Worthwhile or Not? The Pain–Gain Ratio of Screening Routine cMRIs in a Maximum Care University Hospital for Incidental Intracranial Aneurysms Using Artificial Intelligence
Abstract
1. Introduction
2. Materials and Methods
2.1. General Radiologists’ Cohort (GRC)
2.2. Neuroradiologists’ Cohort (NRC)
2.3. Data Management
2.4. Artificial Intelligence Algorithm
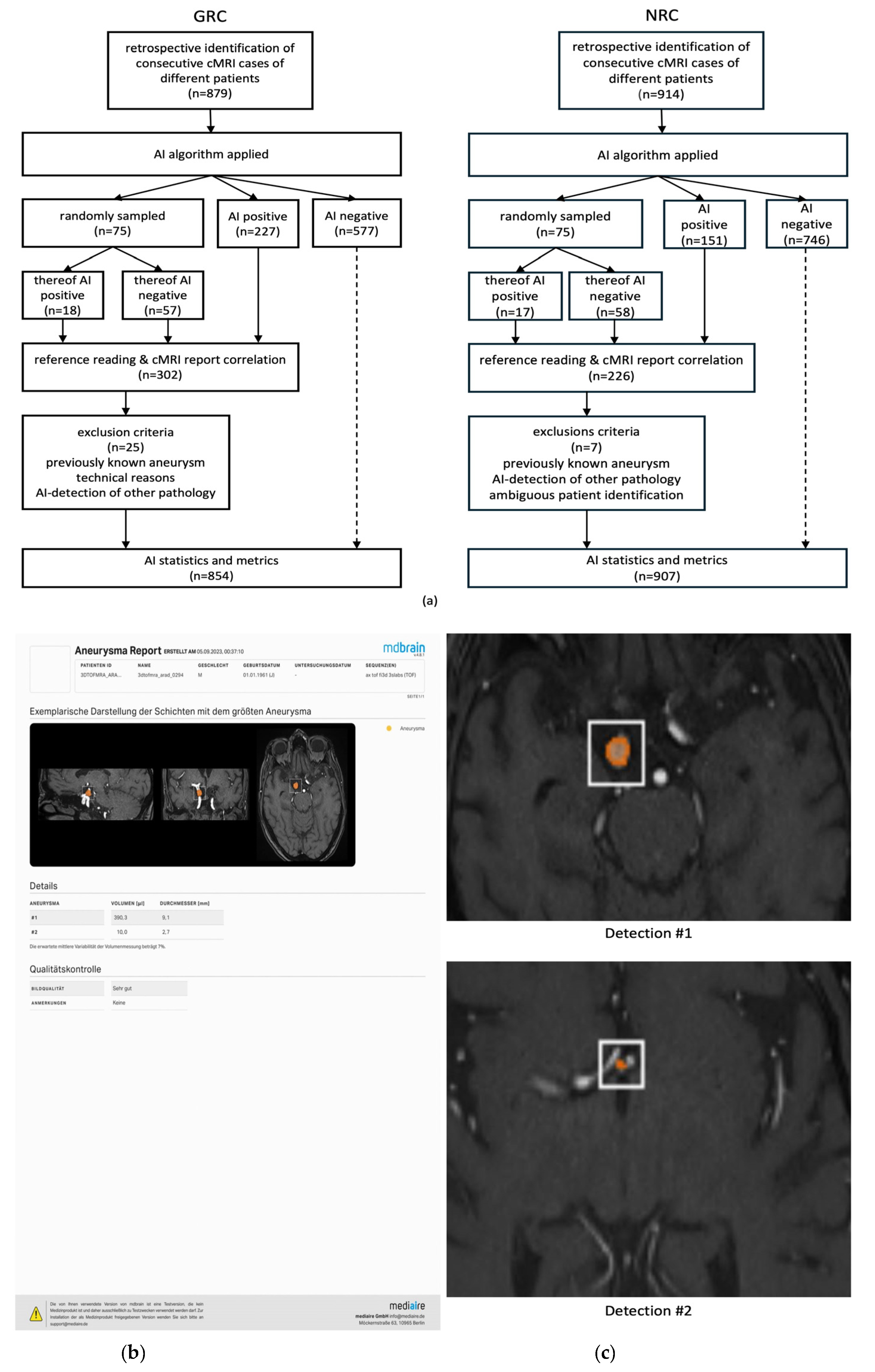
2.5. Reference Reading and Analysis of Initial cMRI Reports
2.6. Patient Consultations
| GRC | NRC | |
|---|---|---|
| cMRIs initially included/statistically analyzed [n] | 879/854 | 914/907 |
| cMRI acquisition period | 9 December 2016—11 April 2023 | 21 October 2020—18 May 2022 |
| patient age [mean ± standard deviation/median age] | 59.8 ± 20.0 years/64 years | 44.4 ± 20.5 years/45 years |
| MRI scanner | 1.5 Tesla MAGNETOM Aera/Avanto (Siemens) | 3 Tesla MAGNETOM Prisma (Siemens) |
| MR sequence used for AI analysis | Isotropic TOF-MRA, voxel size 1 mm | Isotropic TOF-MRA, voxel size 0.5mm |
| image quality evaluated by AI | 767x “good” 111x “acceptable” 1x “rejected” | 914x “good” |
| inclusion criteria for reference reading | 75 randomly sampled cMRIs & all AI+ cMRIs (n = 302) | 75 randomly sampled MRIs & all AI+ MRIs (n = 226) |
cMRI report characteristics | ||
| original reason for cMRI examination: | ||
| 10/3.3% | 7/3.1% |
| 139/46.0% | 56/24.8% |
| - acute or previous SAH [n/%] | 11/3.6% | 1/0.4% |
| - cranial nerve compression syndrome [n/%] | 1/0.3% | 1/0.4% |
| - any other vascular-related question [n/%] | 115/38.1% | 37/16.4% |
| - MRI before electroconvulsive therapy [n/%] | 0/0.0% | 11/4.9% |
| - headaches not suspicious for SAH [n/%] | 12/4.0% | 6/2.7% |
| 153/50.7% | 163/72.1% |
| number of reporting radiologists involved [n] | 83 | 8 |
cMRIs excluded from statistical analysis | ||
| …due to previously known or treated aneurysms [n] | 12 | 4 |
| …due to technical reasons [n] | 11 | 1 |
| …due to AI detected other vasculopathies [n] | 2 | 2 |
2.7. Statistics
3. Results
3.1. GRC Characteristics
3.2. GRC—AI Algorithm Performance
3.3. GRC—Clinical Impact of an AI-Based Routine Screening
| 319 cMRI Findings Suspicious for Intracranial Aneurysms as Detected by the AI Algorithm and/or the Neuroradiologists’ Reference Reading. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reading Score * | Detections [n (%)] | Thereof Initially not Reported [n (%)] | Recommendations (Reference Reading) | Detections [n (%)] | Thereof Initially Not Reported [n (%)] | Prevalence Within RFS [n (%)] | |||
| RFS I ** | RFS II ** | RFS III ** | |||||||
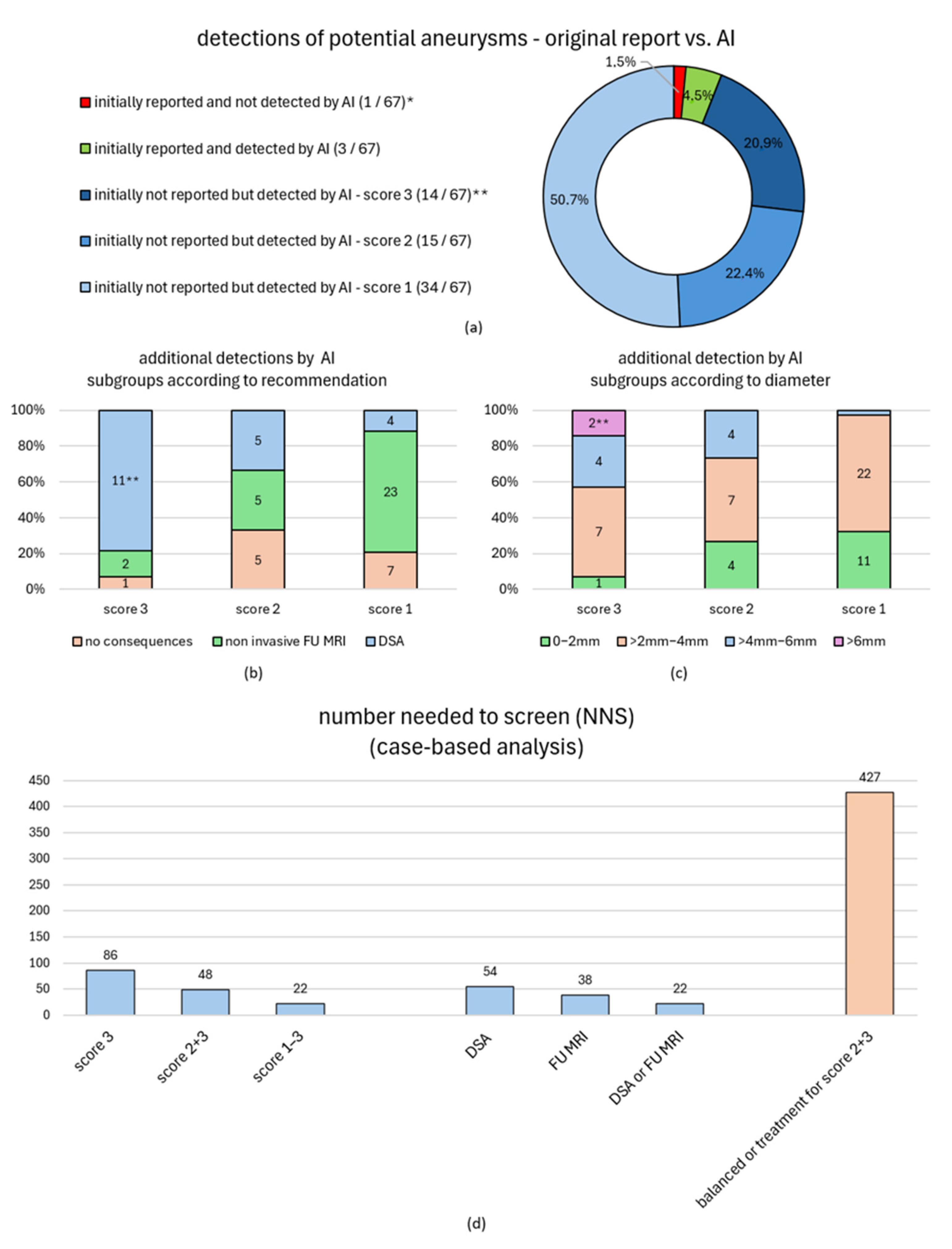
| detected by AI | score 3 * [n (%)] | 16/319 (5.0%) | 14/16 (87.5%) | no consequences | 1/16 (6.3%) | 1/1 (100%) | 16/854 (1.9%) | 32/854 (3.7%) | 66/854 (7.7%) |
| non-invasive FU MRI | 2/16 (12.5%) | 2/2 (100%) | |||||||
| DSA | 13/16 (81.3%) | 11/13 (84.6%) | |||||||
| score 2 * [n (%)] | 16/319 (5.0%) | 15/16 (93.8%) | no consequences | 5/16 (31.3%) | 5/5 (100%) | ||||
| non-invasive FU MRI | 5/16 (31.3%) | 5/5 (100%) | |||||||
