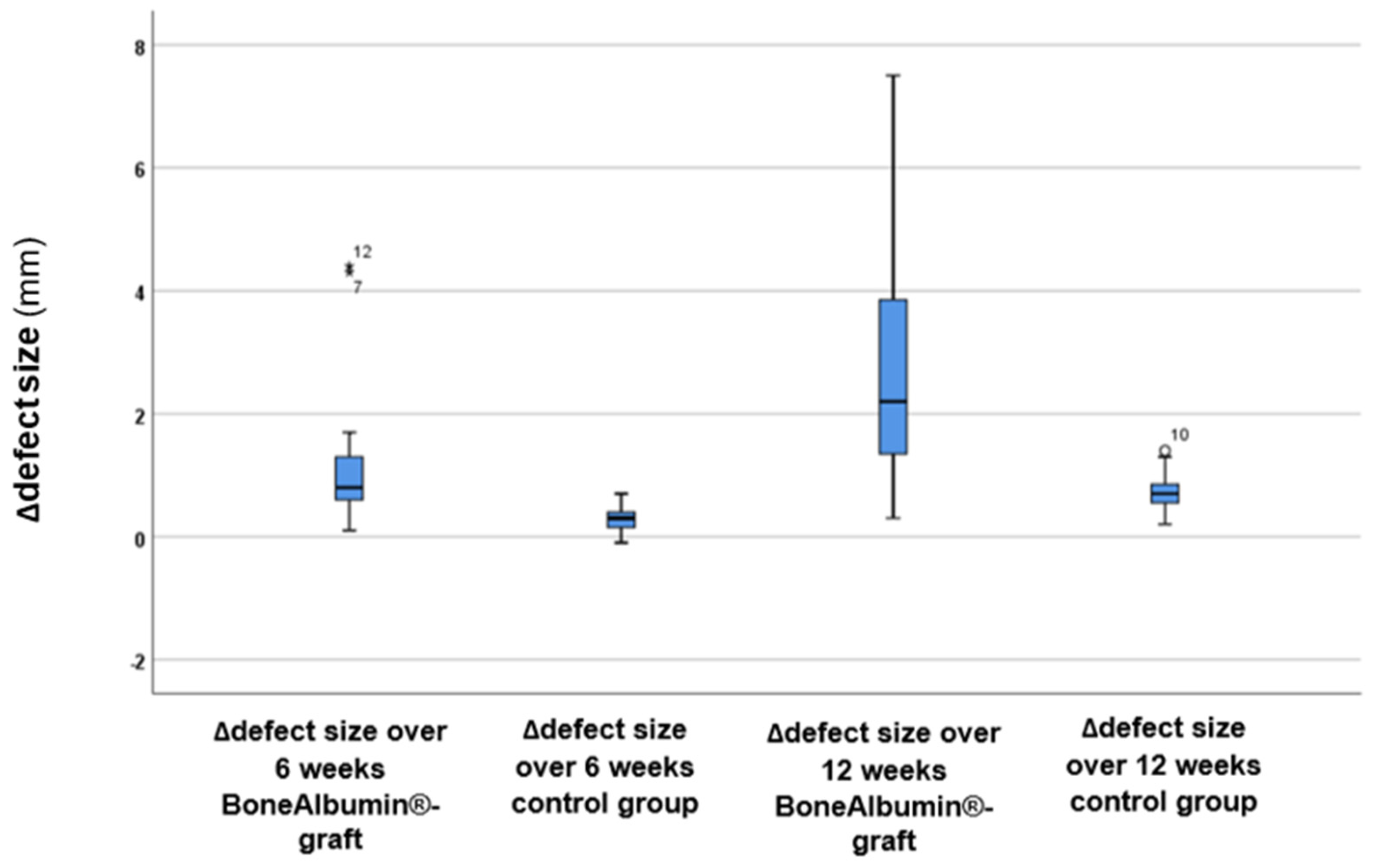
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajendra Santosh, A.B. Odontogenic Cysts. Dent. Clin. N. Am. 2020, 64, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Chaushu, G.; Reiser, V.; Rosenfeld, E.; Masri, D.; Chaushu, L.; Čandrlić, M.; Rider, P.; Perić Kačarević, Ž. Use of a Resorbable Magnesium Membrane for Bone Regeneration After Large Radicular Cyst Removal: A Clinical Case Report. Healthcare 2025, 13, 1068. [Google Scholar] [CrossRef] [PubMed]
- Berretta, L.M.; Melo, G.; Mello, F.W.; Lizio, G.; Rivero, E.R.C. Effectiveness of marsupialisation and decompression on the reduction of cystic jaw lesions: A systematic review. Br. J. Oral Maxillofac. Surg. 2021, 59, E17–E42. [Google Scholar] [CrossRef]
- Buchbender, M.; Koch, B.; Kesting, M.R.; Matta, R.E.; Adler, W.; Seidel, A.; Schmitt, C.M. Retrospective 3D analysis of bone regeneration after cystectomy of odontogenic cysts. J. Xray Sci. Technol. 2020, 28, 1141–1155. [Google Scholar] [CrossRef]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma. 2018, 32, S7–S11. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Q.Y.; Zhu, H.Y. Efficacy of bone grafts in jaw cystic lesions: A systematic review. World J. Clin. Cases 2022, 10, 2801–2810. [Google Scholar] [CrossRef]
- Keating, J.F.; Simpson, A.H.; Robinson, C.M. The management of fractures with bone loss. J. Bone Jt. Surg. Br. 2005, 87, 142–150. [Google Scholar] [CrossRef]
- Sanders, D.W.; Bhandari, M.; Guyatt, G.; Heels-Ansdell, D.; Schemitsch, E.H.; Swiontkowski, M.; Tornetta, P., III; Walter, S. Critical-sized defect in the tibia: Is it critical? Results from the SPRINT trial. J. Orthop. Trauma 2014, 28, 632–635. [Google Scholar] [CrossRef]
- Simonffy, L.; Minya, F.; Trimmel, B.; Lacza, Z.; Dobo-Nagy, C. Albumin-Impregnated Allograft Filling of Surgical Extraction Sockets Achieves Better Bone Remodeling Than Filling with Either Blood Clot or Bovine Xenograft. Int. J. Oral Maxillofac. Implant. 2020, 35, 297–304. [Google Scholar] [CrossRef]
- Minya, F.; Trimmel, B.; Simonffy, L.; Gyulai-Gaal, S.; Lacza, Z.; Dobo-Nagy, C. Alveolar Preservation with Albumin and Gentamycin-Coated Allograft after Third Molar Tooth Removal: A Randomized Clinical Trial. Appl. Sci. 2021, 11, 586. [Google Scholar] [CrossRef]
- Schandl, K.; Horváthy, D.B.; Doros, A.; Majzik, E.; Schwarz, C.M.; Csönge, L.; Abkarovits, G.; Bucsi, L.; Lacza, Z. Bone-Albumin filling decreases donor site morbidity and enhances bone formation after anterior cruciate ligament reconstruction with bone-patellar tendon-bone autografts. Int. Orthop. 2016, 40, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Gyulay, K.K.; Karászi, P.; Rédei, M.; Sólymos, P.; Schandl, K.; Lacza, Z.; Horváthy, D.B. Evaluation of Serum Albumin-Coated Bone Allograft for Bone Regeneration: A Seven-Year Follow-Up Study of 26 Cases. Int. J. Mol. Sci. 2023, 24, 9232. [Google Scholar] [CrossRef] [PubMed]
