Incident Cardiometabolic Comorbidities in Smokers with/Without Chronic Obstructive Pulmonary Disease: A Long-Term Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Clinical Measures
2.3. Follow-Up and Lung Function Decline
2.4. Statistical Analysis
3. Results
3.1. Rate of Lung Function Annual Decline and New COPD Diagnosis During Follow-Up
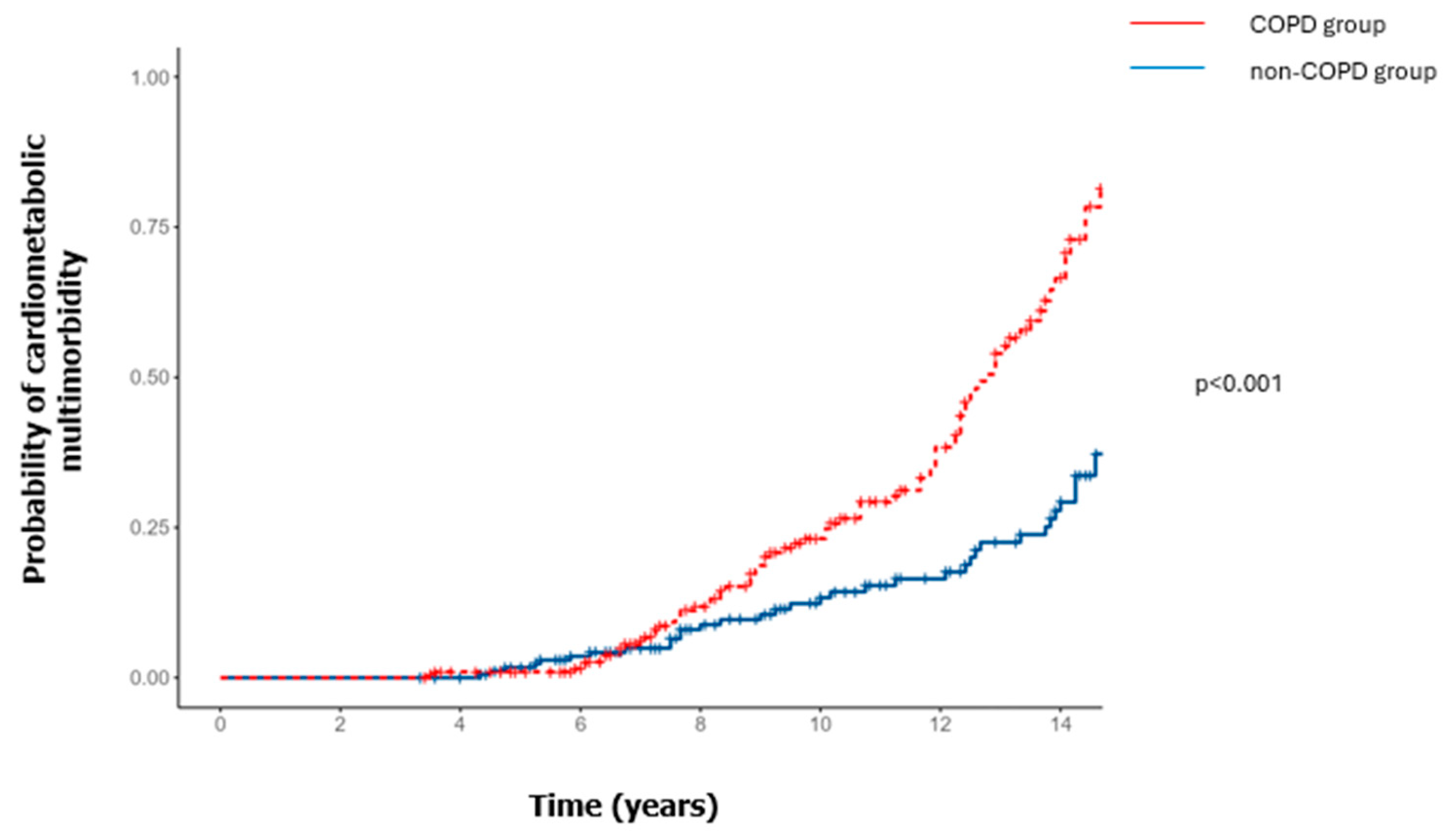
3.2. Incident Cardiometabolic Comorbidities
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collaborators GBDCRD. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kuhn, M.; Prettner, K.; Yu, F.; Yang, T.; Barnighausen, T.; Bloom, D.E.; Wang, C. The global economic burden of chronic obstructive pulmonary disease for 204 countries and territories in 2020–2050: A health-augmented macroeconomic modelling study. Lancet Glob. Health 2023, 11, e1183–e1193. [Google Scholar] [CrossRef] [PubMed]
- Lokke, A.; Lange, P.; Lykkegaard, J.; Ibsen, R.; Andersson, M.; de Fine Licht, S.; Hilberg, O. Economic Burden of COPD by Disease Severity—A Nationwide Cohort Study in Denmark. Int. J. Chron. Obstruct Pulmon Dis. 2021, 16, 603–613. [Google Scholar] [CrossRef]
- Chen, W.; FitzGerald, J.M.; Sin, D.D.; Sadatsafavi, M.; Canadian Respiratory Research Network. Excess economic burden of comorbidities in COPD: A 15-year population-based study. Eur. Respir. J. 2017, 50, 1700393. [Google Scholar] [CrossRef]
- Tan, D.J.; Lodge, C.J.; Walters, E.H.; Bui, D.S.; Pham, J.; Lowe, A.J.; Bowatte, G.; Vicendese, D.; Erbas, B.; Johns, D.P.; et al. Can We Use Lung Function Thresholds and Respiratory Symptoms to Identify Pre-COPD? A Prospective, Population-based Cohort Study. Am. J. Respir. Crit. Care Med. 2024, 209, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur. Respir. J. 2023, 61, 2300239. [Google Scholar] [CrossRef]
- Dai, X.; Gil, G.F.; Reitsma, M.B.; Ahmad, N.S.; Anderson, J.A.; Bisignano, C.; Carr, S.; Feldman, R.; Hay, S.I.; He, J.; et al. Health effects associated with smoking: A Burden of Proof study. Nat. Med. 2022, 28, 2045–2055. [Google Scholar] [CrossRef]
- De Silva, R.; Silva, D.; Piumika, L.; Abeysekera, I.; Jayathilaka, R.; Rajamanthri, L.; Wickramaarachchi, C. Impact of global smoking prevalence on mortality: A study across income groups. BMC Public Health 2024, 24, 1786. [Google Scholar] [CrossRef]
- Divo, M.J.; Celli, B.R.; Poblador-Plou, B.; Calderon-Larranaga, A.; de-Torres, J.P.; Gimeno-Feliu, L.A.; Berto, J.; Zulueta, J.J.; Casanova, C.; Pinto-Plata, V.M.; et al. Chronic Obstructive Pulmonary Disease (COPD) as a disease of early aging: Evidence from the EpiChron Cohort. PLoS ONE 2018, 13, e0193143. [Google Scholar] [CrossRef] [PubMed]