| DSA | 6/16 (37.5%) | 5/6 (83.3%) | |||||||
| score 1 * [n (%)] | 34/319 (10.7%) | 34/34 (100%) | no consequences | 7/34 (20.6%) | 7/7 (100%) | ||||
| non-invasive FU MRI | 23/34 (67.6%) | 23/23 (100%) | |||||||
| DSA | 4/34 (11.8%) | 4/4 (100%) | |||||||
| score 0 *, associated with intracranial artery [n (%)] | 137/319 (42.9%) | 137/137 (100%) | no consequences | 137/137 (100%) | 137/137 (100%) | ||||
| non-invasive FU MRI | 0/137 (0%) | - | |||||||
| DSA | 0/137 (0%) | - | |||||||
| score 0 *, not associated with intracranial artery [n (%)] | 111/319 (34.8%) | 111/111 (100%) | no consequences | 111/111 (100%) | 111/111 (100%) | ||||
| non-invasive FU MRI | 0/111 (0%) | - | |||||||
| DSA | 0/111 (0%) | - | |||||||
| not detected by AI | score 3 *, not detected by AI [n (%)] | 1/319 (0.3%) | 0/1 (0%) | no consequences | 0/1 (0%) | - | |||
| non-invasive FU MRI | 0/1 (0%) | - | |||||||
| DSA | 1/1 (100%) | 0/1 (0%) | |||||||
| score 2 *, not detected by AI [n (%)] | 2/319 (0.6%) | 2/2 (100%) | no consequences | 0/2 (0%) | - | ||||
| non-invasive FU MRI | 0/2 (0%) | - | |||||||
| DSA | 2/2 (100%) | 2/2 (100%) | |||||||
| score 1 *, not detected by AI [n (%)] | 2/319 (0.6%) | 2/2 (100%) | no consequences | 0/2 (0%) | - | ||||
| non-invasive FU MRI | 2/2 (100%) | 2/2 (100%) | |||||||
| DSA | 0/2 (0%) | - | |||||||
| * Likert-based reference reading confidence scores: 0—no aneurysm (AI detection further assessed based on the position relative to intracranial arteries), 1—aneurysm unlikely, 2—aneurysm likely, 3—certain aneurysm. ** Prevalences within the study cohort are calculated based on three differentially sensitive reference standards: The most specific RFS I only considers the reference reading score 3 as positive for an aneurysm, the most sensitive RFS III pools the reference reading scores 1–3 as positive for an aneurysm. The intermediate RFS II considers the reference reading scores 2/3 as positive for an aneurysm. | |||||||||
| General Radiologists’ Cohort (GRC)—Detection-Based Statistics and Subgroup Analysis (Aneurysm Localization and Size) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reading Score | Diameter [mm] | Aneurysm [n(%)] | Thereof Initially Not Reported [n(%)] | Volume [mL] | Aneurysm [n(%)] | Thereof Initially Not Reported [n(%)] | Localization ** | Thereof Initially Not Reported [n(%)] | |
| detected by AI | score 3 * [n(%)] | 0–2 mm >2–4 mm >4–6 mm >6 mm | 1/16 (6.3%) 7/16 (43.8%) 5/16 (31.3%) 3/16 (18.8%) | 1/1 (100%) 7/7 (100%) 4/5 (80.0%) 2/3 (66.7%) | 0–5 mL >5 mL–15 mL >15 ml–30 mL >30 mL | 2/16 (12.5%) 2/16 (12.5%) 4/16 (25.0%) 8/16 (50.0%) | 2/2 (100%) 2/2 (100%) 4/4 (100%) 6/8 (75.0%) | anterior 5/16 (31.3%) posterior 2/16 (12.5%) ICA intradural 6/16 (37.5%) ICA extradural 3/16 (18.8%) | 3/5 (60.0%) 2/2 (100%) 6/6 (100%) 3/3 (100%) |
| score 2 * [n(%)] | 0–2 mm >2–4 mm >4–6 mm >6 mm | 4/16 (25.0%) 8/16 (50.0%) 4/16 (25.0%) 0/16 (0%) | 4/4 (100%) 7/8 (87.5%) 4/4 (100%) - | 0–5 mL >5 mL–15 mL >15 mL–30 mL >30 mL | 5/16 31.3% 4/16 25.0% 3/16 (18.8%) 4/16 (25.0%) | 5/5 (100%) 4/4 (100%) 2/3 (66.7%) 4/4 (100%) | anterior 7/16 (43.8%) posterior 2/16 (12.5%) ICA intradural 1/16 (6.3%) ICA extradural 6/16 (37.5%) | 6/7 (85.7%) 2/2 (100%) 1/1 (100%) 6/6 (100%) | |
