Abstract
Background/Objectives: Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) is a rare neurological disorder primarily affecting pediatric patients but also observed in adults. The radiological hallmark of MERS is a reversible lesion in the splenium of the corpus callosum. Although MERS generally has a favorable prognosis, its variable presentation poses diagnostic challenges. This study examines the clinical variability, diagnostic hurdles, and outcomes of pediatric MERS cases. Methods: Our retrospective study included 19 pediatric patients (11 female and 8 males with an average age of 8.41 years) diagnosed with MERS between 2016 and 2024. Clinical data, including demographic characteristics, prodromal symptoms, neurological features, MRI findings, laboratory results, treatments, and outcomes, were analyzed. Results: Among the 19 patients, 84% were previously healthy, with the remaining 16% having pre-existing medical conditions. The most common prodromal symptoms were fever (68%), vomiting (47%), and diarrhea (32%). Neurological manifestations included seizures (26%), headache (21%), and drowsiness (21%), among others. In terms of etiology, infections were identified in 52% of the patients, with viral agents, particularly rotavirus, being the most common (40%). Hyponatremia was present in 63% of the cohort. The typical MRI splenial lesion underwent complete resolution in all patients. Treatment varied, with 53% of patients receiving electrolyte rehydration, and 21% receiving intravenous immunoglobulin or corticosteroids. All patients, but one, achieved full recovery. Discussion: This study reinforces the clinical heterogeneity of MERS in pediatric patients, emphasizing its favorable prognosis independently of presentation. Viral infections and hyponatremia were the most frequent etiologies.
1. Introduction
Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) is a rare but increasingly recognized clinico-radiological entity, particularly within pediatric populations [1]. Initially described in the early 2000s, MERS predominantly affects children, although it has been described also in adults [1].
In 2011, the term “reversible splenial lesion syndrome” (RESLES) was proposed [2] as a general term for diverse etiologies, while the term MERS was primarily referred to infection-related entities. Currently, the two terms are occasionally employed interchangeably. Recently, a new, more extensive term—cytotoxic lesions of the corpus callosum (CLOCCs)—has been used as a general description for all conditions, including MERS, RESLES, reversible splenial lesions, and transient splenial lesions [3].
MERS clinical manifestations are different and often nonspecific [4,5].
Mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) is a rare neurological syndrome characterized clinically by transient encephalopathic symptoms and radiologically by a reversible lesion in the splenium of the corpus callosum, typically visible on magnetic resonance imaging. Epidemiological data are limited, mainly due to the rarity of the condition and potential underdiagnosis. However, a letter reported a radiological prevalence of approximately 3% in certain pediatric cohorts [6] suggesting that MERS may be more common than previously recognized. The syndrome has been described globally, with a higher concentration of cases reported in East Asia—particularly Japan and China—but also in Europe, Australia, and Turkey. It predominantly affects children and young adults, with a median age of around 16 years and a slight male predominance.
This condition is typically characterized by a prodromal illness consisting of fever, cough, vomiting or diarrhoea, followed by encephalopathy. Common neurological symptoms include altered mental status, seizures, headache, behavioral changes and ataxia [7]. While the condition is typically self-limiting with full recovery, its heterogeneous presentation and the evolving diagnostic criteria represent significant challenges for early diagnosis and management [8].
The radiological hallmark of MERS is a reversible lesion in the corpus callosum, identifiable by Magnetic Resonance Imaging (MRI). Specifically, MRI reveals an ovoid lesion in the mid-splenium of the corpus callosum (SCC), with high signal intensity on diffusion-weighted imaging (DWI), reduced signal intensity on apparent diffusion coefficient (ADC) maps, high signal intensity on FLAIR (fluid-attenuated inversion recovery), and no enhancement after gadolinium infusion [1]. MERS type 1 lesions are confined to the splenium, whereas MERS type 2 lesions extend into the anterior parts of the corpus callosum and/or the periventricular white matter [4,9].
The exact pathophysiology of MERS remains unclear, though several mechanisms have been proposed, including viral infections, autoimmune responses, and metabolic disturbances [7]. Some studies suggest that intramyelinic edema, caused by separation of myelin layers, interstitial edema in tightly packed fibers, and a transient inflammatory infiltrate, may play a role in an immune-mediated mechanism [8]. Other hypotheses propose that hypotonic hyponatremia may lead to water influx into the brain, causing intramyelinic edema and resulting in transient diffusion restriction observed in MRI [1,8].
