No Routine Control Measurements of C-Reactive Protein in Uneventful Postoperative Evolution After Debridement for Infected (Diabetic) Foot Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Definitions, Costs, and Criteria
2.2. Statistical Analyses
3. Results
3.1. Study Population
3.2. Surgical-Site Infections, Pathogens, Therapies, and Outcomes
3.3. C-Reactive Protein Levels Associated to Outcomes
3.4. Multivariate Adjustment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Kenani, N.S.; Alsultan, A.S.; Alosfoor, M.A.; Bahkali, M.I.; Al-Mohrej, O.A. Incidence and predictors of surgical site infections following foot and ankle surgery. J. Musculoskelet. Surg. Res. 2017, 1, 6–9. [Google Scholar]
- Modha, M.R.K.; Morriss-Roberts, C.; Smither, M.; Larholt, J.; Reilly, I. Antibiotic prophylaxis in foot and ankle surgery: A systematic review of the literature. J. Foot Ankle Res. 2018, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, J.M.; Zambelli, R.; Oliveira-Júnior, O.; Avelar, N.C.P.; Polese, J.C.; Leopoldino, A.A.O. Incidence and associated factors of surgical site infection in patients undergoing foot and ankle surgery: A 7-year cohort study. Foot 2024, 59, 102092. [Google Scholar] [CrossRef]
- Meng, J.; Zhu, Y.; Li, Y.; Sun, T.; Zhang, F.; Qin, S.; Zhao, H. Incidence and risk factors for surgical site infection following elective foot and ankle surgery: A retrospective study. J. Orthop. Surg. Res. 2020, 15, 449. [Google Scholar] [CrossRef]
- Tantigate, D.; Jang, E.; Seetharaman, M.; Noback, P.C.; Heijne, A.M.; Greisberg, J.K.; Vosseller, J.T. Timing of Antibiotic Prophylaxis for Preventing Surgical Site Infections in Foot and Ankle Surgery. Foot Ankle Int. 2017, 38, 283–288. [Google Scholar] [CrossRef]
- Michail, M.; Jude, E.; Liaskos, C.; Karamagiolis, S.; Makrilakis, K.; Dimitroulis, D.; Michail, O.; Tentolouris, N. The Performance of Serum Inflammatory Markers for the Diagnosis and Follow-up of Patients with Osteomyelitis. Int. J. Low. Extrem. Wounds 2013, 12, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Garzoni, C.; Ferry, T.; Harbarth, S.; Stern, R.; Assal, M.; Hoffmeyer, P.; Lew, D.; Bernard, L. Postoperative serum pro-calcitonin and C-reactive protein levels in patients with orthopedic infections. Swiss Med. Wkly. 2010, 140, 13124. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Ahmed, S.; Barakat, A.; Mangwani, J.; White, H. Inflammatory response in confirmed non-diabetic foot and ankle infections: A case series with normal inflammatory markers. World J. Orthop. 2023, 14, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Wetzel, O.; Gariani, K.; Kressmann, B.; Jornayvaz, F.R.; Lipsky, B.A.; Uçkay, I. Is routine measurement of the serum C-reactive protein level helpful during antibiotic therapy for diabetic foot infection? Diabetes Obes. Metab. 2021, 23, 637–641. [Google Scholar] [CrossRef]