| score 1 * [n(%)] | 0–2 mm >2–4 mm >4–6 mm >6 mm | 11/34 (32.4%) 22/34 (64.7%) 1/34 (2.9%) 0/34 (0%) | 11/11 (100%) 22/22 (100%) 1/1 (100%) - | 0–5 mL >5 mL–15 mL >15 mL–30 mL >30 mL | 12/34 (35.3%) 14/34 (41.2%) 6/34 (17.6%) 2/34 (5.9%) | 12/12 (100%) 14/14 (100%) 6/6 (100%) 2/2 (100%) | anterior 11/34 (32.4%) posterior 4/34 (11.8%) ICA intradural 10/34 (29.4%) ICA extradural 9/34 (26.5%) | 11/11 (100%) 4/4 (100%) 10/10 (100%) 9/9 (100%) | |
| score 0 *, associated with intracranial artery [n(%)] | 0–2 mm >2–4 mm >4–6 mm >6 mm | 67/137 (48.9%) 52/137 (38.0%) 9/137 (6.6%) 9/137 (6.6%) | 67/67 (100%) 52/52 (100%) 9/9 (100%) 9/9 (100%) | 0–5 mL >5 mL–15 mL >15 mL–30 mL >30 mL | 70/137 (51.1%) 41/137 (29.9%) 8/137 (5.8%) 18/137 (13.1%) | 70/70 (100%) 41/41 (100%) 8/8 (100%) 18/18 (100%) | anterior 23/137 (16.8%) posterior 6/137 (4.4%) ICA intradural 19/137 (13.9%) ICA extradural 28/137 (20.4%) not specified 61/137 (44.5%) | 23/23 (100%) 6/6 (100%) 19/19 (100%) 28/28 (100%) 61/61 (100%) | |
| score 0 *, not associated with intracranial artery [n(%)] | 0–2 mm >2–4 mm >4–6 mm >6 mm | 38/111 (34.2%) 41/111 (36.9%) 13/111 (11.7%) 19/111 (17.1%) | 38/38 (100%) 41/41 (100%) 13/13 (100%) 19/19 (100%) | 0–5 mL >5 mL–15 mL >15 mL–30 mL >30 mL | 38/111 (34.2%) 25/111 (22.5%) 15/111 (13.5%) 34/111 (30.6%) | 38/38 (100%) 25/25 (100%) 15/15 (100%) 34/34 (100%) | - | - | |
| not detected by AI | score 3 *, undetected by AI [n(%)] | 0–2 mm >2–4 mm >4–6 mm >6 mm | 0/1 (0%) 1/1 (100%) 0/1 (0%) 0/1 (0%) | - 0/1 (0%) - - | 0–5 mL >5 mL–15 mL >15 mL–30 mL >30 mL | *** | anterior 1/1 (100%) posterior 0/1 (0%) ICA intradural 0/1 (0%) ICA extradural 0/1 (0%) | 0/1 (0%) - - - | |
| score 2 *, undetected by AI [n(%)] | 0–2 mm >2–4 mm >4–6 mm >6 mm | 0/2 (0%) 2/2 (100%) 0/2 (0%) 0/2 (0%) | - 2/2 (100%) - - | 0–5 mL >5 mL–15 mL >15 mL–30 mL >30 mL | anterior 0/2 (0%) posterior 2/2 (100%) ICA intradural 0/2 (0%) ICA extradural 0/2 (0%) | - 2/2 (100%) - - | |||
| score 1 *, undetected by AI [n(%)] | 0–2 mm >2–4 mm >4–6 mm >6 mm | 0/2 (0%) 1/2 (50.0%) 1/2 (50.0%) 0/2 (0%) | - 1/1 (100%) 1/1 (100%) - | 0–5 mL >5 mL–15 mL >15 mL–30 mL >30 mL | anterior 1/2 (50.0%) posterior 1/2 (50.0%) ICA intradural 0/2 (0%) ICA extradural 0/2 (0%) | 1/1 (100%) 1/1 (100%) - - | |||
| * Likert-based reference reading confidence scores see caption Table 2. ** anterior cerebral circulation (AcomA, ACA, MCA, other arteries anterior circulation), posterior cerebral circulation (basilar artery, V4 segment, PICA, SCA, PCA, other arteries posterior circulation), ICA intradural (including terminal ICA, Pcom, AchA), ICA extradural. *** AI-based volumetry not available (findings not detected by AI). | |||||||||
| %(n) | |||||
|---|---|---|---|---|---|
| RFS I | RFS II | RFS III | |||
| Overall Analysis | |||||
| PPV | GRC (all = 314) | 5.1% (16/314) | 10.2% (32/314) | 21.0% (66/314) | |
