Minimally Invasive Drainage for Diabetic Foot Phlegmon †
Abstract
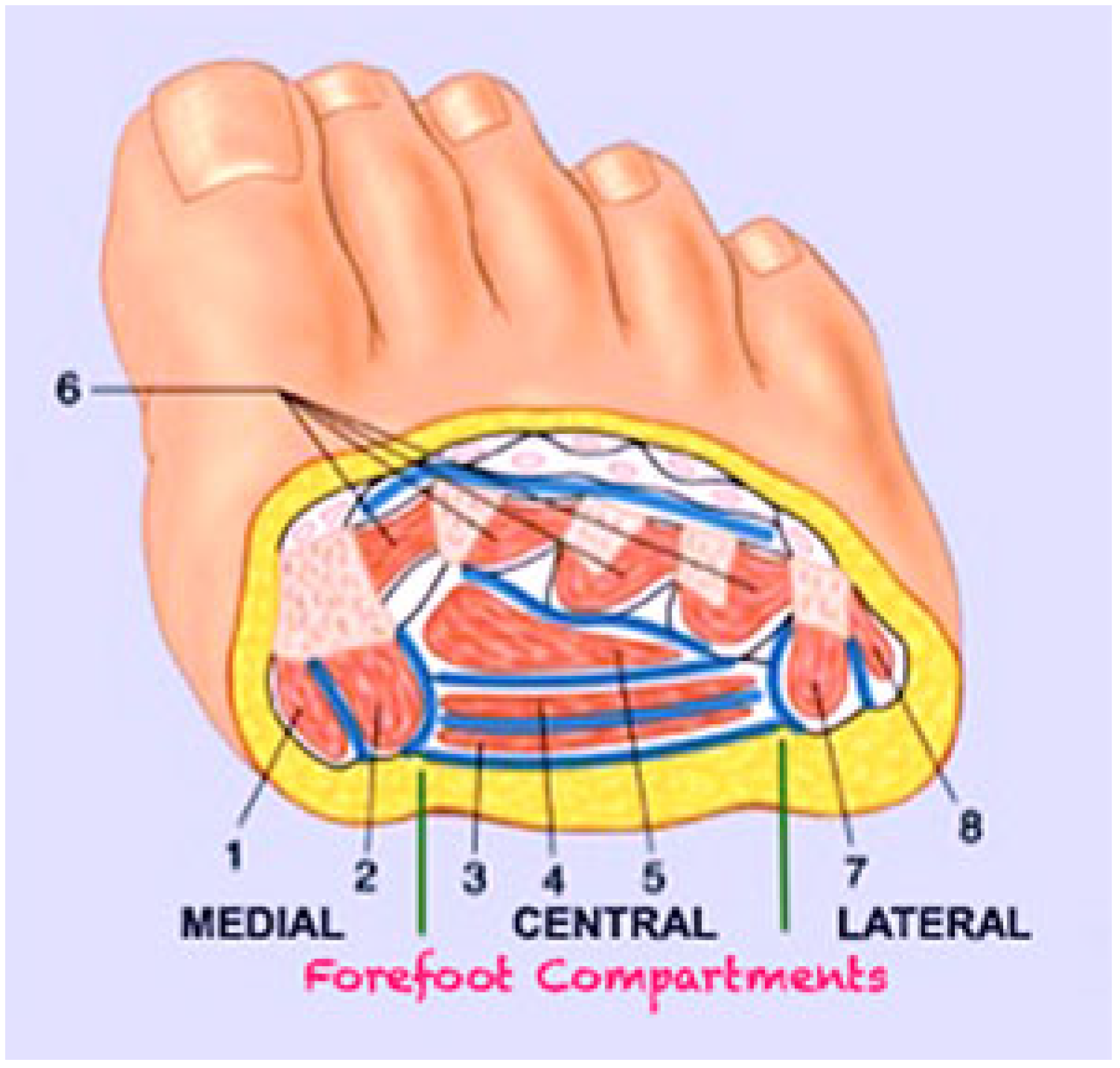
1. Introduction