Although specific treatment guidelines for MERS have not been fully established, management generally consists in supportive care, addressing issues such as seizures, dehydration, and hyponatremia [10]. Antiviral drugs, corticosteroids, and intravenous immunoglobulin (IVIg) are typically reserved for severe cases or when specific underlying causes are identified [11].
The prognosis is generally favorable, with MRI lesions resolving and clinical symptoms regressing within days to weeks [1,12]. While several case reports have improved our understanding of MERS, its clinical heterogeneity in children remains inadequately defined [1]. This variability complicates diagnosis, as MERS may resemble other encephalopathies or be misdiagnosed in favor of more common causes [7]. Moreover, despite a generally favorable outcome, the risk of misdiagnosis or delayed treatment remains a concern, particularly when symptoms overlap with more serious neurological disorders.
Our study has different aims. (1) We explore the clinical variability of MERS in a personal case series of children, examining the spectrum of presenting symptoms, diagnostic challenges, and outcomes. (2) Since most published case series include only Asian patients, we want to investigate whether the clinical, radiological, and laboratory characteristics of western children and adolescents with MERS differ or not from what is already known. In order to reach this purpose, we collected the largest sample of western young patients. (3) We seek to emphasize the importance of considering MERS in the differential diagnosis of acute encephalopathy in children, underscoring the need for heightened clinical awareness and further research to refine our understanding of the condition pathophysiology and optimal management strategies.
2. Materials and Methods
Our retrospective study included 19 patients seen at Ospedale Pediatrico Bambino Gesù (16 patients) and Lecco Hospital (3 patients), on both an outpatient and inpatient basis (Table 1). Eleven patients were female (57.9%) and 8 were male (42.1%). The average age was 8.41 years, with a standard deviation of 4.96 years. All patients were diagnosed with MERS between 2016 and 2024. Participants were eligible for inclusion if they were under 18 years of age and had a confirmed diagnosis of MERS (Mild Encephalitis/Encephalopathy with a Reversible Splenial lesion).

Table 1.
Demographic characteristics, clinical characteristics and instrumental findings of our patients.
Exclusion criteria included the presence of neurodevelopmental disorders, major neurological conditions such as stroke or demyelinating diseases, brain tumors and known genetic syndromes.
Five outpatient cases had previously been diagnosed with MERS in other healthcare institutions and were subsequently followed up at our hospital as part of a comprehensive care management plan. Clinical data, including demographic characteristics, prodromal and neurologic symptoms, neurologic examination, MRI and electroencephalography findings, laboratory findings, treatment, and prognosis were reviewed retrospectively.
3. Results
3.1. Preexisting Illness
Seventeen patients (89%) were previously healthy. Two patients (11%) had already been diagnosed with preexisting illnesses: sleep disorder and migraine (1 patient—5%), aortic coarctation and heart failure (1 patient—5%).
3.2. Prodromal Symptoms
Thirteen patients had prodromal symptoms such as fever (68%), vomiting (47%), diarrhea (32%), reduced appetite (21%), asthenia (16%), weight loss (11%), abdominal pain (5%), dehydration (5%), petechiae (5%), conjunctivitis (5%), and skin rash (5%) No prodromal symptom was detected in 2 patient (11%).
3.3. Neurological Manifestations
Neurological manifestations (Figure 1) were: seizures in 5 patients (26%), headache in 4 (21%), drowsiness in 4 (21%), disorientation in 4 (21%), rambling speech in 2 (11%), diplopia in 2 (11%), nuchal pain in 2 (11%), nuchal rigidity in 1 (5%), catalepsy in 1 (5%), psychomotor agitation in 1 (5%), irritability in 1 (5%), bilateral nystagmus in 1 (5%), sensory disturbance in 1 (5%), and gait disturbance in 1 (5%). Three patients had no neurological manifestation (16%).
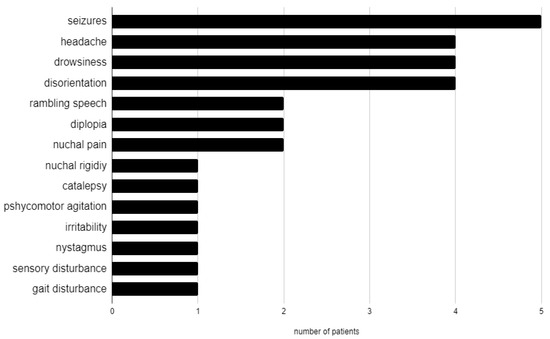
Figure 1.
Neurological manifestations in our cohort.