| NRC (all =182) | 11.5% (21/182) | 19.7% (36/182) | 32.4% (59/182) | ||
| Synthesized | 7.5% (37/496) | 13.7% (68/496) | 25.2% (125/496) | ||
| Sensitivity | GRC (all = 319) | 94.1% (16/17) | 91.4% (32/35) | 93.0% (66/71) | |
| NRC (all=189) | 100% (21/21) | 83.7% (36/43) | 89.4% (59/66) | ||
| Synthesized | 97.4% (37/38) | 87.2% (68/78) | 91.2% (125/137) | ||
| Subgroup Analysis (Diameter) | |||||
| 0–2 mm | PPV | GRC | 0.8% (1/121) | 4.1% (5/121) | 13.2% (16/121) |
| NRC | 2.3% (2/88) | 5.7 (5/88) | 15.9% (14/88) | ||
| Synthesized | 1.4% (3/209) | 4.8% (10/209) | 14.4% (30/209) | ||
| Sensitivity | GRC | 100% (1/1) | 100% (5/5) | 100% (16/16) | |
| NRC | 100% (2/2) | 55.6% (5/9) | 77.7% (14/18) | ||
| Synthesized | 100% (3/3) | 71.4% (10/14) | 88.2% (30 /34) | ||
| >2–4 mm | PPV | GRC | 5.4% (7/130) | 11.5% (15/130) | 28.5% (37/130) |
| NRC | 17.1% (12/70) | 31.4% (22/70) | 51.4% (36/70) | ||
| Synthesized | 9.5% (19/200) | 18.5% (37/200) | 36.5% (73/200) | ||
| Sensitivity | GRC | 87.5% (7/8) | 83.3% (15/18) | 90.2% (37/41) | |
| NRC | 100% (12/12) | 88.0% (22/25) | 92.3% (36/39) | ||
| Synthesized | 95.0% (19/20) | 86.0% (37/43) | 91.3% (73/80) | ||
| >4–6 mm | PPV | GRC | 18.8% (6/32) | 31.3% (10/32) | 31.3% (10/32) |
| NRC | 43.8% (7/16) | 43.8% (7/16) | 43.8% (7/16) | ||
| Synthesized | 27.1% (13/48) | 35.4% (17/48) | 35.4% (17/48) | ||
| Sensitivity | GRC | 100% (6/6) | 100% (10/10) | 90.9% (10/11) | |
| NRC | 100% (7/7) | 100% (7/7) | 100% (7/7) | ||
| Synthesized | 100% (13/13) | 100% (17/17) | 94.4% (17/18) | ||
| >6 mm | PPV | GRC | 9.7% (3/31) | 9.7% (3/31) | 9.7% (3/31) |
| NRC | 0% (0/8) | 0% (0/8) | 0% (0/8) | ||
| Synthesized | 7.7% (3/39) | 7.7% (3/39) | 7.7% (3/39) | ||
| Sensitivity | GRC | 100% (3/3) | 100% (3/3) | 100% (3/3) | |
| NRC | - | - | - | ||
| Synthesized | 100% (3/3) | 100% (3/3) | 100% (3/3) | ||
3.4. Cohort Synthesis (GRC and NRC) and Holistic Analysis
| GRC | NRC | Synthesized | |
|---|---|---|---|
| age [mean ± standard deviation/median] | 59.8 ± 20.0 years/64 years | 44.4 ± 20.5 years/45 years | 51.9 ± 21.7 years/54 years |
| aneurysm prevalence (depending on applied RFSs) | 1.9–7.7% | 2.3–6.5% | 2.1–7.1% |
| findings score 0/1/2/3 [n] | 148/34/16/16 | 123/23/15/21 | 271/57/31/37 |
| diameter [mean ± standard deviation/median] score 1 findings score 2 findings score 3 findings | 2.4 ± 0.8 mm/2.4 mm 2.9 ± 1.2 mm/2.5 mm 4.3 ± 2.1 mm/4.0 mm | 2.2 ± 0.9 mm/2.3 mm 2.5 ± 0.7 mm/2.6 mm 3.5 ± 1.2 mm/3.3 mm | 2.3 ± 0.8 mm/2.3 mm 2.7 ± 1.0 mm/2.6 mm 3.9 ± 1.7 mm/3.5 mm |
| initially non-reported findings (scores 1–3) [%] | 94.4% | 86.4% | 90.5% |
| AI performance (detection-based analysis) | |||
| AI cMRI alert rate [%] | 26.5% | 17.8% | 22.0% |
| 100% AI detection sensitivity of certain aneurysm | >2.7 mm | any size | >2.7 mm |
| algorithm sensitivity for the detection of certain aneurysms/any suspicious finding [%] | 94.1%/93.0% | 100%/89.4% | 97.4%/91.2% |
| PPV range depending on applied RFS sensitivity [%] | 5.1–21.0% | 11.5–32.4% | 7.5–25.2% |