- Van Remoortel, H.; Hornikx, M.; Langer, D.; Burtin, C.; Everaerts, S.; Verhamme, P.; Boonen, S.; Gosselink, R.; Decramer, M.; Troosters, T.; et al. Risk factors and comorbidities in the preclinical stages of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, 30–38. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Celli, B.R.; Agusti, A.; Criner, G.J.; Dransfield, M.T.; Divo, M.; Krishnan, J.K.; Lahousse, L.; Montes de Oca, M.; Salvi, S.S.; et al. COPD and multimorbidity: Recognising and addressing a syndemic occurrence. Lancet Respir. Med. 2023, 11, 1020–1034. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Anthonisen, N.R.; Soriano, J.B.; Agusti, A.G. Mortality in COPD: Role of comorbidities. Eur. Respir. J. 2006, 28, 1245–1257. [Google Scholar] [CrossRef]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef]
- Huber, M.B.; Wacker, M.E.; Vogelmeier, C.F.; Leidl, R. Excess costs of comorbidities in chronic obstructive pulmonary disease: A systematic review. PLoS ONE 2015, 10, e0123292. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Rajwani, A.; Roper, D.; Zhao, S.; Kline, D.; Odei, J.; Brock, G.; Echouffo-Tcheugui, J.B.; Kalyani, R.R.; Bertoni, A.G.; et al. Associations of Cardiometabolic Multimorbidity With All-Cause and Coronary Heart Disease Mortality Among Black Adults in the Jackson Heart Study. JAMA Netw. Open 2022, 5, e2238361. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; Montes de Oca, M.; Mendez, R.A.; Pinto Plata, V.; Cabral, H.J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef]
- Hajiro, T.; Nishimura, K.; Tsukino, M.; Ikeda, A.; Koyama, H.; Izumi, T. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 158, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.; Criner, G.J. Chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; van der Grinten, C.P.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Quanjer, P.H. Standardized lung function testing: Report of the Working Party for the European Community for Steel and Coal. Bull. Eur. Physiopathol. Respir. 1983, 19 (Suppl. S5), 1–95. [Google Scholar]
- Vestbo, J.; Edwards, L.D.; Scanlon, P.D.; Yates, J.C.; Agusti, A.; Bakke, P.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Crim, C.; et al. Changes in forced expiratory volume in 1 second over time in COPD. N. Engl. J. Med. 2011, 365, 1184–1192. [Google Scholar] [CrossRef]
- Donaldson, G.C.; Seemungal, T.A.; Patel, I.S.; Bhowmik, A.; Wilkinson, T.M.; Hurst, J.R.; MacCallum, P.K.; Wedzicha, J.A. Airway and Systemic Inflammation and Decline in Lung Function in Patients With COPD. Chest 2016, 128, 1995–2004. [Google Scholar] [CrossRef]
- Yanbaeva, D.G.; Dentener, M.A.; Creutzberg, E.C.; Wesseling, G.; Wouters, E.F. Systemic effects of smoking. Chest 2007, 131, 1557–1566. [Google Scholar] [CrossRef]