3.4. Putative Etiology
As shown in Table 2, infectious agents were detected in 10 patients (52%), while noninfectious causes were identified in 3 (16%), In 6 patients (32%), no putative etiology could be demonstrated. Among infectious agents, viral infections were detected in 7 out of 10 patients (70%). Particularly, Rotavirus was isolated from stool samples in 4 patients (40%), HHV-6 was identified in 2 patients (20%) through blood specimen, and Parvovirus B19 was detected in 1 patient (10%) from cerebrospinal fluid (CSF) samples. Bacterial infections were detected in 3 patients (30%). Particularly, E. coli was isolated from stool samples in 1 patient (10%), Haemophilus was identified in 1 patient (10%) from tracheal aspirate samples, suspected diagnosis of Meningococcus sepsis was made in 1 patient on the clinical basis (10%), even though the Meningococcus itself was not isolated. Hyponatremia was detected in 12 patients (63%): in 8 patients it was associated with an infectious agent and in 2 patients (11%) with Multisystem Inflammatory Syndrome in Children (MIS-C). In 2 patients (11%), hyponatremia represented an isolated finding.

Table 2.
Putative etiologies in our patients.
3.5. Instrumental Examination
Hyponatremia was found in 12 patients (63%), normal sodium levels in 7 patients (37%). C-reactive protein (CRP) was high in 13 patients (68%). White blood cells were high in 5 patients (26%). Only 9 patients underwent lumbar puncture, and CSF cell count was normal for all of them (100%). An EEG recording was performed in 14 patients. Nine patients (64%) showed an abnormal EEG. Almost half of these patients (4/9 cases) exhibited a slow background activity (BA). In two patients, EEG showed epileptic anomalies and in four EEG recordings there were slow anomalies, without clear epileptic abnormalities. A contrast-enhanced brain MRI with T1, T2-weighted sequences, DWI and FLAIR studies at the onset was performed in all patients. Eighteen patients (95%) showed type I lesions (Figure 2), while 1 patient (5%) had type II lesions. A follow-up MRI was available in 13 patients within a time interval ranging from 1 week to 10 months from the acute episode. In all these cases, the follow-up contrst-enhanced MRI showed complete resolution of the previously observed lesions, including on the T1, T2-weighted sequences DWI and FLAIR studies.
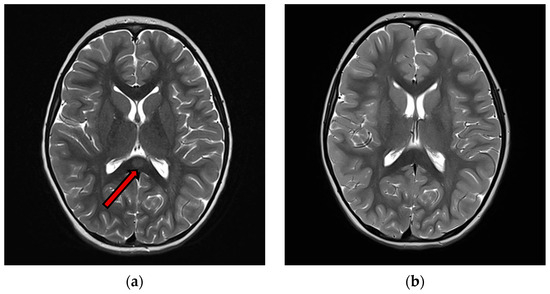
Figure 2.
(a) T2 images of acute phase of MERS Type 1 (b) T2 weighted images of the same patient of acute phase of MERS type 1. An ovoid high signal area is observed in the midmedulla of the body of the corpus callosum (arrow), 3 months after the previous, showing complete resolution of the lesion.
3.6. Treatment and Outcome
As shown in Table 3, glucoelectrolytic rehydration was performed in 10 patients (53%); in 4 cases, it represented the only treatment. Antibiotics were administered to 5 patients (26%), intravenous immunoglobulin (IVIg) to 4 (21%), corticosteroids to 4 (21%), antiviral drugs to 3 (16%), and immunosuppressant drugs (Eculizumab and Anakinra) in 2 (11%). Two children, who presented with seizures, had antiepileptic medications. In 5 patients no pharmacological treatment was administered (26%). While 13 patients recovered completely, only 1 patient had sequelae after the acute event. This patient was the only one in our cohort to experience a convulsive status epilepticus. She received antiepileptic drugs and corticosteroids, and the follow-up brain MRI performed one week after onset showed a complete resolution of splenial lesion. However, she continued to show motor and speech difficulties for other 6 months.

Table 3.
Treatment in our patients.
4. Discussion
This study contributes to the growing body of literature on MERS in youth, with key findings that offer valuable insights concerning its clinical presentation and potential pathophysiology. The main findings from our cohort include: (1) heterogeneity in clinical manifestations, with a wide range of neurological and prodromal, particularly gastrointestinal, symptoms; (2) heterogeneity in potential causes, as we identified both viral and bacterial infections as triggers for MERS, with rotavirus being the most frequent viral agent; and (3) a favorable outcome for most patients, with nearly all children experiencing full recovery.
