Non-Anemic Iron Deficiency Predicts COPD Exacerbations and Hospitalizations: Results from a Prospective Cohort
Abstract
1. Introduction
2. Methods
2.1. Study Design
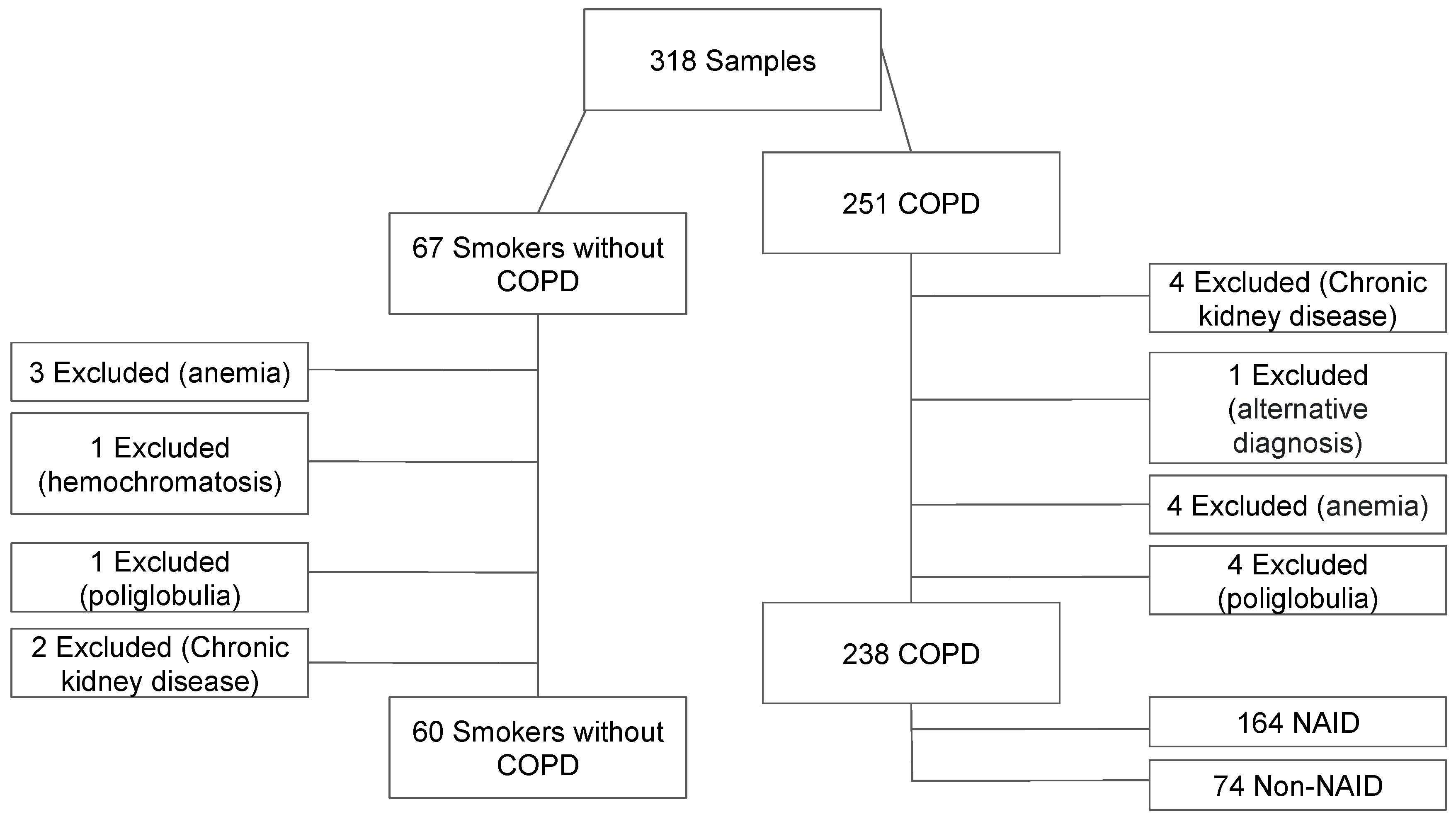
2.2. Participants
2.3. Clinical and Functional Assessment
2.4. Laboratory Analysis
2.5. Follow-Up and Outcomes
