fNIRS Feasibility to Measure Brain Oxygenation Patterns of the Motor Cortex in Relation to Massage and Reflex Locomotion Therapy in Babies
Abstract
1. Introduction
2. Study Objectives
- -
- To test the feasibility of using fNIRS in the measurement of brain oxygenation in healthy and preterm babies.
- -
- To test the potential differences in brain oxygenation between massage therapy and Reflex Locomotion Therapy.
- -
- To test potential sex differences in brain oxygenation after tactile stimuli.
3. Materials and Methods
3.1. Design
3.2. Sample
3.3. Ethical Considerations
3.4. Material
3.5. Variables
3.6. Procedure
3.7. Data Processing
3.8. Statistical Analysis
4. Results
5. Discussion
6. Future Lines of Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HHb | Deoxyhemoglobin |
| fNIRS | Functional near-infrared spectroscopy |
| HbO | Oxygenated hemoglobin |
| NIRS | Near-infrared spectroscopy |
| rSO2 | Regional oxygen saturation |
References
- Duffau, H. Brain plasticity: From pathophysiological mechanisms to therapeutic applications. J. Clin. Neurosci. 2006, 13, 885–897. [Google Scholar] [CrossRef]
- Kolb, B.; Gibb, R. Brain plasticity and behaviour in the developing brain. J. Can. Acad. Child. Adolesc. Psychiatry 2011, 20, 265–276. [Google Scholar]
- Stiles, J. Neural plasticity and cognitive development. Dev. Neuropsychol. 2000, 18, 237–272. [Google Scholar] [CrossRef]
- Knudsen, E.I. Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci. 2004, 16, 1412–1425. [Google Scholar] [CrossRef]
- Lloyd-Fox, S.; Blasi, A.; Elwell, C.E. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010, 34, 269–284. [Google Scholar] [CrossRef]
- Bortfeld, H.; Fava, E.; Boas, D.A. Identifying cortical lateralization of speech processing in infants using near-infrared spectroscopy. Dev. Neuropsychol. 2009, 34, 52–65. [Google Scholar] [CrossRef]
- Aslin, R.N.; Fiser, J. Methodological challenges for understanding cognitive development in infants. Trends Cogn. Sci. 2005, 9, 92–98. [Google Scholar] [CrossRef]
- de Oliveira, S.R.; Machado, A.C.C.P.; de Paula, J.J.; Novi, S.L.; Mesquita, R.C.; Miranda, D.M.; Bouzada, M.C.F. Changes of functional response in sensorimotor cortex of preterm and full-term infants during the first year: An fNIRS study. Early Hum. Dev. 2019, 133, 23–28. [Google Scholar] [CrossRef]
- Munk, N.; Symons, B.; Shang, Y.; Cheng, R.; Yu, G. Noninvasively measuring the hemodynamic effects of massage on skeletal muscle: A novel hybrid near-infrared diffuse optical instrument. J. Bodyw. Mov. Ther. 2012, 16, 22–28. [Google Scholar] [CrossRef]