Most previous studies on pediatric MERS were based on Asian cohorts [5,13,14,15,16,17,18,19,20,21], with western studies on MERS in children being limited to a few case reports and small case series [22,23,24,25,26,27,28,29,30,31]. To the best of our knowledge, our study represents the largest western population with pediatric MERS.
In a recent review by Chen et al. [21], it was reported that more than 60% of the cases of MERS aged under 3 years. The authors suggested that MERS is more likely to occur in young children due to the immaturity of their central nervous system and their increased susceptibility to infections. In our case series, only 4 patients were under the age of 3, while the majority of the population was older.
It is important to note that almost all of our patients were healthy prior to the diagnosis of MERS, with the exception of two cases. The first was an infant who was incidentally diagnosed with MERS during a multidisciplinary follow-up after surgery for aortic coarctation. This finding suggests that, while MERS typically affects previously healthy children, underlying medical conditions or recent surgical interventions could play a role in the development of the disease.
In literature, infections are commonly identified as the most frequent cause of MERS [32]. Our findings are in agreement, supporting the hypothesis that infectious agents may play a role in the immune mechanism contributing to MERS development. Various infectious agents have been linked to MERS in children, including rotavirus, adenovirus, influenza A and B, Epstein-Barr virus, Dengue virus, mumps virus, herpes simplex virus, parainfluenza, parvovirus B19, cytomegalovirus, Mycoplasma pneumoniae, Streptococcus pneumoniae, and Campylobacter jejuni [5]. In our study, viral infections were identified in 7 of 10 infectious cases, with rotavirus being the most frequent agent (4 patients). Additionally, Human Herpesvirus 6 (HHV-6) and Parvovirus B19 were also implicated. Bacterial infections were detected in 3 patients, consistent with prior research showing that bacterial pathogens, though less common, can contribute to MERS [32]. Hyponatremia has been proposed as a potential cause of MERS. In our cohort, the percentage of patients with hyponatremia was 63% (12 out of 19 patients). Diarrhea and vomiting may also contribute to sodium imbalances, as well as the effects of dehydration. The association between hyponatremia and infections in 8 patients supports the hypothesis that electrolyte disturbances may result from the infectious process or the associated inflammatory response. Two patients of ours had overlapping diagnoses of MERS and MIS-C, with a positive serology for SARS-CoV-2 (anti-N and anti-S antibodies), consistent with a previous viral infection. In pediatric patients, MIS-C is a clinical manifestation within the clinical spectrum of COVID-19 [33]. After assessing the radiological outcomes of 45 pediatric patients with MIS-C, Palabiyik et al. [34] showed that the most common finding on brain MRI was MERS. This suggests that in patients with a suspected MERS diagnosis SARS-CoV-2 antigen and antibody tests should be included to evaluate the potential overlap with MIS-C.
The most frequent prodromal symptoms in pediatric patients later diagnosed with MERS are fever, vomiting, diarrhea, abdominal pain, and headache [5]. The prodromal symptoms observed in our patients were generally in agreement with those reported in the literature, with a predominance of fever and gastrointestinal symptoms. As for the neurological symptoms, alterations of consciousness, seizures, drowsiness, headache, monoparesis, abnormal speech, visual hallucinations, and ataxia have been more often reported in the literature. In our cohort, seizures represented the most frequent neurological symptom. Alterations of consciousness showed a higher frequency than that previously reported. Drowsiness, headache, and other neurological symptoms (e.g., abnormal speech, diplopia, and psychomotor agitation) were observed in a significant proportion of patients. The heterogeneous neurological symptoms suggest that MERS can affect different brain networks, with some patients experiencing more localized symptoms and others presenting with global encephalopathy.
As for the instrumental examinations, absence of pleocytosis in the cerebrospinal fluid (CSF) in all our patients supports the hypothesis that MERS is an infection-associated encephalopathy syndrome rather than an infectious encephalitis. In our study, 64% of the patients who underwent EEG showed abnormal results. This is in agreement with the systematic review by Chen et al. [21], reporting an abnormal EEG in 60% of patients with MERS. In our patients, brain MRI showed more frequent Type I than Type II lesions, thus confirming previous findings [5,21].
Regarding treatment, there are no established guidelines for the treatment of MERS [5]. Pulsed therapy with methylprednisolone and IVIg is recommended for patients with infectious encephalopathy, regardless of the pathogen or clinical-radiological syndromes [35], as well as electrolyte correction in case of electrolyte imbalances. A previous review [20] has reported that most patients with MERS could have a full recovery regardless of treatment, suggesting that specific treatments may not be necessary. In our population, the most common treatment was electrolyte rehydration, followed by symptomatic treatment with antibiotics, IVIg, and corticosteroids. Five patients received glucose-electrolyte rehydration without additional medications, while two cases did not receive any treatment. Since pharmacological treatment may be useless, depending on the clinical manifestations, an early diagnosis of MERS could avoid invasive therapies.