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Iron Metabolism Markers in COPD Patients
3.3. Association Between NAID and COPD Severity
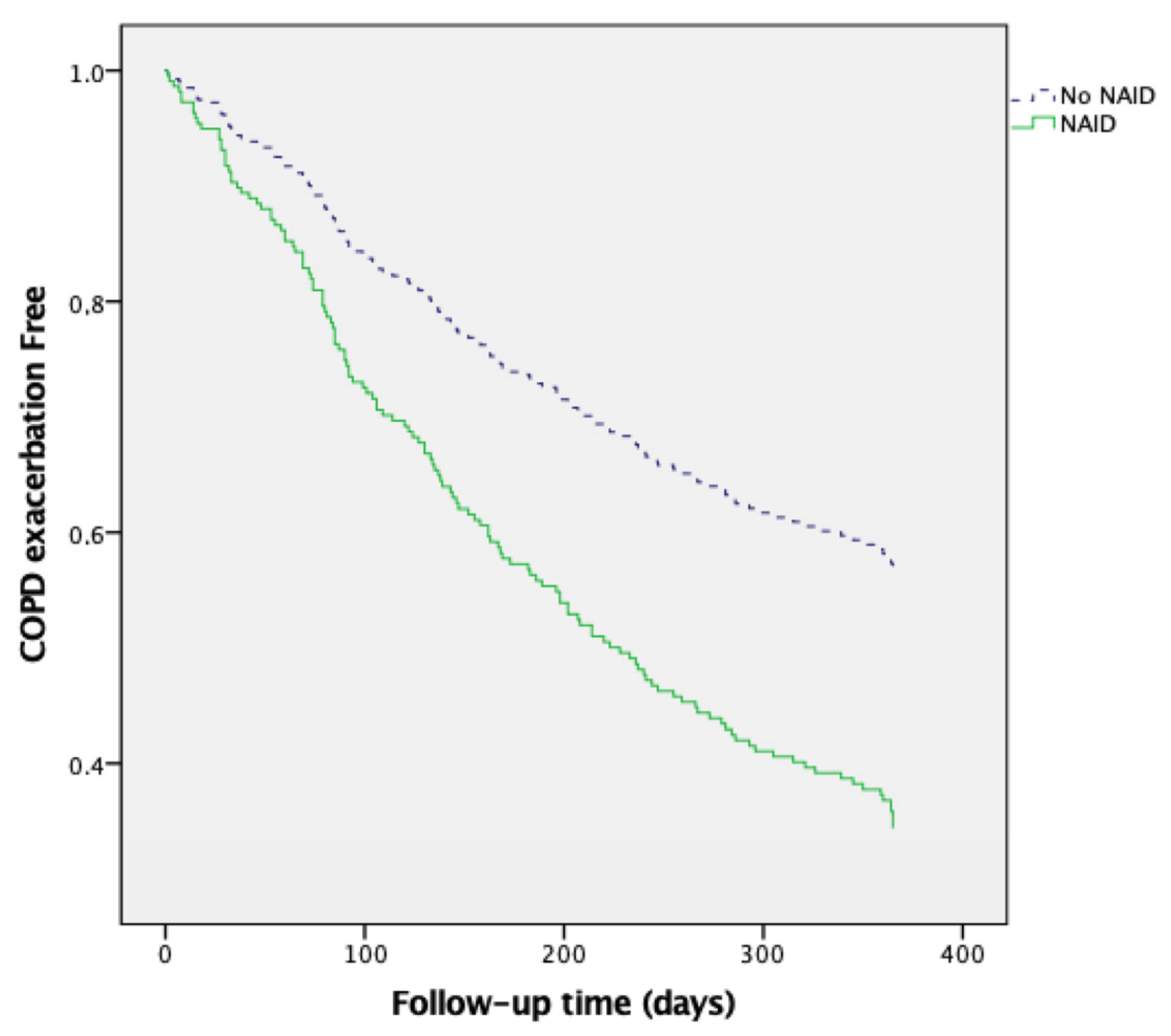
3.4. NAID as a Predictor of COPD Exacerbations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2025 Report. 2025. Available online: https://goldcopd.org/2025-gold-report/ (accessed on 24 December 2024).