- Klára, T.; Csönge, L.; Janositz, G.; Csernátony, Z.; Lacza, Z. Albumin-coated structural lyophilized bone allografts: A clinical report of 10 cases. Cell Tissue Bank 2014, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Chavda, S.; Levin, L. Human Studies of Vertical and Horizontal Alveolar Ridge Augmentation Comparing Different Types of Bone Graft Materials: A Systematic Review. J. Oral Implantol. 2018, 44, 74–84. [Google Scholar] [CrossRef]
- Saima, S.; Jan, S.M.; Shah, A.F.; Yousuf, A.; Batra, M. Bone grafts and bone substitutes in dentistry. J. Oral Res. Rev. 2016, 8, 36–38. [Google Scholar]
- Horváthy, D.B.; Schandl, K.; Schwarz, C.M.; Renner, K.; Hornyák, I.; Szabó, B.T.; Niculescu-Morzsa, E.; Nehrer, S.; Dobó-Nagy, C.; Doros, A.; et al. Serum albumin-coated bone allograft (BoneAlbumin) results in faster bone formation and mechanically stronger bone in aging rats. J. Tissue Eng. Regen. Med. 2019, 13, 416–422. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Vácz, G.; Szabó, T.; Szigyártó, I.C.; Toró, I.; Vámos, B.; Hornyák, I.; Renner, K.; Klára, T.; Szabó, B.T.; et al. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 126–132. [Google Scholar] [CrossRef]
- Evans, T.W. Review article: Albumin as a drug-biological effects of albumin unrelated to oncotic pressure. Aliment. Pharmacol. Ther. 2002, 16, 6–11. [Google Scholar] [CrossRef]
- Maier, R.; Fries, M.R.; Buchholz, C.; Zhang, F.; Schreiber, F. Human versus Bovine Serum Albumin: A Subtle Difference in Hydrophobicity Leads to Large Differences in Bulk and Interface Behavior. Cryst. Growth Des. 2021, 21, 5451–5459. [Google Scholar] [CrossRef]
- Mishra, V.; Heath, R.J. Structural and Biochemical Features of Human Serum Albumin Essential for Eukaryotic Cell Culture. Int. J. Mol. Sci. 2021, 22, 8411. [Google Scholar] [CrossRef]
- Othman, Z.; Cillero Pastor, B.; van Rijt, S.; Habibovic, P. Understanding interactions between biomaterials and biological systems using proteomics. Biomaterials 2018, 167, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Amiji, M.; Park, H.; Park, K. Study on the prevention of surface-induced platelet activation by albumin coating. J. Biomater. Sci. Polym. Ed. 1992, 3, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Horváthy, D.B.; Simon, M.; Schwarz, C.M.; Masteling, M.; Vácz, G.; Hornyák, I.; Lacza, Z. Serum albumin as a local therapeutic agent in cell therapy and tissue engineering. Biofactors 2017, 43, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Sanganeria, P.; Sachar, S.; Chandra, S.; Bahadur, D.; Ray, P.; Khanna, A. Cellular internalization and detailed toxicity analysis of protein-immobilized iron oxide nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 125–134. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Vácz, G.; Cselenyák, A.; Weszl, M.; Kiss, L.; Lacza, Z. Albumin-coated bioactive suture for cell transplantation. Surg. Innov. 2013, 20, 249–255. [Google Scholar] [CrossRef]
- Wang, J.; Cui, L.; Ren, Y.; Zou, Y.; Ma, J.; Wang, C.; Zheng, Z.; Chen, X.; Zeng, R.; Zheng, Y. In vitro and in vivo biodegradation and biocompatibility of an MMT/BSA composite coating upon magnesium alloy AZ31. J. Mater. Sci. Technol. 2020, 47, 52–67. [Google Scholar] [CrossRef]