- Furrer, P.R.; Schömni, M.; Waibel, F.W.A.; Berli, M.C.; Lipsky, B.A.; Uçkay, I. Lack of Benefit of Routine Serum Laboratory Control Samples During Treatment of Diabetic Foot Infection. Arch. Microbiol. Immunol. 2022, 6, 115–122. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, Y.C.; Jeon, D.O.; Cho, H.J.; Im, S.G.; Jang, S.K.; Kang, H.J.; Kim, M.J.; Lee, J.H. High serum C-reactive protein level predicts mortality in patients with stage 3 chronic kidney disease or higher and diabetic foot infections. Kidney Res. Clin. Pract. 2013, 32, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Gaynes, R.P.; Martone, W.J.; Jarvis, W.R.; Emori, T.G. CDC definitions of nosocomial surgical site infections, 1992: A modification of CDC definitions of surgical wound infections. Am. J. Infect. Control 1992, 20, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.R.; Justo, J.A.; Amanatullah, D.F. Local Antibiotic Delivery in Orthopedics: Review of Current Practices and Emerging Technologies. Infect. Dis. Clin. N. Am. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, Y.; Schnetz, M.; Hoffmann, R. Local Administration of Antibiotics in Orthopedics and Traumatology. Z. Orthop. Unfall. 2023, 161, 563–583. [Google Scholar] [CrossRef]
- Smolle, M.A.; Nischwitz, S.P.; Hutan, M.; Trunk, P.; Lumenta, D.; Bernhardt, G.A. Closed-incision negative-pressure wound management in surgery—Literature review and recommendations. Eur. Surg. 2020, 52, 249–267. [Google Scholar] [CrossRef]
- Dupont, C.; Rodenbach, J.; Flachaire, E. The value of C-reactive protein for postoperative monitoring of lower limb arthroplasty. Ann. Readapt. Med. Phys. 2008, 51, 348–357. [Google Scholar] [CrossRef]
- Bejon, P.; Byren, I.; Atkins, B.; Scarborough, M.; Woodhouse, A.; McLardy-Smith, P.; Gundle, R.; Berendt, A.R. Serial measurement of the C-reactive protein is a poor predictor of treatment outcome in prosthetic joint infection. J. Antimicrob. Chemother. 2011, 66, 1590–1593. [Google Scholar] [CrossRef]
- Mederake, M.; Hofmann, U.K.; Benda, S.; Schuster, P.; Fink, B. Diagnostic Value of CRP and Serum WBC Count during Septic Two-Stage Revision of Total Hip Arthroplasties. Antibiotics 2022, 11, 1098. [Google Scholar] [CrossRef]
- Hingsammer, A.M.; Bauer, D.; Renner, N.; Borbas, P.; Böni, T.; Berli, M.C. Correlation of Systemic Inflammatory Markers with Radiographic Stages of Charcot Osteoarthropathy. Foot Ankle Int. 2016, 37, 924–928. [Google Scholar] [CrossRef]
- Lund Håheim, L.; Nafstad, P.; Olsen, I. C-reactive protein variations for different chronic somatic disorders. Scand. J. Public Health 2009, 37, 640–646. [Google Scholar] [CrossRef]