- Farrell, L.A.; O’Rourke, M.B.; Padula, M.P.; Souza-Fonseca-Guimaraes, F.; Caramori, G.; Wark, P.A.B.; Dharmage, S.C.; Hansbro, P.M. The Current Molecular and Cellular Landscape of Chronic Obstructive Pulmonary Disease (COPD): A Review of Therapies and Efforts towards Personalized Treatment. Proteomes 2024, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Amado, C.A.; Martín-Audera, P.; Agüero, J.; Lavín, B.A.; Guerra, A.R.; Boucle, D.; Ferrer-Pargada, D.; Berja, A.; Martín, F.; Casanova, C.; et al. Circulating levels of mitochondrial oxidative stress-related peptides MOTS-c and Romo1 in stable COPD: A cross-sectional study. Front. Med. 2023, 10, 1100211. [Google Scholar] [CrossRef] [PubMed]
- Amado, C.A.; Martín-Audera, P.; Agüero, J.; Ferrer-Pargada, D.; Josa Laorden, B.; Boucle, D.; Berja, A.; Lavín, B.A.; Guerra, A.R.; Ghadban, C.; et al. Alterations in circulating mitochondrial signals at hospital admission for COPD exacerbation. Chronic Respir. Dis. 2023, 20, 14799731231220058. [Google Scholar] [CrossRef] [PubMed]
- Amado, C.A.; Muñoz, P.; García-Unzueta, M.; Agüero, J.; Tello, S.; Fueyo, P.; Vega, C.; Lavín, B.A.; Guerra, R.A.; Casanova, C. High parathyroid hormone predicts exacerbations in COPD patients with hypovitaminosis D. Respir. Med. 2021, 182, 106416. [Google Scholar] [CrossRef]
- Amado, C.A.; García-Unzueta, M.; Agüero, J.; Martín-Audera, P.; Fueyo, P.; Lavín, B.A.; Guerra, A.R.; Muñoz, P.; Tello, S.; Berja, A.; et al. Associations of serum sclerostin levels with body composition, pulmonary function, and exacerbations in COPD patients. Pulmonology 2024, 30, 512–521. [Google Scholar] [CrossRef]
- Boutou, A.K.; Polkey, M.I.; Hopkinson, N.S. Non-anaemic iron deficiency in COPD: A potential therapeutic target? Respirology 2015, 20, 1004–1005, Erratum in Respirology 2016, 21, 196. [Google Scholar] [CrossRef][Green Version]
- Rezvani, A.; Masoompour, S.M.; Azarpira, N.; Monjazeb, R.; Akbarzadeh, M.; Salimi, M.; Shahriarirad, R. Serum levels of erythropoietin in patients with chronic obstructive pulmonary disease and anemia. Sci. Rep. 2023, 13, 6990. [Google Scholar] [CrossRef]
- Okonko, D.O.; Grzweslo, A.; Witkowki, T.; Mandal, A.K.; Slater, R.M.; Roughton, M.; Foldes, G.; Thum, T.; Majda, J.; Banasiak, W.; et al. Effect of intravenous iron sucrose on exercise tolerance in anaemic nonanemic patients with symptomatic chronic heart failure iron deficiency FERRIC-HF: A randomized controlled observer-blinded trial. J. Am. Coll. Cardiol. 2008, 51, 103–112. [Google Scholar] [CrossRef]