In the literature, most pediatric MERS cases report complete recovery without sequelae [15,21]. A case with sequelae was reported by Chen et al. [15], who described a patient with intellectual disability despite normal follow-up brain MRI. While a pediatric case of Type I MERS, following H1N1 influenza virus infection, showed persisting intellectual disability after splenial lesion resolution [36], Type II lesions are more likely associated to neurological sequelae [5]. In our pediatric population, all patients had complete clinical resolution, except for one patient who experienced movement and speech difficulties recovered after a 6-months rehabilitative treatment. In this patient, brain MRI at onset had showed a Type I lesion which had completely recovered after one week.
Limitations of the Present Study and Future Perspectives
Our study has several limitations. First, it is retrospective, which may have introduced which may have introduced both recall and selection bias. Clinical data such as EEG findings, CSF analysis, and follow-up assessments were not uniformly available for all patients, reflecting variability in clinical management across centers and over time. This inconsistency may have influenced our ability to identify certain patterns or associations, particularly regarding long-term neurological outcomes. Secondly, the sample size was relatively small. Lastly, the lack of a standardized follow-up protocol limited our ability to assess the long-term neurological outcomes more comprehensively. Additionally, the absence of a comparative control group, such as pediatric encephalitis patients without splenial lesions, limits our ability to definitively identify features specific to MERS. We have explicitly acknowledged this as a limitation, which should be addressed in future prospective studies with appropriate control cohorts.
We can speculate that some updated techniques of CSF examination may provide important information about MERS pathophysiology. In a retrospective study, enrolling four children with MERS and 14 age-matched healthy subjects, significantly higher levels of pNf-H (phosphorylated neurofilament heavy chain) in the CSF were observed in the MERS cases compared to controls [37]. Neurofilaments are a major structural component of neurons and consist of three subunits: a light chain, a medium chain, and a heavy chain. PNf-H levels in CSF can be used as specific biomarkers for axonal injury or degeneration. Motobayashi et al.’s [37] findings suggest that patients with MERS experience axonal damage, which is often transitory, but can explain the rare cases of MERS with evident clinical sequelae. In our opinion, the study of CSF neurofilaments in larger multicentric studies could shade light about the pathophysiology of the disease. Another important field of research could be represented by the metabolic modifications associated with MERS. From this point of view, spectroscopic MRI (sMRI) of the brain might be a useful examination in patients with MERS. Recent studies have utilized advanced MRI techniques, including MR spectroscopy (MRS), to investigate the characteristics of reversible splenial lesions. Ueda et al. [38] reported on a case of mild encephalopathy where MRS revealed slightly elevated choline levels, suggesting increased glial cell membrane turnover without neuronal damage. The signal abnormalities persisted for 90 days, indicating that such lesions may not always be rapidly reversible. Similarly, Lin and Yu [39] described a patient with a reversible focal splenial lesion associated with staphylococcal meningitis, where MRS showed relatively elevated lactate levels, highlighting metabolic disturbances as a potential underlying mechanism. Additionally, Shimizu et al. [40] conducted extensive neuroimaging of a transient splenial lesion, emphasizing the importance of comprehensive imaging in understanding the pathophysiology of such lesions. These studies underscore the utility of MRS and advanced MRI in elucidating the metabolic and structural aspects of reversible splenial lesions, providing valuable insights into their diagnosis and management.
Author Contributions
Conceptualization, M.R., M.P. and M.V.; methodology, M.R. and M.P.; investigation, M.R., M.P., V.M. and M.V.; writing—original draft preparation, M.R. and M.P. writing—review and editing, M.V.; supervision, R.M., L.M. and M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health with Current Research funds.
Institutional Review Board Statement
The study was approved by the Institutional Review Board of our institution (protocol code: RAP-2025-0002 and date of approval 1 March 2025).
Informed Consent Statement
This study is retrospective; the local IRB does not require informed consent.