| FPR range depending on applied RFS sensitivity [%] | 79.0–94.9% | 67.6–88.5% | 61.9–92.5% |
| NNS (case-based analysis) | |||
| certain aneurysm (score 3) [n] | 86 | 70 | 77 |
| any suspicious finding (scores 1–3) [n] | 22 | 26 | 24 |
| recommended FU MRI [n] | 38 | 57 | 48 |
| recommended DSA [n] | 54 | 46 | 49 |
| recommended DSA or FU MRI [n] | 22 | 26 | 24 |
| UIATS balanced or treatment for scores 2/3 [n] | 427 | 152 | 221 |
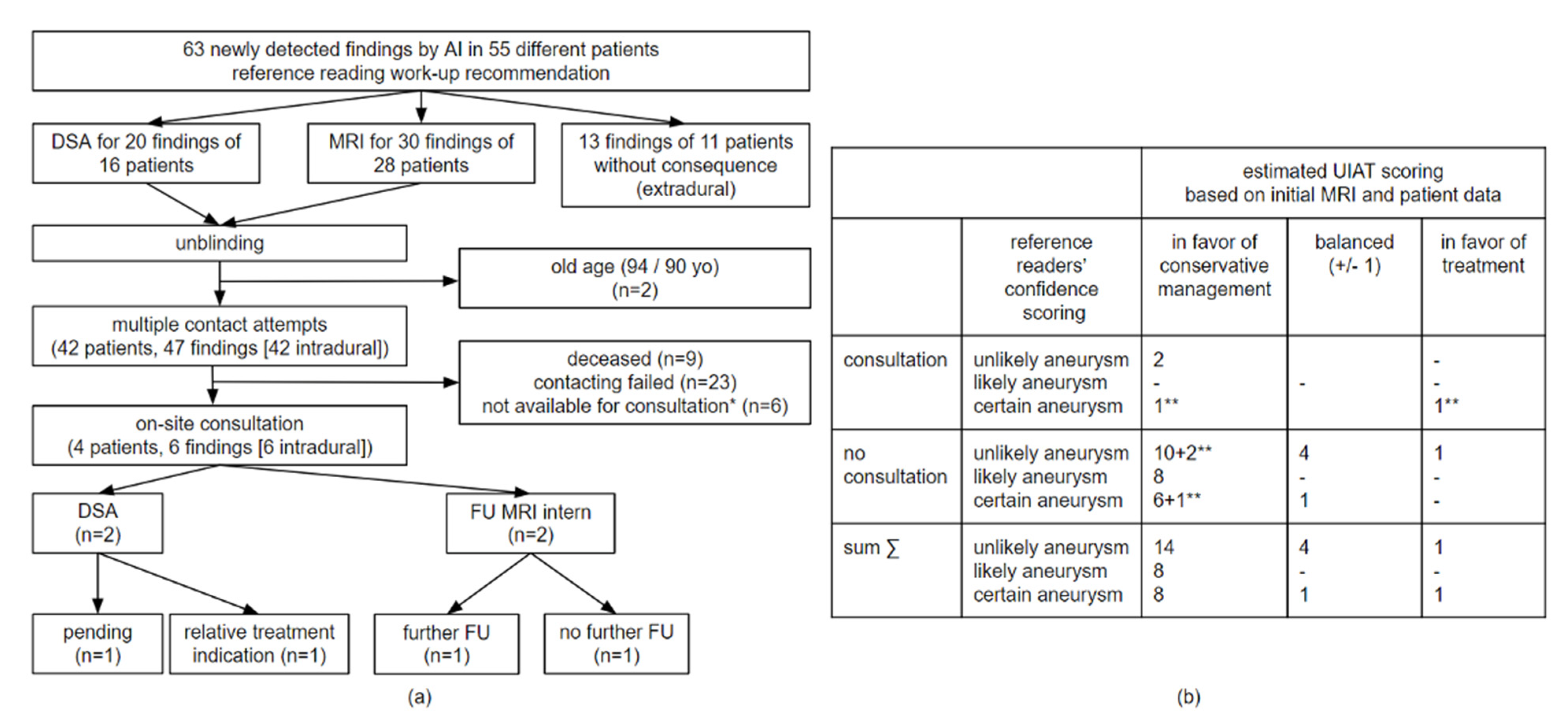
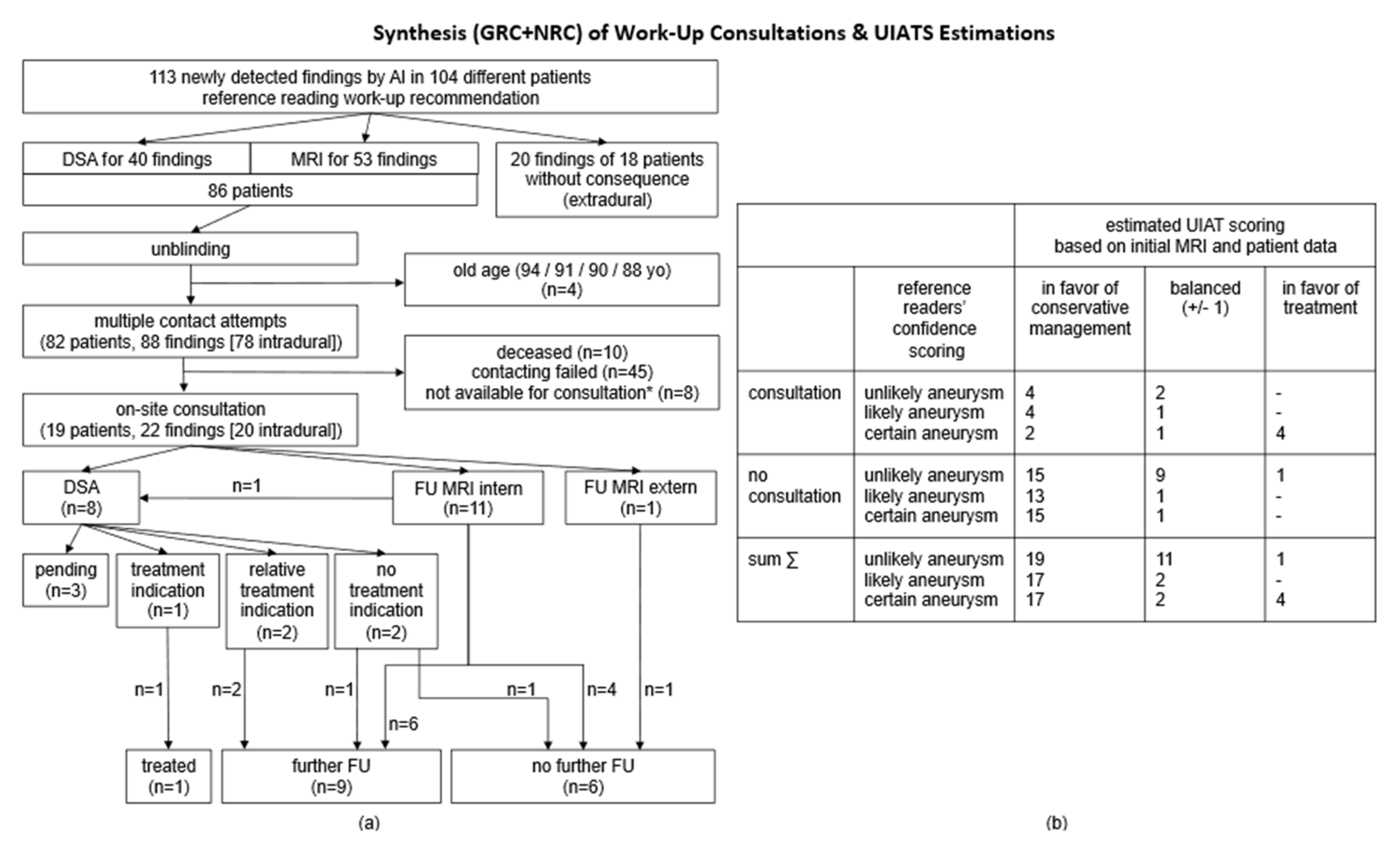
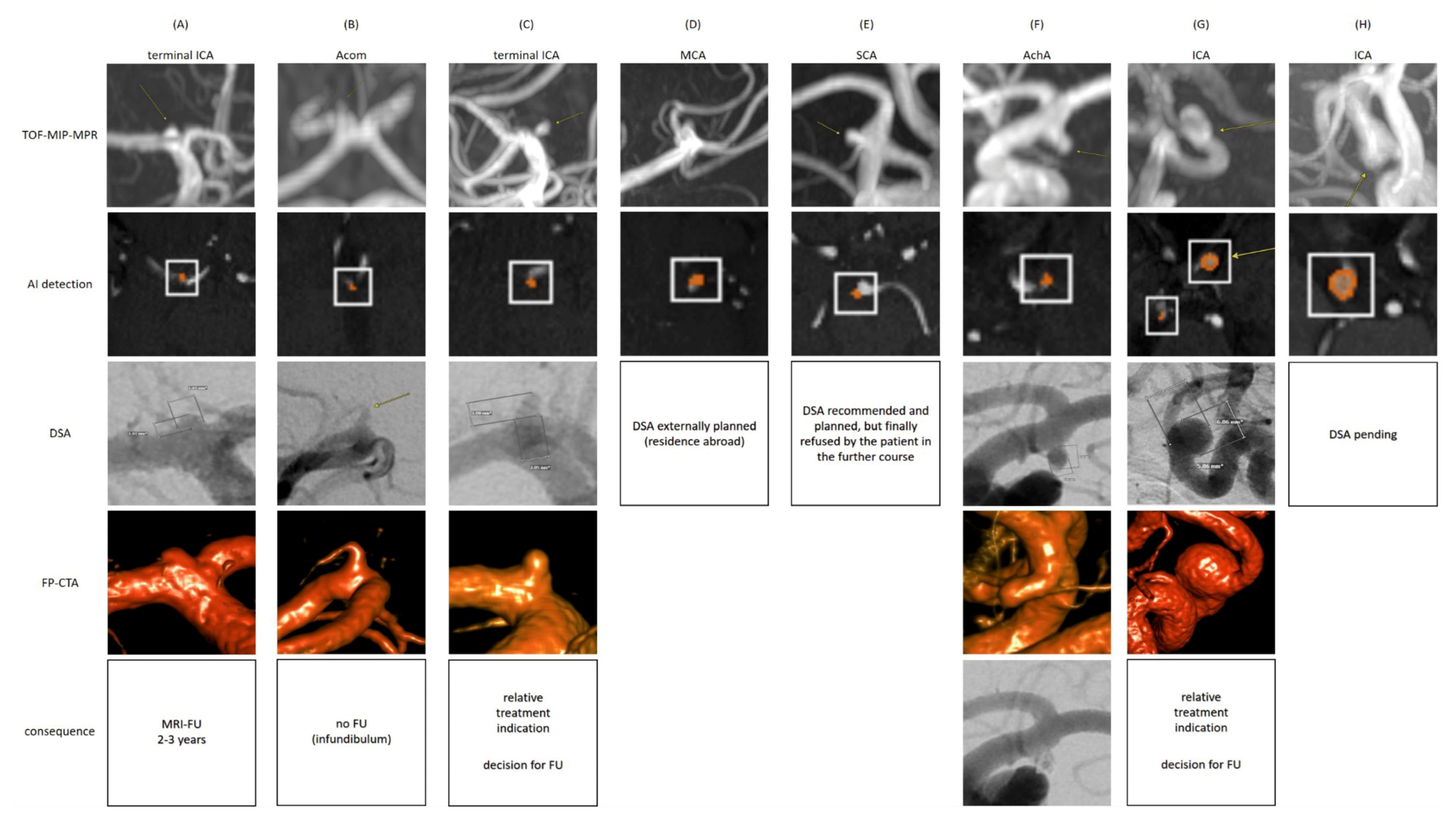
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACA | anterior cerebral artery |
| AchA | anterior choroidal artery |
| AcomA | anterior communicating artery |
| AI | artificial intelligence |
| AI+ | MRI with AI detections |
| AI- | cMRIs without AI detections |
| AVM | arteriovenous malformation |
| cMRI | cranial magnetic resonance imaging, |
| CTA | computed tomography angiography |
| DSA | digital subtraction angiography |
| FU | follow-up |
| GRC | general radiologists’ cohort |
| ICA | internal carotid artery |
| MCA | middle cerebral artery |
| MRA | magnetic resonance angiography |
| NNS | number needed to screen |
| NRC | neuroradiologist’s cohort |
| PCA | posterior cerebral artery |
| PcomA | posterior communicating artery |
| PICA | posterior inferior cerebellar artery |
| PPV | positive predictive value |
| RFS | reference standard |
| SAH | subarachnoid hemorrhage |
| SCA | superior cerebellar artery |
| TOF-MRA | time of flight magnetic resonance angiography |
| UIAT score | unruptured intracranial aneurysm treatment score |
| yo | years old |
References
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Faes, L.; Kale, A.U.; Wagner, S.K.; Fu, D.J.; Bruynseels, A.; Mahendiran, T.; Moraes, G.; Shamdas, M.; Kern, C.; et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: A systematic review and meta-analysis. Lancet Digit. Health 2019, 1, e271–e297. [Google Scholar] [CrossRef] [PubMed]
- Oren, O.; Gersh, B.J.; Bhatt, D.L. Artificial intelligence in medical imaging: Switching from radiographic pathological data to clinically meaningful endpoints. Lancet Digit. Health 2020, 2, e486–e488. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.; Huemmer, C.; Preuhs, A.; Buizza, G.; Hoppe, B.F.; Dinkel, J.; Koliogiannis, V.; Fink, N.; Goller, S.S.; Schwarze, V.; et al. Nonradiology Health-Care Professionals Significantly Benefit from AI Assistance in Emergency-Related Chest Radiography Interpretation. CHEST 2024, 166, 157–170. [Google Scholar] [CrossRef]
- Rudolph, J.; Schachtner, B.; Fink, N.; Koliogiannis, V.; Schwarze, V.; Goller, S.; Trappmann, L.; Hoppe, B.F.; Mansour, N.; Fischer, M.; et al. Clinically focused multi-cohort benchmarking as a tool for external validation of artificial intelligence algorithm performance in basic chest radiography analysis. Sci. Rep. 2022, 12, 12764. [Google Scholar] [CrossRef]