- Lakhal-Littleton, S.; Cleland, J.G.F. Iron deficiency and supplementation in heart failure. Nat. Rev. Cardiol. 2024, 21, 463–486. [Google Scholar] [CrossRef]
- Martín-Ontiyuelo, C.; Rodó-Pin, A.; Sancho-Muñoz, A.; Martinez-Llorens, J.M.; Admetlló, M.; Molina, L.; Gea, J.; Barreiro, E.; Chiaradía, D.A.R. Is iron deficiency modulating physical activity in COPD? Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 211–214. [Google Scholar] [CrossRef]
- Pizzini, A.; Aichner, M.; Sonnweber, T.; Tancevski, I.; Weiss, G.; Löffler-Ragg, J. The Significance of iron deficiency and anemia in a real-life COPD cohort. Int. J. Med. Sci. 2020, 17, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Hardang, I.M.; Søyseth, V.; Kononova, N.; Hagve, T.A.; Einvik, G. COPD: Iron Deficiency and Clinical Characteristics in Patients With and Without Chronic Respiratory Failure. Chronic Obstr. Pulm. Dis. 2024, 11, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Barberan-Garcia, A.; Rodríguez, D.A.; Blanco, I.; Gea, J.; Torralba, Y.; Arbillaga-Etxarri, A.; Barberà, J.A.; Vilaró, J.; Roca, J.; Orozco-Levi, M. Non-anaemic iron deficiency impairs response to pulmonary rehabilitation in COPD. Respirology 2015, 20, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.A.; Nazir, A.; Qureshi, S.A.; Haleem, N.; Khattak, N.; Rashid, M.A.; Iltaf, S. Iron deficiency without anaemia in copd patients: Assessing exercise capacity and exacerbation frequency. J. Ayub Med. Coll. Abbottabad 2024, 36, 113–118. [Google Scholar] [CrossRef]
- Barbui, T.; Thiele, J.; Gisslinger, H.; Finazzi, G.; Vannucchi, A.M.; Tefferi, A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: Document summary and in-depth discussion. Blood Cancer J. 2018, 8, 15. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Anaemia-How Is It Defined. 2008. Available online: https://www.who.int/data/nutrition/nlis/info/anaemia (accessed on 10 January 2023).
- García-Río, F.; Calle, M.; Burgos, F.; Casan, P.; Del Campo, F.; Galdiz, J.B.; Giner, J.; González-Mangado, N.; Ortega, F.; Puente Maestu, L. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Spirometry. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Arch. Bronconeumol. 2013, 49, 388–401. [Google Scholar] [CrossRef]
- Barreiro, E.; Bustamante, V.; Cejudo, P.; Gáldiz, J.B.; Gea, J.; de Lucas, P.; Martínez-Llorens, J.; Ortega, F.; Puente-Maestu, L.; Roca, J.; et al. SEPAR Guidelines for the evaluation and treatment of muscle dysfunction in patients with chronic obstructive pulmonary disease. Arch. Bronconeumol. 2015, 51, 384–395. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, W.; Qi, G.; Wei, X. Sex-specific association of total mineral intake with pulmonary function in middle-aged and older adults with chronic obstructive pulmonary disease. Sci. Rep. 2024, 14, 29228. [Google Scholar] [CrossRef]