Data Availability Statement
The data are extracted from our patients’ medical records, therefore they cannot be shared due to privacy reasons.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MERS | Mild encephalitis/encephalopathy with reversible splenial lesion |
| MRI | Magnetic Resonance Imaging |
| RESLES | Reversible splenial lesion syndrome |
| CLOCCs | cytotoxic lesions of the corpus callosum |
| SCC | splenium of the corpus callosum |
| DWI | diffusion-weighted imaging |
| ADC | apparent diffusion coefficient |
| FLAIR | fluid-attenuated inversion recovery |
| IVIg | intravenous immunoglobulin |
| MIS-C | Multisystem Inflammatory Syndrome in Children |
| HHV-6 | Human Herpesvirus 6 |
| CSF | cerebrospinal fluid |
| pNf-H | phosphorylated neurofilament heavy chain |
| sMRI | spectroscopic MRI |
References
- Tada, H.; Takanashi, J.; Barkovich, A.J.; Oba, H.; Maeda, M.; Tsukahara, H.; Suzuki, M.; Yamamoto, T.; Shimono, T.; Ichiyama, T.; et al. Clinically Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion. Neurology 2004, 63, 1854–1858. [Google Scholar] [CrossRef]
- Garcia-Monco, J.C.; Cortina, I.E.; Ferreira, E.; Martínez, A.; Ruiz, L.; Cabrera, A.; Beldarrain, M.G. Reversible Splenial Lesion Syndrome (RESLES): What’s in a Name? J. Neuroimaging 2011, 21, e1–e14. [Google Scholar] [CrossRef] [PubMed]
- Aksu Uzunhan, T.; Maraş Genç, H.; Kutlubay, B.; Kalın, S.; Bektaş, G.; Yapıcı, Ö.; Çıracı, S.; Sözen, H.G.; Şevketoğlu, E.; Palabıyık, F.; et al. Cytotoxic Lesions of the Corpus Callosum in Children: Etiology, Clinical and Radiological Features, and Prognosis. Brain Dev. 2021, 43, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, M.; Tanabe, T.; Shimakawa, S.; Nakamura, M.; Murata, S.; Shabana, K.; Shinohara, J.; Odanaka, Y.; Matsumura, H.; Maki, K.; et al. Clinico-Radiological Spectrum of Reversible Splenial Lesions in Children. Brain Dev. 2014, 36, 330–336. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, Y.; Kang, J.; Duan, C.; Yi, Z.; Yang, C.; Li, F.; Liu, K.; Song, Z. A Cohort Study of Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion in Children. Brain Behav. 2021, 11, e2306. [Google Scholar] [CrossRef]
- Gómez Iglesias, P.; López Valdés, E.; Vega Bayoll, M.; Gómez Ruíz, M.N. Mild Encephalitis/Encephalopathy with Reversible Splenial Lesion: A Little-Known Entity with Favourable Prognosis. Neurol. Engl. Ed. 2020, 35, 581–583. [Google Scholar] [CrossRef]
- Ka, A.; Britton, P.; Troedson, C.; Webster, R.; Procopis, P.; Ging, J.; Chua, Y.W.; Buckmaster, A.; Wood, N.; Jones, C.; et al. Mild Encephalopathy with Reversible Splenial Lesion: An Important Differential of Encephalitis. Eur. J. Paediatr. Neurol. 2015, 19, 377–382. [Google Scholar] [CrossRef]
- Takanashi, J.; Tada, H.; Maeda, M.; Suzuki, M.; Terada, H.; Barkovich, A.J. Encephalopathy with a Reversible Splenial Lesion Is Associated with Hyponatremia. Brain Dev. 2009, 31, 217–220. [Google Scholar] [CrossRef]
- Gallucci, M.; Limbucci, N.; Paonessa, A.; Caranci, F. Reversible Focal Splenial Lesions. Neuroradiology 2007, 49, 541–544. [Google Scholar] [CrossRef]
- Marsala, S.Z.; Antichi, E.; Pistacchi, M.; Gioulis, M.; Candeago, R.M.; Montemurro, R.T.; Gentile, M.; D’Andrea, P.; Ferracci, F. Mild Encephalitis with a Reversible Splenial Lesion: A Clinical Benign Condition, Often Underrecognized—Clinical Case and Literature Review. J. Neurosci. Rural Pract. 2017, 8, 281–283. [Google Scholar] [CrossRef]