- Son, J.; Shin, J.Y.; Kim, H.D.; Jung, K.H.; Park, K.H.; Park, S.J. Development and Validation of Deep Learning Models for Screening Multiple Abnormal Findings in Retinal Fundus Images. Ophthalmology 2020, 127, 85–94. [Google Scholar] [CrossRef]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef]
- Brown, R.D.; Broderick, J.P. Unruptured intracranial aneurysms: Epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014, 13, 393–404. [Google Scholar] [CrossRef]
- Ajiboye, N.; Chalouhi, N.; Starke, R.M.; Zanaty, M.; Bell, R. Unruptured Cerebral Aneurysms: Evaluation and Management. Sci. World J. 2015, 2015, 954954. [Google Scholar] [CrossRef]
- Korja, M.; Silventoinen, K.; Laatikainen, T.; Jousilahti, P.; Salomaa, V.; Kaprio, J. Cause-specific mortality of 1-year survivors of subarachnoid hemorrhage. Neurology 2013, 80, 481–486. [Google Scholar] [CrossRef]
- Schievink, W.I.; Wijdicks, E.F.M.; Piepgras, D.G.; Chu, C.P.; O’Fallon, W.M.; Whisnant, J.P. The poor prognosis of ruptured intracranial aneurysms of the posterior circulation. J. Neurosurg. 1995, 82, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Kreiter, K.T.; Copeland, D.; Bernardini, G.L.; Bates, J.E.; Peery, S.; Claassen, J.; Du, Y.E.; Stern, Y.; Connolly, E.S.; Mayer, S.A. Predictors of Cognitive Dysfunction After Subarachnoid Hemorrhage. Stroke 2002, 33, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Brown, R.D.; Beseoglu, K.; Juvela, S.; Raymond, J.; Morita, A.; Torner, J.C.; Derdeyn, C.P.; Raabe, A.; Mocco, J.; et al. The unruptured intracranial aneurysm treatment score. Neurology 2015, 85, 881–889. [Google Scholar] [CrossRef]
- Greving, J.P.; Wermer, M.J.H.; Brown, R.D.; Morita, A.; Juvela, S.; Yonekura, M.; Ishibashi, T.; Torner, J.C.; Nakayama, T.; E Rinkel, G.J.; et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, S.; Sprengers, M.; Peluso, J.; Daams, J.; Verbaan, D.; van Rooij, W.; Majoie, C. A systematic review and meta-analysis of Woven EndoBridge single layer for treatment of intracranial aneurysms. Interv. Neuroradiol. 2020, 26, 455–460. [Google Scholar] [CrossRef]
- Wiebers, D.O.; International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef]
- Rinkel, G.J.; Ruigrok, Y.M. Preventive screening for intracranial aneurysms. Int. J. Stroke Off. J. Int. Stroke Soc. 2022, 17, 30–36. [Google Scholar] [CrossRef]
- Schmidt, C.; Stahl, R.; Mueller, F.; Fischer, T.D.; Forbrig, R.; Brem, C.; Isik, H.; Seelos, K.; Thon, N.; Stoecklein, S.; et al. Evaluation of an AI-Powered Routine Screening of Clinically Acquired cMRIs for Incidental Intracranial Aneurysms. Diagnostics 2025, 15, 254. [Google Scholar] [CrossRef]
- Chen, G.; Wei, X.; Lei, H.; Liqin, Y.; Yuxin, L.; Yakang, D.; Daoying, G. Automated computer-assisted detection system for cerebral aneurysms in time-of-flight magnetic resonance angiography using fully convolutional network. Biomed. Eng. Online 2020, 19, 38. [Google Scholar] [CrossRef]
- Lehnen, N.C.; Haase, R.; Schmeel, F.C.; Vatter, H.; Dorn, F.; Radbruch, A.; Paech, D. Automated Detection of Cerebral Aneurysms on TOF-MRA Using a Deep Learning Approach: An External Validation Study. AJNR Am. J. Neuroradiol. 2022, 43, 1700–1705. [Google Scholar] [CrossRef]