- Wacka, E.; Nicikowski, J.; Jarmuzek, P.; Zembron-Lacny, A. Anemia and Its Connections to Inflammation in Older Adults: A Review. J. Clin. Med. 2024, 13, 2049. [Google Scholar] [CrossRef]
- Wawer, A.A.; Jennings, A.; Fairweather-Tait, S.J. Iron status in the elderly: A review of recent evidence. Mech. Ageing Dev. 2018, 175, 55–73. [Google Scholar] [CrossRef]
- Philip, K.E.J.; Sadaka, A.S.; Polkey, M.I.; Hopkinson, N.S.; Steptoe, A.; Fancourt, D. The prevalence and associated mortality of non-anaemic iron deficiency in older adults: A 14 years observational cohort study. Br. J. Haematol. 2020, 189, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Enjuanes, C.; Bruguera, J.; Grau, M.; Cladellas, M.; Gonzalez, G.; Merono, O.; Moliner-Borja, P.; Verdu, J.M.; Farre, N.; Comin-Colet, J. Iron status in chronic heart failure: Impact on symptoms, functional class and submaximal exercise capacity. Rev. Espanola Cardiol. 2016, 69, 247–255. [Google Scholar] [CrossRef]
- Greenwood, S.A.; Oliveira, B.A.; Asgari, E.; Ayis, S.; Baker, L.A.; Beckley-Hoelscher, N.; Goubar, A.; Banerjee, D.; Bhandari, S.; Chilcot, J.; et al. A Randomized Trial of Intravenous Iron Supplementation and Exercise on Exercise Capacity in Iron-Deficient Nonanemic Patients with CKD. Kidney Int. Rep. 2023, 8, 1496–1505. [Google Scholar] [CrossRef]
- Schols, A.M.; Broekhuizen, R.; Weling-Scheepers, C.A.; Wouters, E.F. Body composition and mortality in chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2005, 82, 53–59. [Google Scholar] [CrossRef]
- Nickol, A.H.; Frise, M.C.; Cheng, H.Y.; McGahey, A.; McFadyen, B.M.; Harris-Wright, T.; Bart, N.K.; Curtis, M.K.; Khandwala, S.; O’Neill, D.P.; et al. A cross-sectional study of the prevalence and associations of iron deficiency in a cohort of patients with chronic obstructive pulmonary disease. BMJ Open 2015, 5, e007911. [Google Scholar] [CrossRef]
- Kunitomo, Y.; Putcha, N.; Fawzy, A.; Raju, S.; McCormack, M.C.; Wise, R.A.; Hansel, N.N.; Balasubramanian, A. Iron deficiency and risk of all-cause hospitalization in a clinical cohort of patients with COPD. Chronic Obstr. Pulm. Dis. 2025, 12, 72–81. [Google Scholar]
- Oppenheimer, S.J. Iron and its relation to immunity and infectious disease. J. Nutr. 2001, 131 (Suppl. S2), 616S–635S. [Google Scholar] [CrossRef]

| Demographic Characteristics and Comorbidities | Control Group n = 60 | COPD n = 238 | p | NAID and COPD n = 164 | Non-NAID and COPD n = 74 | p |