- Ueda, N.; Minami, S.; Akimoto, M. Mycoplasma Pneumoniae-Associated Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion: Report of Two Pediatric Cases and a Comprehensive Literature Review. BMC Infect. Dis. 2016, 16, 671. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, J.; Imamura, A.; Hayakawa, F.; Terada, H. Differences in the Time Course of Splenial and White Matter Lesions in Clinically Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion (MERS). J. Neurol. Sci. 2010, 292, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Chen, L.; Chen, Q.; Lin, Z.; Yang, F. Clinically Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion of Corpus Callosum in Chinese Children. Brain Dev. 2017, 39, 321–326. [Google Scholar] [CrossRef]
- Nakata, T.; Natsume, J.; Yamamoto, H.; Ito, Y.; Suzuki, T.; Kawaguchi, M.; Shiraki, A.; Kumai, S.; Sawamura, F.; Suzui, R.; et al. Underlying Disorders in Children with Infection-Related Acute Encephalopathy. Pediatr. Neurol. 2024, 155, 126–132. [Google Scholar] [CrossRef]
- Chen, W.-X.; Liu, H.-S.; Yang, S.-D.; Zeng, S.-H.; Gao, Y.-Y.; Du, Z.-H.; Li, X.-J.; Lin, H.-S.; Liang, H.-C.; Mai, J.-N. Reversible Splenial Lesion Syndrome in Children: Retrospective Study and Summary of Case Series. Brain Dev. 2016, 38, 915–927. [Google Scholar] [CrossRef]
- Qing, Y.; Xiong, W.; Da-xiang, H.; Juan, Z.; Fei, W.; Yong-qiang, Y. Statistical Analysis of the Apparent Diffusion Coefficient in Patients with Clinically Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion Indicates That the Pathology Extends Well beyond the Visible Lesions. Magn. Reson. Med. Sci. 2020, 19, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.; Abe, S.; Ikeno, M.; Shima, T.; Shimizu, T.; Okumura, A. A Nationwide Survey of Adenovirus-Associated Encephalitis/Encephalopathy in Japan. Brain Dev. 2024, 46, 10–17. [Google Scholar] [CrossRef]
- Hirayama, Y.; Saito, Y.; Maegaki, Y. “Symptomatic” Infection-Associated Acute Encephalopathy in Children with Underlying Neurological Disorders. Brain Dev. 2017, 39, 243–247. [Google Scholar] [CrossRef]
- Tsubouchi, Y.; Itamura, S.; Saito, Y.; Yamashita, E.; Shinohara, Y.; Okazaki, T.; Ohno, K.; Nishimura, Y.; Oguri, M.; Maegaki, Y. Use of High b Value Diffusion-Weighted Magnetic Resonance Imaging in Acute Encephalopathy/Encephalitis During Childhood. Brain Dev. 2018, 40, 116–125. [Google Scholar] [CrossRef]
- Mizuguchi, M.; Ichiyama, T.; Imataka, G.; Okumura, A.; Goto, T.; Sakuma, H.; Takanashi, J.; Murayama, K.; Yamagata, T.; Yamanouchi, H.; et al. Guidelines for the Diagnosis and Treatment of Acute Encephalopathy in Childhood. Brain Dev. 2021, 43, 2–31. [Google Scholar] [CrossRef]
- Chen, H.; Yu, X.; Chen, Y.; Wu, H.; Wu, Z.; Zhong, J.; Tang, Z. Reversible Splenial Lesion Syndrome in Children: A Retrospective Study of 130 Cases. Front. Neurol. 2023, 14, 1241549. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, G.; Schotsmans, K.; Maréchal, E.; Crols, R. Mild Encephalitis with Reversible Splenial (MERS) Lesion Syndrome Due to Influenza B Virus. Pract. Neurol. 2018, 18, 391–392. [Google Scholar] [CrossRef]
- Feraco, P.; Porretti, G.; Marchiò, G.; Bellizzi, M.; Recla, M. Mild Encephalitis/Encephalopathy with Reversible Splenial Lesion (MERS) Due to Cytomegalovirus: Case Report and Review of the Literature. Neuropediatrics 2018, 49, 068–071. [Google Scholar] [CrossRef]
- Bektaş, G.; Akçay, N.; Boydağ, K.; Şevketoğlu, E. Reversible Splenial Lesion Syndrome Associated with SARS-CoV-2 Infection in Two Children. Brain Dev. 2021, 43, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Britton, P.N.; Dale, R.C.; Blyth, C.C.; Macartney, K.; Crawford, N.W.; Marshall, H.; Clark, J.E.; Elliott, E.J.; Webster, R.I.; Cheng, A.C.; et al. Influenza-Associated Encephalitis/Encephalopathy Identified by the Australian Childhood Encephalitis Study 2013–2015. Pediatr. Infect. Dis. J. 2017, 36, 1021–1026. [Google Scholar] [CrossRef]