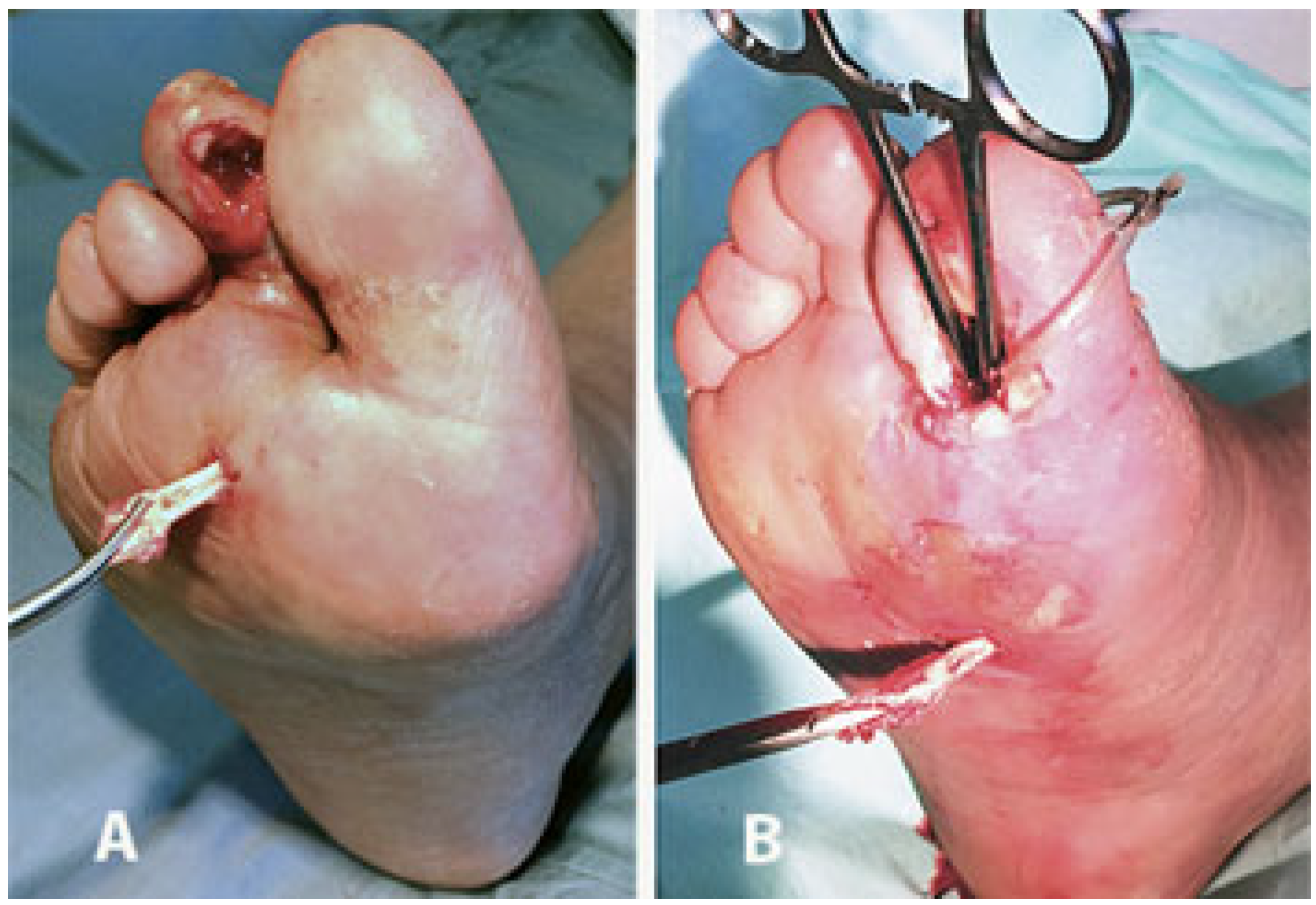
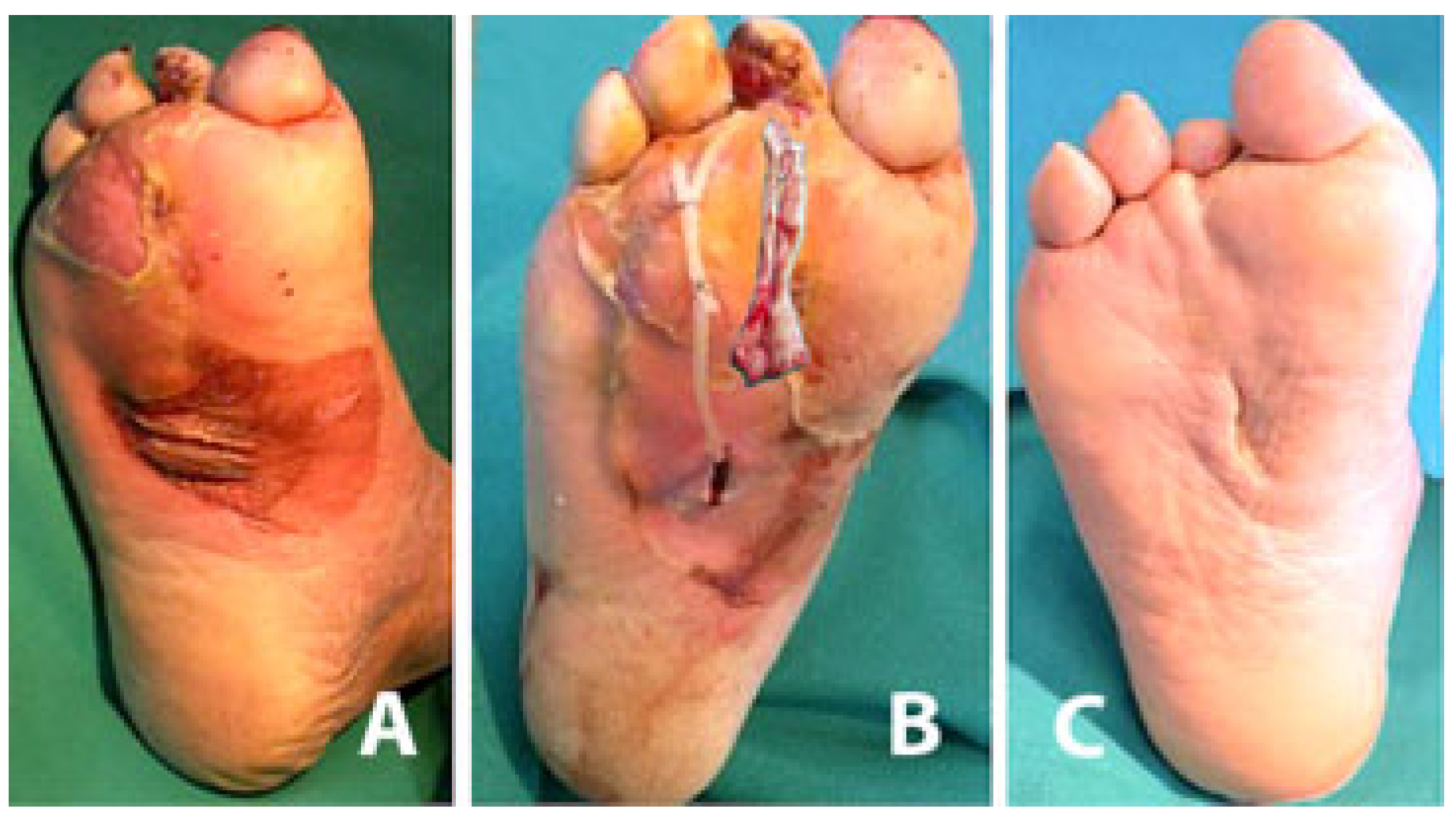