|---|---|---|---|---|---|---|
| Age (years) | 66.1 ± 6.1 | 66.9 ± 8.1 | 0.455 | 66.9 ± 8.2 | 66.7 ± 7.7 | 0.796 |
| Male sex n (%) | 37 (61.7) | 152 (63.9) | 0.752 | 89 (54.3) | 63 (85.1) | <0.001 |
| Current smokers n (%) | 30 (50) | 87 (36.6) | 0.057 | 23 (32.1) | 64 (39) | 0.239 |
| CAT score | 3 (1–6) | 10 (6–16) | <0.001 | 11 (7–17) | 9 (4–14) | 0.007 |
| Charlson | 1 (0–2) | 1 (1–2) | 0.012 | 1 (1–2) | 1 (1–2) | 0.900 |
| mMRC score 0/I/II/III/IV n (%) | 47 (78.3)/8 (13.3)/5 (8.3)/0 (0) | 81 (34.0)/69 (29.0)/61 (25.6)/27 (11.3) | <0.001 | 48 (29.3)/51 (31.1)/45 (27.4)/20 (12.2) | 33 (44.6)/18 (24.3)/16 (21.6)/7 (9.5) | 0.149 |
| GOLD 1/2/3/4 n (%) | - | 35 (14.7)/112 (47.1)/69 (29.0)/22 (9.2) | - | 24 (14.6)/76 (46.3)/51 (31.1)/13 (7.9) | 11 (14.9)/36 (48.6)/18 (24.3)/9 (12.2) | 0.603 |
| GOLD A/B/E n (%) | - | 60 (25.2)/72 (30.2)/106 (44.5) | - | 33 (20.1)/54(32.9)/77 (47.0) | 27 (36.5)/18 (24.3)/29 (39.2) | 0.025 |
| 1 or more admissions in the previous year n (%) | - | 170 (71.4) | - | 117 (71.3) | 53 (71.6) | 0.965 |
| ICS treatment n (%) | - | 91 (38.2) | - | 26 (35.1) | 65 (39.6) | 0.509 |
| Long-term oxygen therapy n (%) | - | 14 (5.88) | 9 (5.49) | 5 (6.76) | 0.768 | |
| DM n (%) | 8 (13.3) | 28 (11.8) | 0.739 | 6 (8.1) | 22 (13.4) | 0.240 |
| HTN n (%) | 11 (18.3) | 105 (44.1) | <0.001 | 39 (52.7) | 66 (40.2) | 0.073 |
| Dyslipidemia n (%) | 16 (26.7) | 95 (39.9) | 0.058 | 29 (39.2) | 66 (40.2) | 0.878 |
| Previous cardiovascular events n (%) | 3 (5) | 29 (12.2) | 0.108 | 10 (13.5) | 19 (11.6) | 0.674 |
| Pulmonary Function and Physical Performance Parameters | Control Group n = 60 | COPD n = 238 | p | NAID and COPD n = 164 | Non-NAID and COPD n = 74 | p |
| FVC (mL) | 3403 ± 917 | 2938 ± 862 | 0.001 | 2821 ± 838 | 3197 ± 865 | 0.002 |
| FVC (%) | 102.6 ± 18.6 | 87.8 ± 19.0 | <0.001 | 88.3 ±18.6 | 86.9 ± 19.9 | 0.608 |
| FEV1 (mL) | 2595 (2072–3150) | 1390 (985–1900) | <0.001 | 1320 (940–1800) | 1575 (1122–2110) | 0.006 |
| FEV1 (%) | 99.3 ± 20.4 | 57.1 ± 20.4 | <0.001 | 56.9 ±19.9 | 57.7 ± 20.8 | 0.759 |
| FEV1/FVC | 75.3 (80–72.5) | 50.1 (39.9–60.3) | <0.001 | 48.9 (40.1–58.9) | 54.1 (39.4–61.7) | 0.263 |
| DLCO (%) | 84 (74.3–92.3) | 65.8 (52.5–81) | 0.014 | 62 (51–81) | 69 (56–83) | 0.260 |
| DLCO (mmol/min/kPa) | 6.595 (5.03–8.13) | 4.78 (3.51–6.43) | 0.008 | 4.55 (3.43–6.01) | 5.91 (3.86–6.71) | 0.065 |
| KCO (%) | 94 (74–108) | 70 (44–92) | 0.042 | 74 (56–93) | 88 (69–100) | 0.590 |
| KCO (mmol/min/kPa/L) | 1.33 ± 0.24 | 1.01 ± 0.55 | 0.001 | 0.99 ± 0.6 | 1.05 ± 0.44 | 0.053 |
| Weight (Kg) | 74.0 (63.7–88.0) | 73 (63.8–84.5) | 0.699 | 70.5 (61–81) | 78.8 (66.8–96.1) | <0.001 |