- Delgado, G.E.; Krämer, B.K.; Siekmeier, R.; Yazdani, B.; März, W.; Leipe, J.; Kleber, M.E. Influence of smoking and smoking cessation on biomarkers of endothelial function and their association with mortality. Atherosclerosis 2020, 292, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Thomas, J.; Sadatsafavi, M.; FitzGerald, J.M. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Lancet Respir. Med. 2015, 3, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chou, O.H.I.; Cheung, B.M. The Association Between Systemic Arterial Hypertension and Chronic Obstructive Pulmonary Disease. Results from the U.S. National Health and Nutrition Examination Survey 1999–2018: A Cross-sectional Study. Chronic Obstr. Pulm. Dis. 2023, 10, 190–198. [Google Scholar] [CrossRef]
- Kinney, G.L.; Baker, E.H.; Klein, O.L.; Black-Shinn, J.L.; Wan, E.S.; Make, B.; Regan, E.; Bowler, R.P.; Lutz, S.M.; Young, K.A.; et al. Pulmonary Predictors of Incident Diabetes in Smokers. Chronic Obstr. Pulm. Dis. 2016, 3, 739–747. [Google Scholar] [CrossRef]
- Li, G.; Lu, Y.; Qiao, Y.; Hu, D.; Ke, C. Role of Pulmonary Function in Predicting New-Onset Cardiometabolic Diseases and Cardiometabolic Multimorbidity. Chest 2022, 162, 421–432. [Google Scholar] [CrossRef]
- Whittaker, H.; Nordon, C.; Rubino, A.; Morris, T.; Xu, Y.; De Nigris, E.; Müllerová, H.; Quint, J.K. Frequency and severity of respiratory infections prior to COPD diagnosis and risk of subsequent postdiagnosis COPD exacerbations and mortality: EXACOS-UK health care data study. Thorax 2023, 78, 760–766. [Google Scholar] [CrossRef]
- Ding, B.; Zaha, R.; Makita, N.; Graham, S.; Lambrelli, D.; Huse, S.; Müllerová, H.; Nordon, C.; Muro, S. History of Respiratory Events Prior to a First COPD Diagnosis and Future Exacerbations: A Longitudinal Observational Cohort Database Study in Japan. Int. J. Chronic Obstr. Pulm. Dis. 2023, 7, 247–258. [Google Scholar] [CrossRef]
- Fan, J.; Sun, Z.; Yu, C.; Guo, Y.; Pei, P.; Yang, L.; Chen, Y.; Du, H.; Sun, D.; Pang, Y.; et al. Multimorbidity patterns and association with mortality in 0.5 million Chinese adults. Chin. Med J. 2022, 135, 648–657. [Google Scholar] [CrossRef] [PubMed]


| All Participants (n = 391) | COPD (n = 207) | Non-COPD (n = 184) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (year) | 56 (10) | 61 (8) | 51 (11) | <0.001 |
| Male, No (%) | 321 (82) | 189 (91) | 131 (71) | <0.001 |
| BMI (kg/m2) | 28.0 (4.6) | 28.2 (4.2) | 27.7 (5.1) | 0.32 |
| Active smokers, No (%) | 173 (44) | 75 (36) | 98 (53) | 0.002 |
| Former smokers, No (%) | 218 (56) | 132 (64) | 86 (47) | |
| Pack-years | 41.0 (21.9) | 47.4 (22.4) | 33.9 (18.9) | <0.001 |