- Teicher, M.H.; Samson, J.A.; Anderson, C.M.; Ohashi, K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016, 17, 652–666. [Google Scholar] [CrossRef]
- Field, T. Massage therapy research review. Complement. Ther. Clin. Pract. 2014, 20, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Weerapong, P.; Hume, P.A.; Kolt, G.S. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005, 35, 235–256. [Google Scholar] [CrossRef]
- Moraska, A.; Pollini, R.A.; Boulanger, K.; Brooks, M.Z.; Teitlebaum, L. Physiological adjustments to stress measures following massage therapy: A review of the literature. Evid. Based Complement. Altern. Med. 2010, 7, 409–418. [Google Scholar] [CrossRef]
- Sánchez-González, J.L.; Díez-Villoria, E.; Pérez-Robledo, F.; Sanz-Esteban, I.; Llamas-Ramos, I.; Llamas-Ramos, R.; de la Fuente, A.; Bermejo-Gil, B.M.; Canal-Bedia, R.; Martín-Nogueras, A.M. Synergy of Muscle and Cortical Activation through Vojta Reflex Locomotion Therapy in Young Healthy Adults: A Pilot Randomized Controlled Trial. Biomedicines 2023, 11, 3203. [Google Scholar] [CrossRef]
- Kanda, T.; Pidcock, F.S.; Hayakawa, K.; Yamori, Y.; Shikata, Y. Motor outcome differences between two groups of children with spastic diplegia who received different intensities of early onset physiotherapy followed for 5 years. Brain Dev. 2004, 26, 118–126. [Google Scholar] [CrossRef]
- Llamas-Ramos Sánchez-González, J.L.; Alvarado-Omenat, J.J.; Rodriguez-Pérez, V.; Llamas-Ramos, I. Brain electrical activity and oxygenation by Reflex Locomotion Therapy and massage in preterm and term infants. A protocol study. Neuroimage 2024, 298, 120765. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef]
- Gemignani, J.; Gervain, J. Comparing different pre-processing routines for infant fNIRS data. Dev. Cogn. Neurosci. 2021, 48, 100943. [Google Scholar] [CrossRef]
- Aasted, C.M.; Yücel, M.A.; Cooper, R.J.; Petkov, M.P.; Boas, D.A.; Cooper, R.J.; Dubb, J.; Tsuzuki, D. Anatomical guidance for functional near-infrared spectroscopy: AtlasViewer tutorial. Neurophoton 2015, 2, 020801. [Google Scholar] [CrossRef]
- Shibata, M.; Fuchino, Y.; Naoi, N.; Kohno, S.; Kawai, M.; Okanoya, K.; Myowa-Yamakoshi, M. Broad cortical activation in response to tactile stimulation in newborns. Neuroreport 2012, 23, 373–377. [Google Scholar] [CrossRef]