- Talukder, N.T.; Feezel, A.; Lankford, J.E. Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion Associated with Systemic Mycoplasma Pneumoniae Infection in North America: A Case Report. J. Med. Case Rep. 2022, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Tuscano, A.; Zoppo, M.; Canavese, C.; Cogoni, M.; Scolfaro, C. Transient Blindness Associated with Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion (MERS): A Case Report and Review of Literature. Ital. J. Pediatr. 2020, 46, 152. [Google Scholar] [CrossRef]
- Le Soudéer, L.; Truong, J.; Le Gal, J.; Escoda, S. Shigella-Associated Mild Encephalitis with Reversible Splenial Lesion in Hospital Center Delafontaine, Saint-Denis, France: A Case Report. BMC Pediatr. 2022, 22, 421. [Google Scholar] [CrossRef]
- Varol, F.; Ergul, N.; Sahin, E.G.; Can, Y.Y.; Ergul, U.; Guven, S.; Cam, H. Can Plasma Exchange Therapy Be an Option for the Treatment of SARS-CoV-2 Related Splenial Lesion Syndrome: Two Cases from the Pediatric Intensive Care Unit. Transfus. Apher. Sci. 2022, 61, 103491. [Google Scholar] [CrossRef]
- Oger, V.; Bost, C.; Salah, L.; Yazbeck, E.; Maurey, H.; Bellesme, C.; Sevin, C.; Adamsbaum, C.; Chrétien, P.; Benaiteau, M.; et al. Mild Encephalitis/Encephalopathy with Reversible Splenial Lesion Syndrome: An Unusual Presentation of Anti-GFAP Astrocytopathy. Eur. J. Paediatr. Neurol. 2020, 26, 89–91. [Google Scholar] [CrossRef]
- Grosset, L.; Hosseini, H.; Bapst, B.; Hodel, J.; Cleret De Langavant, L.; Faugeras, F.; Bachoud-Lévi, A.-C.; Seddik, L. Mild Encephalopathy with reversible Splenial Lesion: Description of Nine Cases and Review of the Literature. Seizure 2021, 88, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Brar, J.S.S.; Gupta, S.; Mohideen, S.M.H.; Liauw, L.; Lath, N. The Pancreatic and Extrapancreatic Manifestations of IgG4-Related Disease. Diagn. Interv. Radiol. 2019, 24, 83–88. [Google Scholar] [CrossRef]
- Gupte, A.; Sriram, S.; Gunasekaran, V.; Chaudhari, K.; Kamat, D. The Triad of COVID-19 in Children: Acute COVID-19, Multisystem Inflammatory Syndrome, and Long COVID—Part II. Pediatr. Ann. 2025, 54, e40–e44. [Google Scholar] [CrossRef] [PubMed]
- Palabiyik, F.; Akcay, N.; Sevketoglu, E.; Hatipoglu, N.; Sari, E.E.; Inci, E. Imaging of Multisystem Inflammatory Disease in Children (MIS-C) Associated With COVID-19. Acad. Radiol. 2021, 28, 1200–1208. [Google Scholar] [CrossRef]
- Mizuguchi, M.; Yamanouchi, H.; Ichiyama, T.; Shiomi, M. Acute Encephalopathy Associated with Influenza and Other Viral Infections. Acta Neurol. Scand. 2007, 115, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Matsushige, T.; Inoue, H.; Shirabe, K.; Fukano, R.; Ichiyama, T. Serum and Cerebrospinal Fluid Cytokine Profile of Patients with 2009 Pandemic H1N1 Influenza Virus-Associated Encephalopathy. Cytokine 2011, 54, 167–172. [Google Scholar] [CrossRef]
- Motobayashi, M.; Fukuyama, T.; Okuno-Yuguchi, J.; Tsukahara, K.; Nahaharu, S.; Hagimoto, R.; Kinoshita, T.; Nakazawa, Y.; Inaba, Y. Subclinical neuroaxonal damage in patients with clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Pediatr. Neurol. 2017, 74, e3–e4. [Google Scholar] [CrossRef]
- Ueda, F.; Yoshie, Y.; Aburano, H.; Hashimoto, M.; Matsui, O.; Gabata, T. Splenial and white matter lesions showing transiently-reduced diffusion in mild encephalopathy monitored with MR spectroscopy and imaging. Magn. Reson. Med. Sci. 2014, 13, 271–275. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, C. Reversible Focal Splenium Lesion–MRS Study of a Different Etiology. Acta Neurol. Taiwan 2009, 18, 203–206. [Google Scholar]
- Shimizu, H.; Kataoka, H.; Yagura, H.; Hirano, M.; Taoka, T.; Ueno, S. Extensive neuroimaging of a transient lesion in the splenium of the corpus callosum. Eur. J. Neurol. 2007, 14, e37–e39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).