- Nakao, T.; Hanaoka, S.; Nomura, Y.; Sato, I.; Nemoto, M.; Miki, S.; Maeda, E.; Yoshikawa, T.; Hayashi, N.; Abe, O. Deep neural network-based computer-assisted detection of cerebral aneurysms in MR angiography. J. Magn. Reson. Imaging JMRI 2018, 47, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Sichtermann, T.; Faron, A.; Sijben, R.; Teichert, N.; Freiherr, J.; Wiesmann, M. Deep Learning-Based Detection of Intracranial Aneurysms in 3D TOF-MRA. AJNR Am. J. Neuroradiol. 2019, 40, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Stember, J.N.; Chang, P.; Stember, D.M.; Liu, M.; Grinband, J.; Filippi, C.G.; Meyers, P.; Jambawalikar, S. Convolutional Neural Networks for the Detection and Measurement of Cerebral Aneurysms on Magnetic Resonance Angiography. J. Digit. Imaging 2019, 32, 808–815. [Google Scholar] [CrossRef]
- Ueda, D.; Yamamoto, A.; Nishimori, M.; Shimono, T.; Doishita, S.; Shimazaki, A.; Katayama, Y.; Fukumoto, S.; Choppin, A.; Shimahara, Y.; et al. Deep Learning for MR Angiography: Automated Detection of Cerebral Aneurysms. Radiology 2019, 290, 187–194. [Google Scholar] [CrossRef]
- Yang, X.; Blezek, D.J.; Cheng, L.T.E.; Ryan, W.J.; Kallmes, D.F.; Erickson, B.J. Computer-aided detection of intracranial aneurysms in MR angiography. J. Digit. Imaging 2011, 24, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Shi, Z.; Lu, L.; Miao, Z.; Wang, H.; Zhou, Z.; Zhang, F.; Wang, R.; Luo, X.; Xu, F.; et al. A deep-learning model for intracranial aneurysm detection on CT angiography images in China: A stepwise, multicentre, early-stage clinical validation study. Lancet Digit. Health 2024, 6, e261–e271. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.; Wang, S.H.; Zhang, Y.D. A Review of Deep Learning on Medical Image Analysis. Mob. Netw. Appl. 2021, 26, 351–380. [Google Scholar] [CrossRef]
- Bo, Z.H.; Qiao, H.; Tian, C.; Guo, Y.; Li, W.; Liang, T.; Li, D.; Liao, D.; Zeng, X.; Mei, L.; et al. Toward human intervention-free clinical diagnosis of intracranial aneurysm via deep neural network. Patterns 2021, 2, 100197. [Google Scholar] [CrossRef]
- Çiçek, Ö.; Abdulkadir, A.; Lienkamp, S.S.; Brox, T.; Ronneberger, O. 3D U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2016; Ourselin, S., Joskowicz, L., Sabuncu, M.R., Unal, G., Wells, W., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 424–432. [Google Scholar] [CrossRef]
- UCAS Japan Investigators. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N. Engl. J. Med. 2012, 366, 2474–2482. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, Y.; Delcourt, C.; Shan, J.; Li, Q.; Xu, J.; Hackett, M.L. Is Regular Screening for Intracranial Aneurysm Necessary in Patients with Autosomal Dominant Polycystic Kidney Disease? A Systematic Review and Meta-analysis. Cerebrovasc. Dis. 2017, 44, 75–82. [Google Scholar] [CrossRef]