- André, V.; Durier, V.; Beuchée, A.; Roué, J.M.; Lemasson, A.; Hausberger, M.; Sizun, J.; Henry, S. Higher tactile sensitivity in preterm infants at term-equivalent age: A pilot study. PLoS ONE 2020, 15, e0229270. [Google Scholar] [CrossRef]
- Miguel, H.O.; Gonçalves, Ó.F.; Sampaio, A. Behavioral response to tactile stimuli relates to brain response to affective touch in 12-month-old infants. Dev. Psychobiol. 2020, 62, 107–115. [Google Scholar] [CrossRef]
- Jönsson, E.H.; Kotilahti, K.; Heiskala, J.; Wasling, H.B.; Olausson, H.; Croy, I.; Mustaniemi, H.; Hiltunen, P.; Tuulari, J.J.; Scheinin, N.M.; et al. Affective and non-affective touch evoke differential brain responses in 2-month-old infants. Neuroimage 2018, 169, 162–171. [Google Scholar] [CrossRef]
- Fuchino, Y.; Kato, I.; Htun, Y.; Takano, Y.; Konishi, Y.; Koyano, K.; Nakamura, S.; Tanaka, N.; Kusaka, T.; Konishi, Y. Developmental changes in neonatal hemodynamics during tactile stimulation using whole-head functional near-infrared spectroscopy. Neuroimage 2023, 284, 120465. [Google Scholar] [CrossRef]
- Lai, M.M.; D’Acunto, G.; Guzzetta, A.; Boyd, R.N.; Rose, S.E.; Fripp, J.; Finnigan, S.; Ngenda, N.; Love, P.; Whittingham, K.; et al. PREMM: Preterm early massage by the mother: Protocol of a randomised controlled trial of massage therapy in very preterm infants. BMC Pediatr. 2016, 16, 146. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, C.; Cheng, L.; Li, H. Effects of massage therapy on preterm infants and their mothers: A systematic review and meta-analysis of randomized controlled trials. Front. Pediatr. 2023, 11, 1198730. [Google Scholar] [CrossRef]
- Li, Q.; Becker, B.; Wernicke, J.; Chen, Y.; Zhang, Y.; Li, R.; Le, J.; Kou, J.; Zhao, W.; Kendrick, K.M. Foot massage evokes oxytocin release and activation of orbitofrontal cortex and superior temporal sulcus. Psychoneuroendocrinology 2019, 101, 193–203. [Google Scholar] [CrossRef]
- Chen, Y.F.; Mao, M.C.; Zhu, G.Y.; Sun, C.C.; Zhao, J.W.; He, H.X.; Chen, Y.H.; Xu, D.S. The changes of neuroactivity of Tui Na (Chinese massage) at Hegu acupoint on sensorimotor cortex in stroke patients with upper limb motor dysfunction: A fNIRS study. BMC Complement. Med. Ther. 2023, 23, 334. [Google Scholar] [CrossRef]
- Sagar, S.M.; Dryden, T.; Myers, C. Research on therapeutic massage for cancer patients: Potential biologic mechanisms. J. Soc. Integr. Oncol. 2007, 5, 155–162. [Google Scholar] [CrossRef]
- Pinti, P.; Dina, L.M.; Smith, T.J. Ecological functional near-infrared spectroscopy in mobile children: Using short separation channels to correct for systemic contamination during naturalistic neuroimaging. Neurophotonics 2024, 11, 045004. [Google Scholar] [CrossRef]