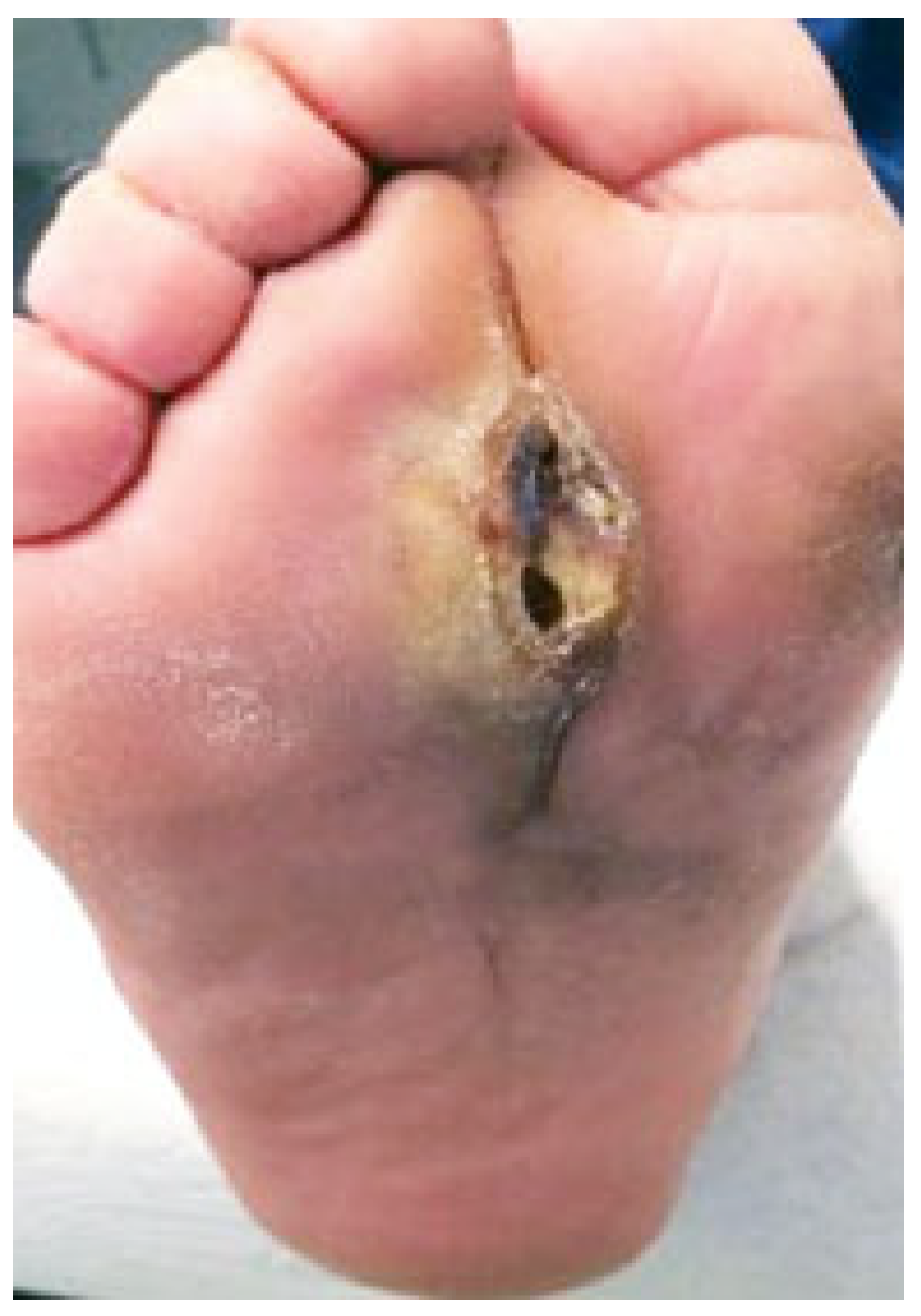