| Lung function | ||||
| Post FEV1% predicted | 80 (23) | 66 (19) | 96 (14) | <0.001 |
| GOLD I (≥80% pred.), No (%) | - | 46 (22) | - | - |
| GOLD II (50–79% pred.), No (%) | - | 122 (59) | - | |
| GOLD III/IV (≤49% pred.), No (%) | - | 39 (19) | - | |
| DLCO % predicted | 80 (21) | 76 (22) | 86 (18) | <0.001 |
| Respiratory symptoms | ||||
| Chronic bronchitis, No (%) | 152 (39) | 82 (39) | 70 (38) | 0.55 |
| Dyspnea (mMRC) | 1.2 (0.8) | 1.3 (0.9) | 1.1 (0.8) | 0.07 |
| ≥1 Hospital admission due to respiratory problems, No (%) | 182 (35) | 121 (40) | 58 (27) | 0.032 |
| Pre-COPD **, No (%) | 73 (40) | |||
| Cardiometabolic Multimorbidity (n = 133) | No Cardiometabolic Multimorbidity (n = 258) | p Value | |
|---|---|---|---|
| Spirometry status | |||
| Non-COPD, No (%) | 51 (28) | 133 (51) | 0.017 |
| COPD, No (%) | 81 (39) | 126 (61) | |
| GOLD I (≥80% pred.), No (%) | 19 (23) | 27 (21) | |
| GOLD II (50–79% pred.), No (%) | 48 (59) | 74 (58) | |
| GOLD III/IV (≤49% pred.), No (%) | 14 (17) | 25 (20) | |
| Demographics | |||
| Age (year) | 59 (9) | 55 (11) | <0.001 |
| Male, No (%) | 125 (94) | 195 (75) | <0.001 |
| BMI (kg/m2) | 29.4 (4.3) | 27.1 (4.5) | <0.001 |
| Active smokers, No (%) | 57 (43) | 116 (45) | 0.78 |
| Former smokers, No (%) | 76 (57) | 142 (55) | |
| Pack-years | 44.6 (23.1) | 39.2 (21.0) | 0.02 |
| Lung function | |||
| Post FEV1 % predicted | 76 (21) | 82 (24) | 0.01 |
| DLCO % predicted | 80 (20) | 82 (20) | 0.432 |
| FEV1 rate of decline, mL/yr | 37 (36) | 33 (44) | 0.411 |
| Respiratory symptoms | |||
| Chronic bronchitis, No (%) | 53 (40) | 99 (38) | 0.71 |
| Dyspnoea (mMRC) | 1.4 (1.0) | 1.2 (0.8) | 0.13 |
| ≥1 Hospital admission due to respiratory problems, No (%) | 77 (58) | 119 (46) | 0.02 |
| Univariable | Multivariable (Adjusted) # | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Cardiometabolic multimorbidity (≥2 cardiometabolic comorbidities) | ||||
| COPD diagnosis | 1.76 (1.23 to 2.51) | 0.002 | 1.59 (1.03 to 2.46) | 0.036 |
| Pack-years | 1.01 (1.00 to 1.01) | 0.049 | 1.00 (0.99 to 1.01) | 0.613 |
| Greater decliners (ΔFEV1 ≥ 40 mL/year) | 1.30 (0.92 to 1.84) | 0.131 | 1.57 (1.10 to 2.24) | 0.013 |
| ≥1 Hospital admission due to respiratory problems | 1.15 (0.82–1.63) | 0.418 | 1.09 (0.76 to 1.57) | 0.629 |
| Cerebro-cardiovascular events | ||||
| COPD diagnosis | 2.39 (1.60 to 3.57) | <0.001 | 1.68 (1.08 to 2.62) | 0.021 |
| Pack-years | 1.01 (1.01 to 1.02) | <0.001 | 1.00 (1.00 to 1.01) | 0.229 |
| Greater decliners (ΔFEV1 ≥ 40 mL/year) | 0.95 (0.65 to 1.38) | 0.772 | 1.05 (0.72 to 1.55) | 0.785 |
| ≥1 Hospital admission due to respiratory problems | 2.27 (1.54 to 3.34) | <0.001 | 2.42 (1.63 to 3.60) | <0.001 |