- Taga, G.; Asakawa, K.; Maki, A.; Konishi, Y.; Koizumi, H. Brain imaging in awake infants by near-infrared optical topography. Proc. Natl. Acad. Sci. USA 2003, 100, 10722–10727. [Google Scholar] [CrossRef] [PubMed]
- Kozberg, M.G.; Chen, B.R.; DeLeo, S.E.; Bouchard, M.B.; Hillman, E.M. Resolving the transition from negative to positive blood oxygen level-dependent responses in the developing brain. Proc. Natl. Acad. Sci. USA 2013, 110, 4380–4385. [Google Scholar] [CrossRef] [PubMed]
- Issard, C.; Gervain, J. Variability of the hemodynamic response in infants: Influence of experimental design and stimulus complexity. Dev. Cogn. Neurosci. 2018, 33, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Homae, F.; Watanabe, H.; Otobe, T.; Nakano, T.; Go, T.; Konishi, Y.; Taga, G. Development of global cortical networks in early infancy. J. Neurosci. 2010, 30, 4877–4882. [Google Scholar] [CrossRef]
- Emberson, L.L.; Richards, J.E.; Aslin, R.N. Top-down modulation in the infant brain: Learning-induced expectations rapidly affect the sensory cortex at 6 months. Proc. Natl. Acad. Sci. USA 2015, 112, 9585–9590. [Google Scholar] [CrossRef]
- Aslin, R.N.; Shukla, M.; Emberson, L.L. Hemodynamic correlates of cognition in human infants. Annu. Rev. Psychol. 2015, 66, 349–379. [Google Scholar] [CrossRef]
- Lloyd-Fox, S.; Begus, K.; Halliday, D.; Pirazzoli, L.; Blasi, A.; Papademetriou, M.; Darboe, M.K.; Prentice, A.M.; Johnson, M.H.; Moore, S.E.; et al. Cortical specialisation to social stimuli from the first days to the second year of life: A rural Gambian cohort. Dev. Cogn. Neurosci. 2017, 25, 92–104. [Google Scholar] [CrossRef]

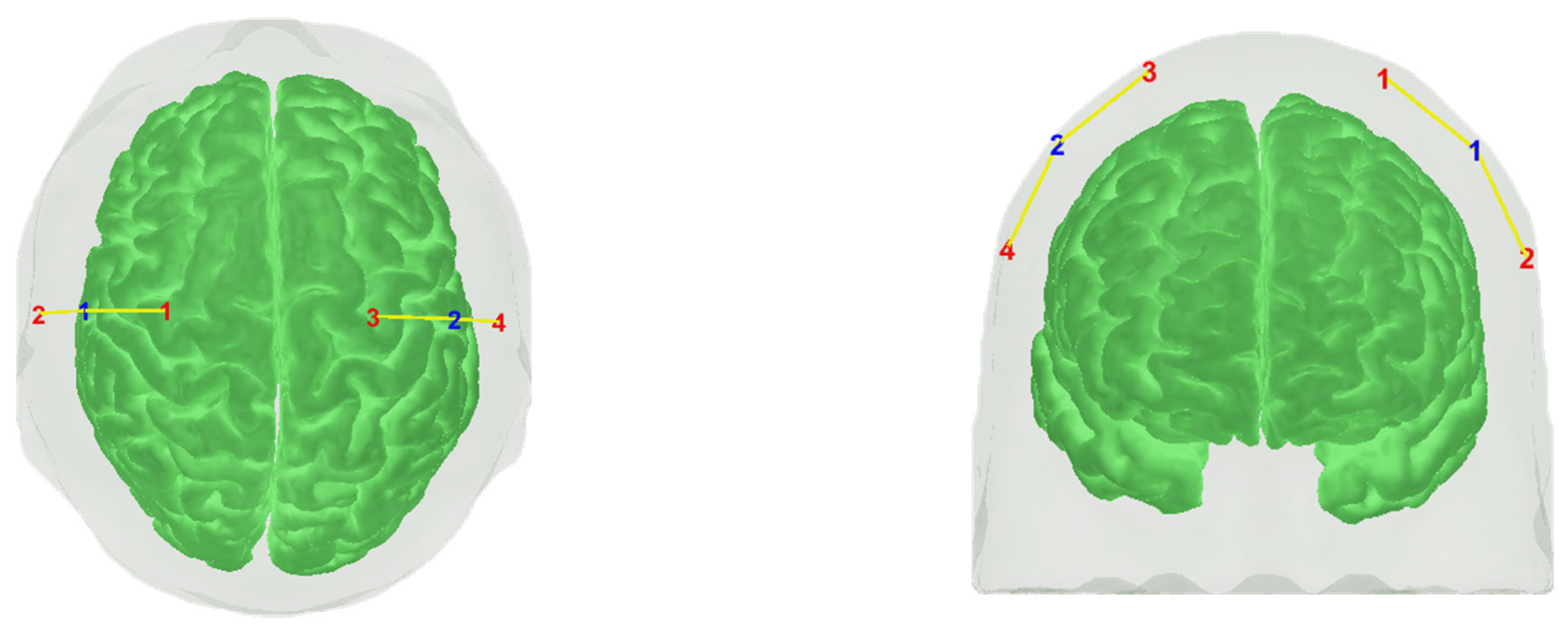

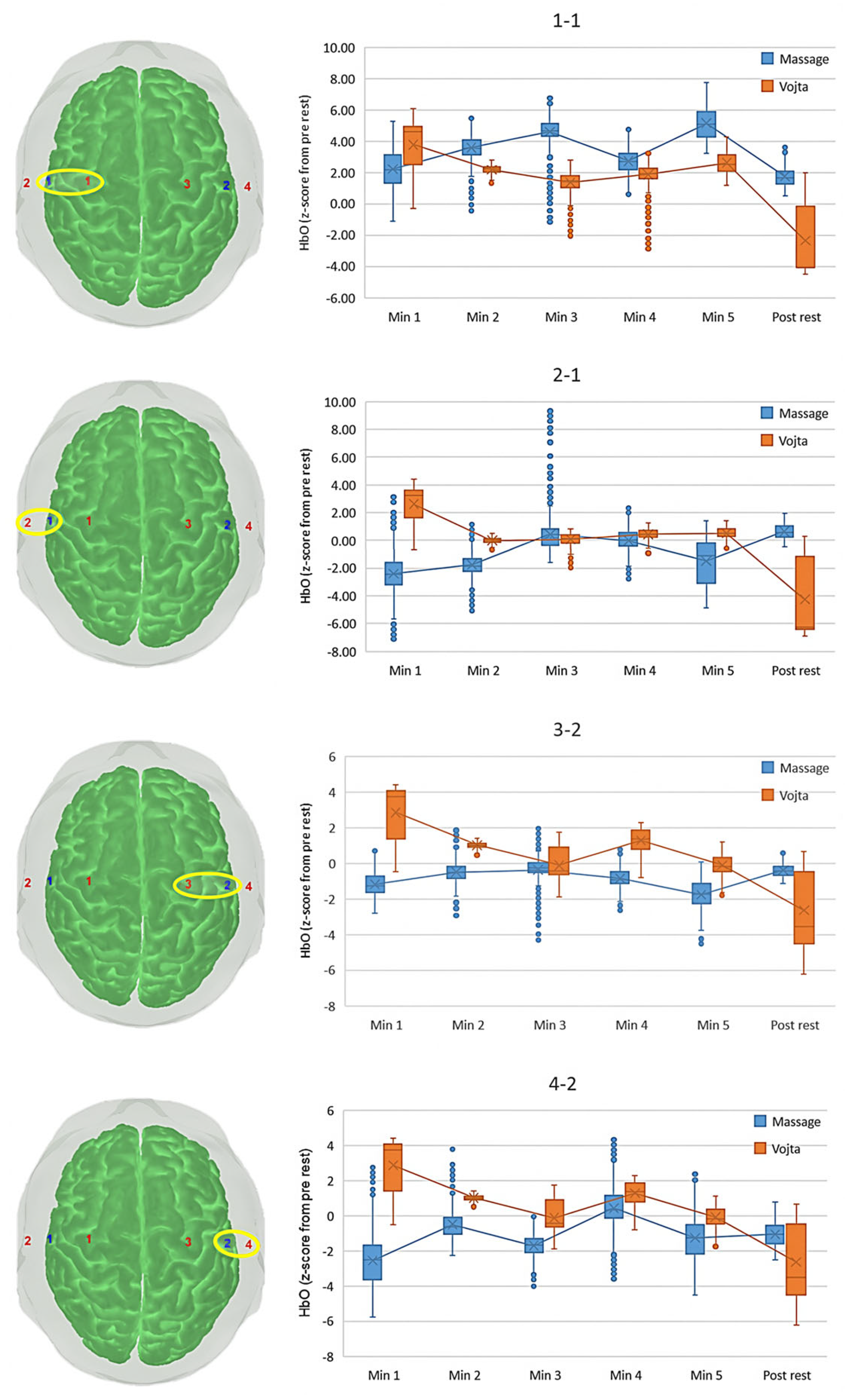
| Optode | Time Interval | Massage | Vojta | p-Values (Post Hoc) |
|---|---|---|---|---|
| Left superior (1-1) | Min 1 | 2.156 (1.479) | 3.816 (1.804) | <0.001 |
| Min 2 | 3.577 (0.814) | 2.199 (0.251) | <0.001 | |
| Min 3 | 4.618 (1.032) | 1.317 (0.639) | <0.001 | |
| Min 4 | 2.687 (0.841) | 1.838 (0.660) | <0.001 | |
| Min 5 | 5.112 (1.006) | 2.726 (0.598) | <0.001 | |
| Post rest | 1.752 (0.693) | −1.709 (2.546) | <0.001 | |
| p-value (post hoc) | <0.001 | <0.001 | ||
| Left inferior (2-1) | Min 1 | −2.369 (1.596) | 2.688 (1.341) | <0.001 |
| Min 2 | −1.782 (0.843) | −0.011 (0.197) § | <0.001 | |
| Min 3 | 0.438 (1.436) | 0.017 (0.425) § | <0.001 | |