| BMI (Kg/m2) | 27.0 (23.7–31.0) | 26.9 (24.0–31.2) | 0.758 | 26.7 (23.8–30.2) | 28.7 (24.1–32.8) | 0.002 |
| 6MWD (m) | 522 (423–570) | 447 (348–500) | <0.001 | 430 (330–500) | 462 (390–510) | 0.029 |
| Maximum hand grip strength (Kg) | 33.5 (22.8–40) | 29 (23–36) | 0.144 | 26 (22–33) | 34 (27–40) | <0.001 |
| FFMI (Kg/m2) | 19.0 (16.4–20.8) | 18.6 (16.0–20.4) | <0.001 | 17.9 (15.5–20.2) | 20.6 (17.6–22.6) | <0.001 |
| Laboratory Parameters | Control Group n = 60 | COPD n = 238 | p | NAID and COPD n = 164 | Non-NAID and COPD n = 74 | p |
| Albumin (g/dL) | 4.7 (4.5–4.9) | 4.6 (4.4–4.8) | 0.03 | 4.6 (4.3–4.8) | 4.6 (4.5–4.9) | 0.184 |
| Creatinine (mg/dL) | 0.82 (0.68–0.97) | 0.79 (0.68–0.91) | 0.377 | 0.78 (0.67–0.89) | 0.84 (0.71–0.95) | 0.042 |
| CK (UI/L) | 72.5 (52.0–115.3) | 80.5 (54.3–117.8) | 0.472 | 79 (52–113) | 92.5 (57.5–128.8) | 0.236 |
| Hb (g/dL) | 14.1 ± 1.3 | 14.0 ± 1.3 | 0.321 | 14.514.8 ± 1.2 | 14.8 ± 1.2 | 0.536 |
| NAID n(%) | 28 (46.7) | 164 (68.9) | 0.001 | - | - | - |
| Iron (µg/dL) | 66.1 ± 6.1 | 66.9 ± 8.1 | 0.188 | 88.6 ± 35.7 | 108.1 ± 26.3 | <0.001 |
| Ferritin (ng/mL) | 105 (50–203) | 78.4 (33.8–167.3) | 0.097 | 47.4 (25.1–82.0) | 216 (163–318) | <0.001 |
| Transferrin (mg/dL) | 243 (223–267) | 253 (228–279) | 0.085 | 258 (239–297) | 240 (221–256) | <0.001 |
| Transferrin saturation (%) | 29.9 ± 11.4 | 26.6 ± 10.4 | 0.037 | 24.2 ± 10.4 | 32.2 ± 8.0 | <0.001 |
| sTFR1 (mg/L) | 1.853 (0.984–2.872) | 2.231 (1.393–3.642) | 0.019 | 2.250 (1.464–3.857) | 2.102 (1.332–3.236) | 0.221 |
| NAID (Unadjusted) | NAID (Adjusted) | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Age (years) | 1.005 (0.971–1.039) | 0.795 | 0.986 (0.939–1.035) | 0.570 | |
| Sex | |||||
| Male | 1 | 1 | |||
| Female | 4.826 (2.372–9.820) | <0.001 | 2.075 (0.716–6.015) | 0.179 | |
| Smoking status | |||||
| Former | 1 | 1 | |||
| Current | 1.419 (0.792–2.544) | 0.240 | 0.790 (0.332–1.879) | 0.594 | |
| GOLD | |||||
| A or B | 1 | 1 | |||
| E | 1.373 (0.786–2.401) | 0.266 | 0.841 (0.393–1.800) | 0.656 | |
| mMRC Dyspnea score | 1.305 (0.986–1.728) | 0.063 | 1.079 (0.682–1.710) | 0.745 | |
| Charlson | 1.098 (0.822–1.468) | 0.526 | 1.227 (0.831–1.811) | 0.304 | |
| ICS treatment | 0.825 (0.466–1.460) | 0.509 | 1.628 (0.718–3.694) | 0.244 | |
| FFMI (kg/m2) | 0.707.022 (0.620–0.806) | <0.001 | 0.734 (0.619–0.871) | <0.001 | |
| 6MWD (m) | 0.996 (0.994–0.999) | 0.005 | 0.995 (0.991–0.999) | 0.010 | |
| FEV1 (%) | 0.998 (0.985–1.011) | 0.758 | 1.007 (0.978–1.036) | 0.645 | |
| FVC (%) | 1.004 (0.989–1.018) | 0.606 | 1.016 (0.989–1.044) | 0.251 | |