- Black, S.; Kushner, I.; Samols, D. C-reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef] [PubMed]
- Kipp, J.A.; LeSavage, L.K.; Evans, J.K.; Denmeade, T.A.; Blazek, C.D. Diabetic Osteomyelitis: Oral versus Intravenous Antibiotics at a Single Level 1 Academic Medical Trauma Center. J. Foot Ankle Surg. 2024, 63, 490–494. [Google Scholar] [CrossRef]
- Haddad, N.; Ajaz, J.; Mansour, L.; Kasemodel, R.; Jarvis, J.; Jarad, J.; Gorski, H.; Carr, M. A Review of the Clinical Utilization of Oral Antibacterial Therapy in the Treatment of Bone Infections in Adults. Antibiotics 2023, 13, 4. [Google Scholar] [CrossRef]
- Tominaga, H.; Setoguchi, T.; Ishidou, Y.; Nagano, S.; Yamamoto, T.; Komiya, S. Risk factors for surgical site infection and urinary tract infection after spine surgery. Eur. Spine J. 2016, 25, 3908–3915. [Google Scholar] [CrossRef]
- Huttner, A.; Albrich, W.C.; Bochud, P.-Y.; Gayet-Agéron, A.; Rossel, A.; von Dach, E.; Harbarth, S.; Kaiser, L. PIRATE project: Point-of-care, informatics-based randomised controlled trial for decreasing overuse of antibiotic therapy in Gram-negative bacteraemia. BMJ Open 2017, 7, 017996. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, H.; Zhou, N.; Zhao, W.; Wu, D.; Shen, B. Combined Effects of Single Nucleotide Polymorphisms (SNPs) within C-reactive Protein (CRP) and Environmental Parameters on Risk and Prognosis for Diabetic Foot Osteomyelitis Patients. Exp. Clin. Endocrinol. Diabetes 2020, 28, 528–539. [Google Scholar] [CrossRef]
- Costa, L.; Soares, D.; Aido, R.; Sousa, R. The value of monitoring inflammatory markers after total joint arthroplasty. Hard Tissue 2013, 2, 17. [Google Scholar] [CrossRef]
- Rohe, S.; Böhle, S.; Matziolis, G.; Jacob, B.; Wassilew, G.; Brodt, S. C-reactive protein during the first 6 postoperative days after total hip arthroplasty cannot predict early periprosthetic infection. Arch. Orthop. Trauma. Surg. 2023, 143, 3495–3503. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.; Schlumberger, M.; Beyersdorff, J.; Schuster, P. C-reactive protein is not a screening tool for late periprosthetic joint infection. J. Orthop. Traumatol. 2020, 21, 2. [Google Scholar] [CrossRef]
- Lee, S.H.; Chu, C.T.; Chang, C.H.; Hu, C.C.; Chen, S.Y.; Lu, T.W.; Lin, Y.C. Do Serum C-Reactive Protein Trends Predict Treatment Outcome in Patients with Knee Periprosthetic Joint Infection Undergoing Two-Stage Exchange Arthroplasty? Diagnostics 2022, 12, 1030. [Google Scholar] [CrossRef]
- Pérez-Prieto, D.; Portillo, M.E.; Puig-Verdié, L.; Alier, A.; Martínez, S.; Sorlí, I.; Horcajada, J.P.; Monllau, J.C. C-reactive protein may misdiagnose prosthetic joint infections, particularly chronic and low-grade infections. Int. Orthop. 2017, 41, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
| Remission | Failures | p | |
|---|---|---|---|