| Min 4 | −0.069 (1.013) | 0.422 (0.408) | <0.001 | |
| Min 5 | −1.453 (1.645) | 0.584 (0.379) | <0.001 | |
| Post rest | 0.645 (0.505) | −3.603 (2.923) | <0.001 | |
| p-value (post hoc) | <0.001; 3-post rest p = 0.002 | <0.001 | ||
| Right superior (3-2) | Min 1 | −1.173 (0.706) | 2.909 (1.581) | <0.001 |
| Min 2 | −0.520 (0.527) | 1.012 (0.147) | <0.001 | |
| Min 3 | −0.397 (0.766) § | −0.155 (0.843) § | <0.001 | |
| Min 4 | −0.851 (0.490) | 1.332 (0.638) | <0.001 | |
| Min 5 | −1.771 (0.728) | −1.104 (0.547) § | <0.001 | |
| Post rest | −0.389 (0.357) § | −2.140 (2.315) | <0.001 | |
| p-value (post hoc) | <0.001; 2-post rest p = 0.018 | <0.001 but Min3-Min5 | ||
| Right inferior (4-2) | Min 1 | −2.515 (1.521) | 2.912 (1.578) | p < 0.001 |
| Min 2 | −0.511 (0.782) | 1.019 (0.145) | p < 0.001 | |
| Min 3 | −1.834 (0.690) | −0.164 (0.840) § | p < 0.001 | |
| Min 4 | 0.790 (1.100) | 1.328 (0.636) | p < 0.001 | |
| Min 5 | −1.616 (1.021) | −0.095 (0.545) § | p < 0.001 | |
| Post rest | −1.012 (0.606) | −2.144 (2.311) | p < 0.001 | |
| p-value (post hoc) | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llamas-Ramos, R.; Sánchez-González, J.L.; Alvarado-Omenat, J.J.; Sanz-Esteban, I.; Serrano, J.I.; Llamas-Ramos, I. fNIRS Feasibility to Measure Brain Oxygenation Patterns of the Motor Cortex in Relation to Massage and Reflex Locomotion Therapy in Babies. J. Clin. Med. 2025, 14, 3818. https://doi.org/10.3390/jcm14113818
Llamas-Ramos R, Sánchez-González JL, Alvarado-Omenat JJ, Sanz-Esteban I, Serrano JI, Llamas-Ramos I. fNIRS Feasibility to Measure Brain Oxygenation Patterns of the Motor Cortex in Relation to Massage and Reflex Locomotion Therapy in Babies. Journal of Clinical Medicine. 2025; 14(11):3818. https://doi.org/10.3390/jcm14113818
Chicago/Turabian StyleLlamas-Ramos, Rocío, Juan Luis Sánchez-González, Jorge Juan Alvarado-Omenat, Ismael Sanz-Esteban, J. Ignacio Serrano, and Inés Llamas-Ramos. 2025. "fNIRS Feasibility to Measure Brain Oxygenation Patterns of the Motor Cortex in Relation to Massage and Reflex Locomotion Therapy in Babies" Journal of Clinical Medicine 14, no. 11: 3818. https://doi.org/10.3390/jcm14113818
APA StyleLlamas-Ramos, R., Sánchez-González, J. L., Alvarado-Omenat, J. J., Sanz-Esteban, I., Serrano, J. I., & Llamas-Ramos, I. (2025). fNIRS Feasibility to Measure Brain Oxygenation Patterns of the Motor Cortex in Relation to Massage and Reflex Locomotion Therapy in Babies. Journal of Clinical Medicine, 14(11), 3818. https://doi.org/10.3390/jcm14113818