| B | Wald | p | HR | 95% IC | ||
|---|---|---|---|---|---|---|
 Inferior |  Superior | |||||
| Age | 0.021 | 3.677 | 0.055 | 1.022 | 1.000 | 1.044 |
| Sex | −0.248 | 1.794 | 0.180 | 0.780 | 0.543 | 1.122 |
| Current smoker | 0.068 | 0.122 | 0.727 | 1.071 | 0.730 | 1.571 |
| Charlson | −0.191 | 3.706 | 0.054 | 0.826 | 0.680 | 1.003 |
| mMRC | −0.114 | 1.209 | 0.271 | 0.892 | 0.727 | 1.094 |
| FEV1 (% predicted) | −0.015 | 8.510 | 0.004 | 0.985 | 0.976 | 0.995 |
| GOLD E | −0.186 | 1.084 | 0.298 | 0.831 | 0.586 | 1.178 |
| NAID | 0.611 | 8.622 | 0.003 | 1.843 | 1.225 | 2.772 |
| B | Wald | p | HR | 95% IC | ||
|---|---|---|---|---|---|---|
 Inferior |  Superior | |||||
| Age | 0.044 | 4.387 | 0.036 | 1.045 | 1.003 | 1.089 |
| Sex | 0.048 | 0.021 | 0.885 | 1.050 | 0.546 | 2.019 |
| Current smoker | <0.001 | <0.001 | 0.999 | 1.000 | 0.492 | 2.029 |
| Charlson | −0.019 | 0.019 | 0.891 | 0.981 | 0.745 | 1.292 |
| mMRC | 0.349 | 3.459 | 0.063 | 1.417 | 0.981 | 2.047 |
| FEV1 (% predicted) | −0.032 | 8.912 | 0.003 | 0.968 | 0.948 | 0.989 |
| GOLD E | 0.323 | 1.028 | 0.311 | 1.381 | 0.740 | 2.580 |
| NAID | 0.940 | 4.808 | 0.028 | 2.559 | 1.105 | 5.926 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amado, C.A.; Ghadban, C.; Agüero, J.; Lavín, B.A.; Martín-Audera, P.; Guerra, A.R.; Berja, A.; Aranda, N.; Guzun, A.; Insua, A.I.; et al. Non-Anemic Iron Deficiency Predicts COPD Exacerbations and Hospitalizations: Results from a Prospective Cohort. J. Clin. Med. 2025, 14, 4154. https://doi.org/10.3390/jcm14124154
Amado CA, Ghadban C, Agüero J, Lavín BA, Martín-Audera P, Guerra AR, Berja A, Aranda N, Guzun A, Insua AI, et al. Non-Anemic Iron Deficiency Predicts COPD Exacerbations and Hospitalizations: Results from a Prospective Cohort. Journal of Clinical Medicine. 2025; 14(12):4154. https://doi.org/10.3390/jcm14124154
Chicago/Turabian StyleAmado, Carlos A., Cristina Ghadban, Juan Agüero, Bernardo A. Lavín, Paula Martín-Audera, Armando R. Guerra, Ana Berja, Nieves Aranda, Anastasia Guzun, Ana Isabel Insua, and et al. 2025. "Non-Anemic Iron Deficiency Predicts COPD Exacerbations and Hospitalizations: Results from a Prospective Cohort" Journal of Clinical Medicine 14, no. 12: 4154. https://doi.org/10.3390/jcm14124154
APA StyleAmado, C. A., Ghadban, C., Agüero, J., Lavín, B. A., Martín-Audera, P., Guerra, A. R., Berja, A., Aranda, N., Guzun, A., Insua, A. I., & García-Unzueta, M. (2025). Non-Anemic Iron Deficiency Predicts COPD Exacerbations and Hospitalizations: Results from a Prospective Cohort. Journal of Clinical Medicine, 14(12), 4154. https://doi.org/10.3390/jcm14124154