- Bor, A.S.E.; Rinkel, G.J.E.; Van Norden, J.; Wermer, M.J.H. Long-term, serial screening for intracranial aneurysms in individuals with a family history of aneurysmal subarachnoid haemorrhage: A cohort study. Lancet Neurol. 2014, 13, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Takao, H.; Nojo, T.; Ohtomo, K. Screening for Familial Intracranial Aneurysms. Acad. Radiol. 2008, 15, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Wu, X.; Matouk, C.C.; Forman, H.P.; Gandhi, D.; Sanelli, P. MR Angiography Screening and Surveillance for Intracranial Aneurysms in Autosomal Dominant Polycystic Kidney Disease: A Cost-effectiveness Analysis. Radiology 2019, 291, 400–408. [Google Scholar] [CrossRef]
- Rao, V.M.; Levin, D.C.; Parker, L.; Cavanaugh, B.; Frangos, A.J.; Sunshine, J.H. How Widely Is Computer-Aided Detection Used in Screening and Diagnostic Mammography? J. Am. Coll. Radiol. 2010, 7, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Fenton, J.J.; Taplin, S.H.; Carney, P.A.; Abraham, L.; Sickles, E.A.; D’Orsi, C.; Berns, E.A.; Cutter, G.; Hendrick, R.E.; Barlow, W.E.; et al. Influence of Computer-Aided Detection on Performance of Screening Mammography. N. Engl. J. Med. 2007, 356, 1399–1409. [Google Scholar] [CrossRef]
- Gilbert, F.J.; Astley, S.M.; Gillan, M.G.C.; Agbaje, O.F.; Wallis, M.G.; James, J.; Boggis, C.R.; Duffy, S.W. Single Reading with Computer-Aided Detection for Screening Mammography. N. Engl. J. Med. 2008, 359, 1675–1684. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, F.; Schmidt, C.C.; Stahl, R.; Forbrig, R.; Fischer, T.D.; Brem, C.; Seelos, K.; Isik, H.; Rudolph, J.; Hoppe, B.F.; et al. Worthwhile or Not? The Pain–Gain Ratio of Screening Routine cMRIs in a Maximum Care University Hospital for Incidental Intracranial Aneurysms Using Artificial Intelligence. J. Clin. Med. 2025, 14, 4121. https://doi.org/10.3390/jcm14124121
Mueller F, Schmidt CC, Stahl R, Forbrig R, Fischer TD, Brem C, Seelos K, Isik H, Rudolph J, Hoppe BF, et al. Worthwhile or Not? The Pain–Gain Ratio of Screening Routine cMRIs in a Maximum Care University Hospital for Incidental Intracranial Aneurysms Using Artificial Intelligence. Journal of Clinical Medicine. 2025; 14(12):4121. https://doi.org/10.3390/jcm14124121
Chicago/Turabian StyleMueller, Franziska, Christina Carina Schmidt, Robert Stahl, Robert Forbrig, Thomas David Fischer, Christian Brem, Klaus Seelos, Hakan Isik, Jan Rudolph, Boj Friedrich Hoppe, and et al. 2025. "Worthwhile or Not? The Pain–Gain Ratio of Screening Routine cMRIs in a Maximum Care University Hospital for Incidental Intracranial Aneurysms Using Artificial Intelligence" Journal of Clinical Medicine 14, no. 12: 4121. https://doi.org/10.3390/jcm14124121
APA StyleMueller, F., Schmidt, C. C., Stahl, R., Forbrig, R., Fischer, T. D., Brem, C., Seelos, K., Isik, H., Rudolph, J., Hoppe, B. F., Kunz, W. G., Thon, N., Ricke, J., Ingrisch, M., Stoecklein, S., Liebig, T., & Rueckel, J. (2025). Worthwhile or Not? The Pain–Gain Ratio of Screening Routine cMRIs in a Maximum Care University Hospital for Incidental Intracranial Aneurysms Using Artificial Intelligence. Journal of Clinical Medicine, 14(12), 4121. https://doi.org/10.3390/jcm14124121