- Yamazoe, H.; Tanabe, T. Preparation of water-insoluble albumin film possessing nonadherent surface for cells and ligand binding ability. J. Biomed. Mater. Res. A 2008, 86, 228–234. [Google Scholar] [CrossRef]
- Yamazoe, H.; Uemura, T.; Tanabe, T. Facile cell patterning on an albumin-coated surface. Langmuir 2008, 24, 8402–8404. [Google Scholar] [CrossRef]
- Wei, J.; Yoshinari, M.; Takemoto, S.; Hattori, M.; Kawada, E.; Liu, B.; Oda, Y. Adhesion of mouse fibroblasts on hexamethyldisiloxane surfaces with wide range of wettability. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 66–75. [Google Scholar] [CrossRef]
- Timothy, M.M.; Massicotte, M.P.; Peter, D.W. ECMO Biocompatibility: Surface Coatings, Anticoagulation, and Coagulation Monitoring. In Extracorporeal Membrane Oxygenation—Advances in Therapy; Michael, S.F., Ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Preston, T.J.; Ratliff, T.M.; Gomez, D.; Olshove, V.E., Jr.; Nicol, K.K.; Sargel, C.L.; Chicoine, L.G. Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit. J. Extracorpor. Technol. 2010, 42, 199–202. [Google Scholar] [CrossRef]
- Mijiritsky, E.; Gardin, C.; Ferroni, L.; Lacza, Z.; Zavan, B. Albumin-impregnated bone granules modulate the interactions between mesenchymal stem cells and monocytes under in vitro inflammatory conditions. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110678. [Google Scholar] [CrossRef]
- Skaliczki, G.; Schandl, K.; Weszl, M.; Major, T.; Kovács, M.; Skaliczki, J.; Szendrői, M.; Dobó-Nagy, C.; Lacza, Z. Serum albumin enhances bone healing in a nonunion femoral defect model in rats: A computer tomography micromorphometry study. Int. Orthop. 2013, 37, 741–745. [Google Scholar] [CrossRef]
- Budán, F.; Szigeti, K.; Weszl, M.; Horváth, I.; Balogh, E.; Kanaan, R.; Berényi, K.; Lacza, Z.; Máthé, D.; Gyöngyi, Z. Novel radiomics evaluation of bone formation utilizing multimodal (SPECT/X-ray CT) in vivo imaging. PLoS ONE 2018, 13, e0204423. [Google Scholar] [CrossRef]
- Weszl, M.; Skaliczki, G.; Cselenyák, A.; Kiss, L.; Major, T.; Schandl, K.; Bognár, E.; Stadler, G.; Peterbauer, A.; Csönge, L.; et al. Freeze-dried human serum albumin improves the adherence and proliferation of mesenchymal stem cells on mineralized human bone allografts. J. Orthop. Res. 2012, 30, 489–496. [Google Scholar] [CrossRef]
- Kuten Pella, O.; Hornyák, I.; Horváthy, D.; Fodor, E.; Nehrer, S.; Lacza, Z. Albumin as a Biomaterial and Therapeutic Agent in Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 10557. [Google Scholar] [CrossRef]
- Martín, M.L.; Pfaffen, V.; Valenti, L.E.; Giacomelli, C.E. Albumin biofunctionalization to minimize the Staphylococcus aureus adhesion on solid substrates. Colloids Surf. B Biointerfaces 2018, 167, 156–164. [Google Scholar] [CrossRef]
- Cometta, S.; Bock, N.; Suresh, S.; Dargaville, T.R.; Hutmacher, D.W. Antibacterial Albumin-Tannic Acid Coatings for Scaffold-Guided Breast Reconstruction. Front. Bioeng. Biotechnol. 2021, 9, 638577. [Google Scholar] [CrossRef]