| Hypertension | ||||
| COPD diagnosis | 2.40 (1.69 to 3.40) | <0.001 | 1.68 (1.12 to 2.52) | 0.012 |
| Pack-years | 1.01 (1.00 to 1.01) | 0.044 | 1.00 (0.99 to 1.00) | 0.298 |
| Greater decliners (ΔFEV1 ≥ 40 mL/year) | 0.88 (0.62 to 1.24) | 0.462 | 1.01 (0.71 to 1.44) | 0.954 |
| ≥1 Hospital admission due to respiratory problems | 0.82 (0.58 to 1.15) | 0.248 | 0.80 (0.57 to 1.14) | 0.217 |
| Dyslipidemia | ||||
| COPD diagnosis | 0.67 (0.48 to 0.92) | 0.012 | 0.66 (0.46 to 0.97) | 0.035 |
| Pack-years | 1.00 (0.99 to 1.01) | 0.911 | 1.00 (1.00 to 1.01) | 0.352 |
| Greater decliners (ΔFEV1 ≥ 40 mL/year) | 1.59 (1.16 to 2.19) | 0.004 | 1.55 (1.12 to 2.14) | 0.008 |
| ≥1 Hospital admission due to respiratory problems | 0.97 (0.71 to 1.34) | 0.871 | 0.90 (0.65 to 1.25) | 0.537 |
| Diabetes mellitus | ||||
| COPD diagnosis | 0.81 (0.47 to 1.38) | 0.432 | 0.82 (0.45 to 1.49) | 0.505 |
| Pack-years | 1.00 (0.99 to 1.02) | 0.507 | 1.00 (0.99 to 1.01) | 0.868 |
| Greater decliners (ΔFEV1 ≥ 40 mL/year) | 1.33 (0.78 to 2.28) | 0.291 | 1.41 (0.82 to 2.43) | 0.211 |
| ≥1 Hospital admission due to respiratory problems | 2.27 (1.28 to 4.02) | 0.005 | 1.97 (1.09 to 3.55) | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero-Cortina, B.; Maldonado-Guaje, A.; Rodriguez-Sanz, J.; Boldova-Loscertales, A.; Cubero-Marin, P.; Marin-Oto, M.; Sanz-Rubio, D.; Marin, J.M. Incident Cardiometabolic Comorbidities in Smokers with/Without Chronic Obstructive Pulmonary Disease: A Long-Term Cohort Study. J. Clin. Med. 2024, 13, 7627. https://doi.org/10.3390/jcm13247627
Herrero-Cortina B, Maldonado-Guaje A, Rodriguez-Sanz J, Boldova-Loscertales A, Cubero-Marin P, Marin-Oto M, Sanz-Rubio D, Marin JM. Incident Cardiometabolic Comorbidities in Smokers with/Without Chronic Obstructive Pulmonary Disease: A Long-Term Cohort Study. Journal of Clinical Medicine. 2024; 13(24):7627. https://doi.org/10.3390/jcm13247627
Chicago/Turabian StyleHerrero-Cortina, Beatriz, Aura Maldonado-Guaje, Jorge Rodriguez-Sanz, Ana Boldova-Loscertales, Pablo Cubero-Marin, Marta Marin-Oto, David Sanz-Rubio, and Jose M. Marin. 2024. "Incident Cardiometabolic Comorbidities in Smokers with/Without Chronic Obstructive Pulmonary Disease: A Long-Term Cohort Study" Journal of Clinical Medicine 13, no. 24: 7627. https://doi.org/10.3390/jcm13247627
APA StyleHerrero-Cortina, B., Maldonado-Guaje, A., Rodriguez-Sanz, J., Boldova-Loscertales, A., Cubero-Marin, P., Marin-Oto, M., Sanz-Rubio, D., & Marin, J. M. (2024). Incident Cardiometabolic Comorbidities in Smokers with/Without Chronic Obstructive Pulmonary Disease: A Long-Term Cohort Study. Journal of Clinical Medicine, 13(24), 7627. https://doi.org/10.3390/jcm13247627