| n = 36 | n = 29 | n = 7 | value |
| Male sex, n = 15 | 12 (41%) | 3 (43%) | 0.94 |
| Median age | 61 years | 53 years | 0.58 |
| American Society of Anesthesiologists’ Score 3 points | 13 (45%) | 4 (57%) | 0.56 |
| Charcot neuroarthropathy, n = 5 | 2 (7%) | 3 (43%) | 0.01 |
| Mainly bone infection, n = 24 (more than soft tissues) | 19 (66%) | 5 (71%) | 0.77 |
| Infected osteosynthesis material in situ, n = 7 | 6 (21%) | 1 (14%) | 0.70 |
| Median number of surgical debridement procedures | 1 intervention | 1 intervention | 0.15 |
| Median duration of postoperative antibiotic therapy | 42 days | 47 days | 0.54 |
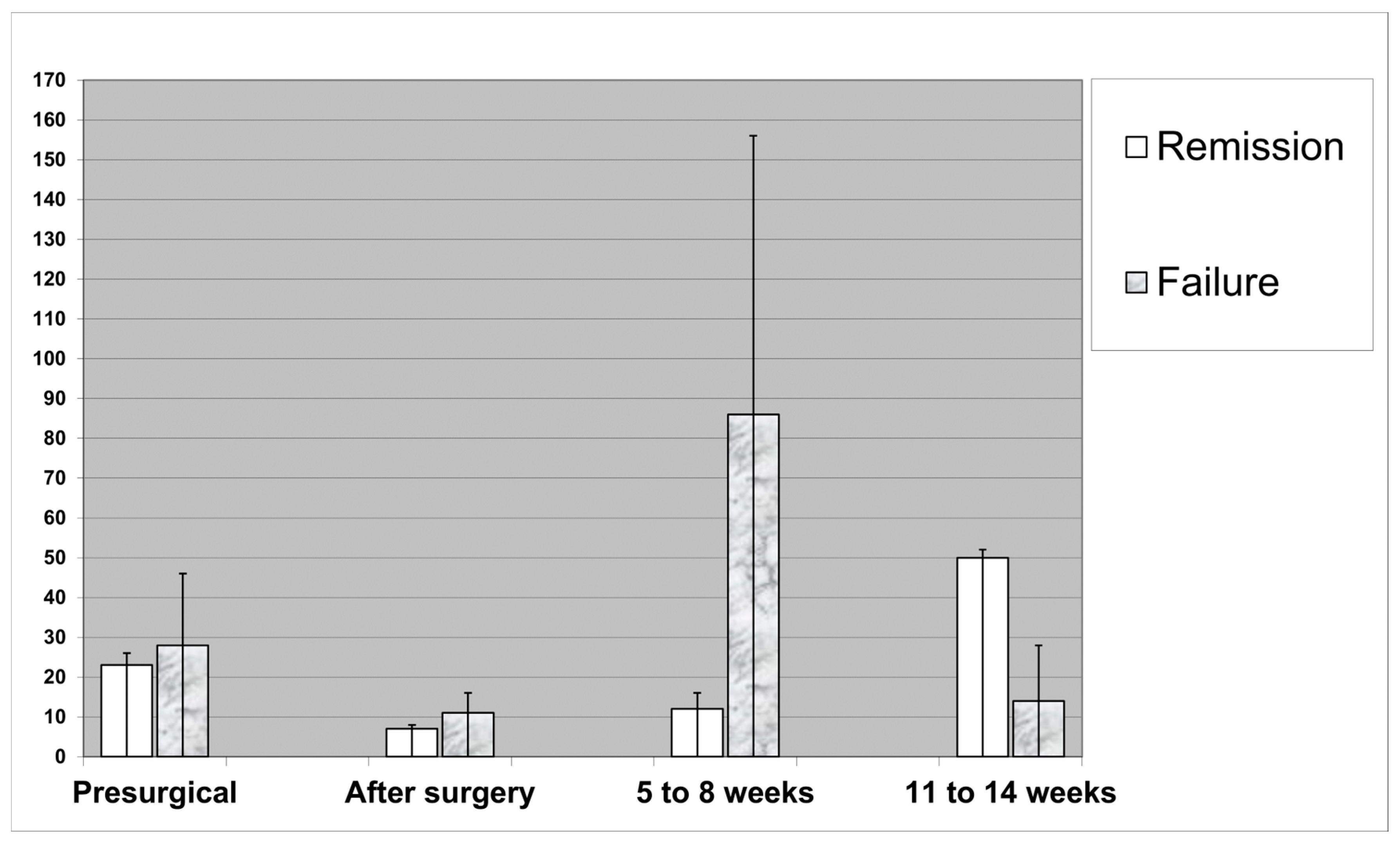
| Median CRP level on admission | 23 mg/L | 28 mg/L | 0.55 |
| Median CRP level after surgery | 7 mg/L | 11 mg/L | 0.22 |
| Median drop inn CRP level (ratio second/admission values) | 25% | 26% | 0.52 |
| Median CRP level at end of therapy (11–14 weeks) | 49 mg/L | 14 mg/L | 0.38 |
| n = 36 | Multivariate Results |
|---|---|
| Age (continuous variable) | 1.1, 0.97–1.23 |
| Diabetes mellitus | 1.0 (omitted from final model) |
| Osteosynthesis material | 1.0 (omitted from final model) |
| Concomitant Charcot foot pathology | 0.1, 0.01–5.2 |
| Serum CRP level on admission (continuous variable) | 1.0, 0.97–1.02 |
| Serum CRP level immediately postoperatively | 1.0, 0.94–1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebe, J.; Soldevila-Boixader, L.; Yιldιz, İ.; Furrer, P.R.; Jans, P.; Viehöfer, A.; Wirth, S.; Uckay, İ. No Routine Control Measurements of C-Reactive Protein in Uneventful Postoperative Evolution After Debridement for Infected (Diabetic) Foot Surgery. J. Clin. Med. 2025, 14, 4122. https://doi.org/10.3390/jcm14124122
Liebe J, Soldevila-Boixader L, Yιldιz İ, Furrer PR, Jans P, Viehöfer A, Wirth S, Uckay İ. No Routine Control Measurements of C-Reactive Protein in Uneventful Postoperative Evolution After Debridement for Infected (Diabetic) Foot Surgery. Journal of Clinical Medicine. 2025; 14(12):4122. https://doi.org/10.3390/jcm14124122
Chicago/Turabian StyleLiebe, Jonas, Laura Soldevila-Boixader, İnci Yιldιz, Pascal R. Furrer, Peter Jans, Arnd Viehöfer, Stephan Wirth, and İlker Uckay. 2025. "No Routine Control Measurements of C-Reactive Protein in Uneventful Postoperative Evolution After Debridement for Infected (Diabetic) Foot Surgery" Journal of Clinical Medicine 14, no. 12: 4122. https://doi.org/10.3390/jcm14124122
APA StyleLiebe, J., Soldevila-Boixader, L., Yιldιz, İ., Furrer, P. R., Jans, P., Viehöfer, A., Wirth, S., & Uckay, İ. (2025). No Routine Control Measurements of C-Reactive Protein in Uneventful Postoperative Evolution After Debridement for Infected (Diabetic) Foot Surgery. Journal of Clinical Medicine, 14(12), 4122. https://doi.org/10.3390/jcm14124122