- An, Y.H.; Bradley, J.; Powers, D.L.; Friedman, R.J. The prevention of prosthetic infection using a cross-linked albumin coating in a rabbit model. J. Bone Jt. Surg. Br. 1997, 79, 816–819. [Google Scholar] [CrossRef]
- Márton, K.; Tamás, S.B.; Orsolya, N.; Béla, C.; Ferenc, D.; Péter, N.; Csaba, D.N.; Lajos, C.; Zsombor, L.; Eitan, M.; et al. Microarchitecture of the Augmented Bone Following Sinus Elevation with an Albumin Impregnated Demineralized Freeze-Dried Bone Allograft (BoneAlbumin) versus Anorganic Bovine Bone Mineral: A Randomized Prospective Clinical, Histomorphometric, and Micro-Computed Tomography Study. Materials 2018, 11, 202. [Google Scholar] [CrossRef]
- Scarano, A.; Degidi, M.; Iezzi, G.; Pecora, G.; Piattelli, M.; Orsini, G.; Caputi, S.; Perrotti, V.; Mangano, C.; Piattelli, A. Maxillary sinus augmentation with different biomaterials: A comparative histologic and histomorphometric study in man. Implant. Dent. 2006, 15, 197–207. [Google Scholar] [CrossRef]
- Handschel, J.; Simonowska, M.; Naujoks, C.; Depprich, R.A.; Ommerborn, M.A.; Meyer, U.; Kübler, N.R. A histomorphometric meta-analysis of sinus elevation with various grafting materials. Head Face Med. 2009, 5, 12. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Study Group (n = 15) | Control Group (n = 15) | p-Value | ||
|---|---|---|---|---|---|
| Age (mean years ± SD) | 41.6 ± 12.5 | 43.6 ± 14.37 | 0.37 * | ||
| Sex (men/women) | 7/8 | 9/6 | 0.46 ** | ||
| Complication (fistula), n (%) | 2 | (13.3%) | 1 | (6.6%) | 0.67 ** |
| Control Group (n = 15) | BoneAlbumin® Group (n = 15) | |
|---|---|---|
| Postoperative (mean mm ± SD) | 17.97 ± 4.98 | 18.4 ± 6.56 |
| 6-month follow-up (mean mm ± SD) | 16.69 ± 4.53 | 18.11 ± 6.53 |
| 12-month follow-up (mean mm ± SD) | 15.23 ± 3.67 | 17.66 ± 6.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangics, A.; Répássy, G.D.; Hargas, D.; Dobó-Nagy, C.; Gyulai-Gaál, S.; Molnár, A.; Simonffy, L. Bone Grafting with Albumin-Impregnated Bone Allograft After Odontogenic Cyst Removal. J. Clin. Med. 2025, 14, 4173. https://doi.org/10.3390/jcm14124173
Rangics A, Répássy GD, Hargas D, Dobó-Nagy C, Gyulai-Gaál S, Molnár A, Simonffy L. Bone Grafting with Albumin-Impregnated Bone Allograft After Odontogenic Cyst Removal. Journal of Clinical Medicine. 2025; 14(12):4173. https://doi.org/10.3390/jcm14124173
Chicago/Turabian StyleRangics, Anna, Gábor Dénes Répássy, Dóra Hargas, Csaba Dobó-Nagy, Szabolcs Gyulai-Gaál, András Molnár, and László Simonffy. 2025. "Bone Grafting with Albumin-Impregnated Bone Allograft After Odontogenic Cyst Removal" Journal of Clinical Medicine 14, no. 12: 4173. https://doi.org/10.3390/jcm14124173
APA StyleRangics, A., Répássy, G. D., Hargas, D., Dobó-Nagy, C., Gyulai-Gaál, S., Molnár, A., & Simonffy, L. (2025). Bone Grafting with Albumin-Impregnated Bone Allograft After Odontogenic Cyst Removal. Journal of Clinical Medicine, 14(12), 4173. https://doi.org/10.3390/jcm14124173