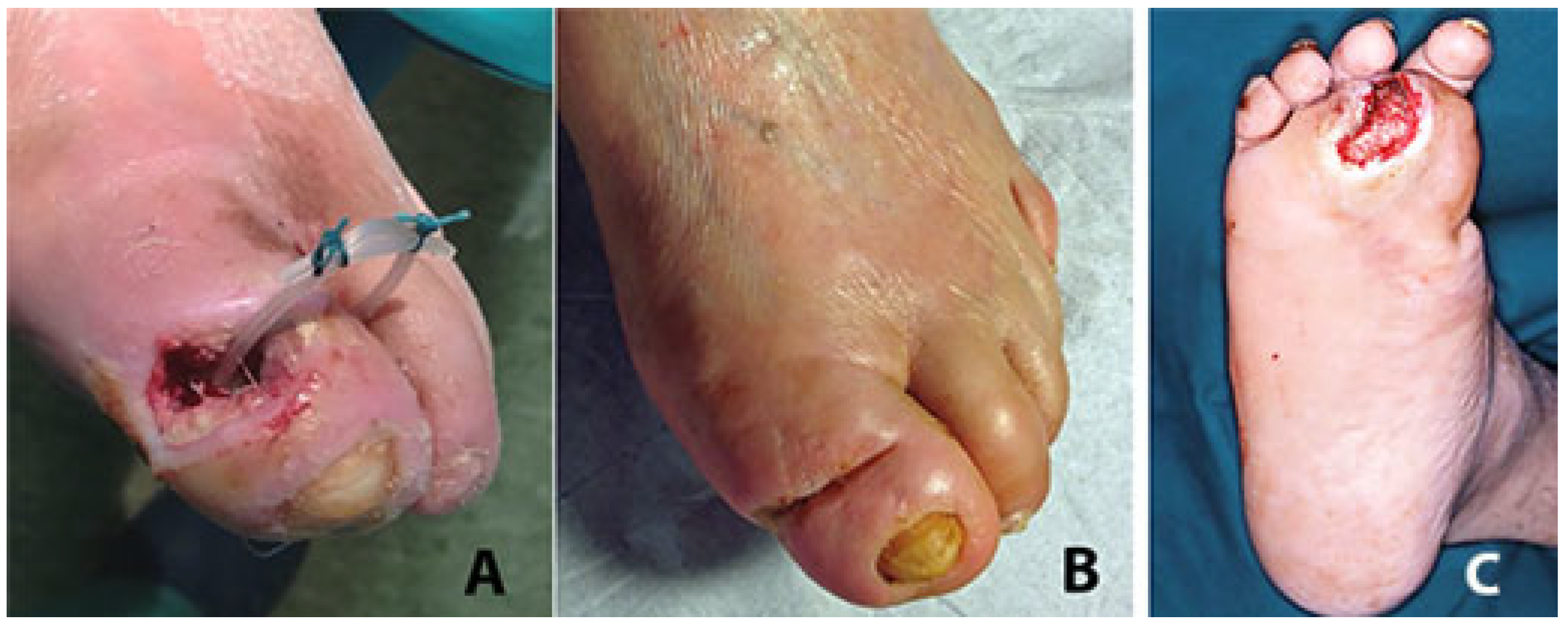
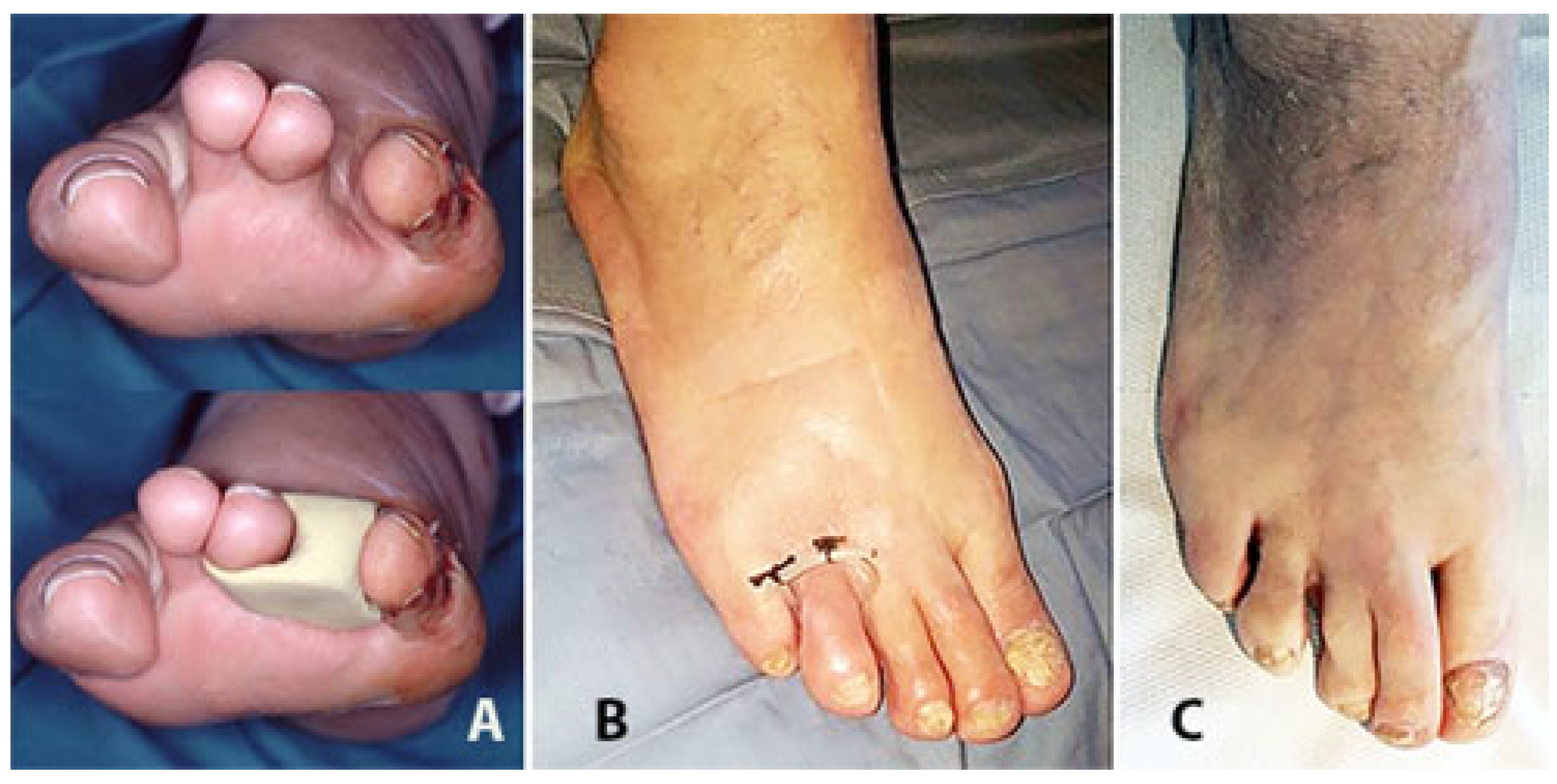
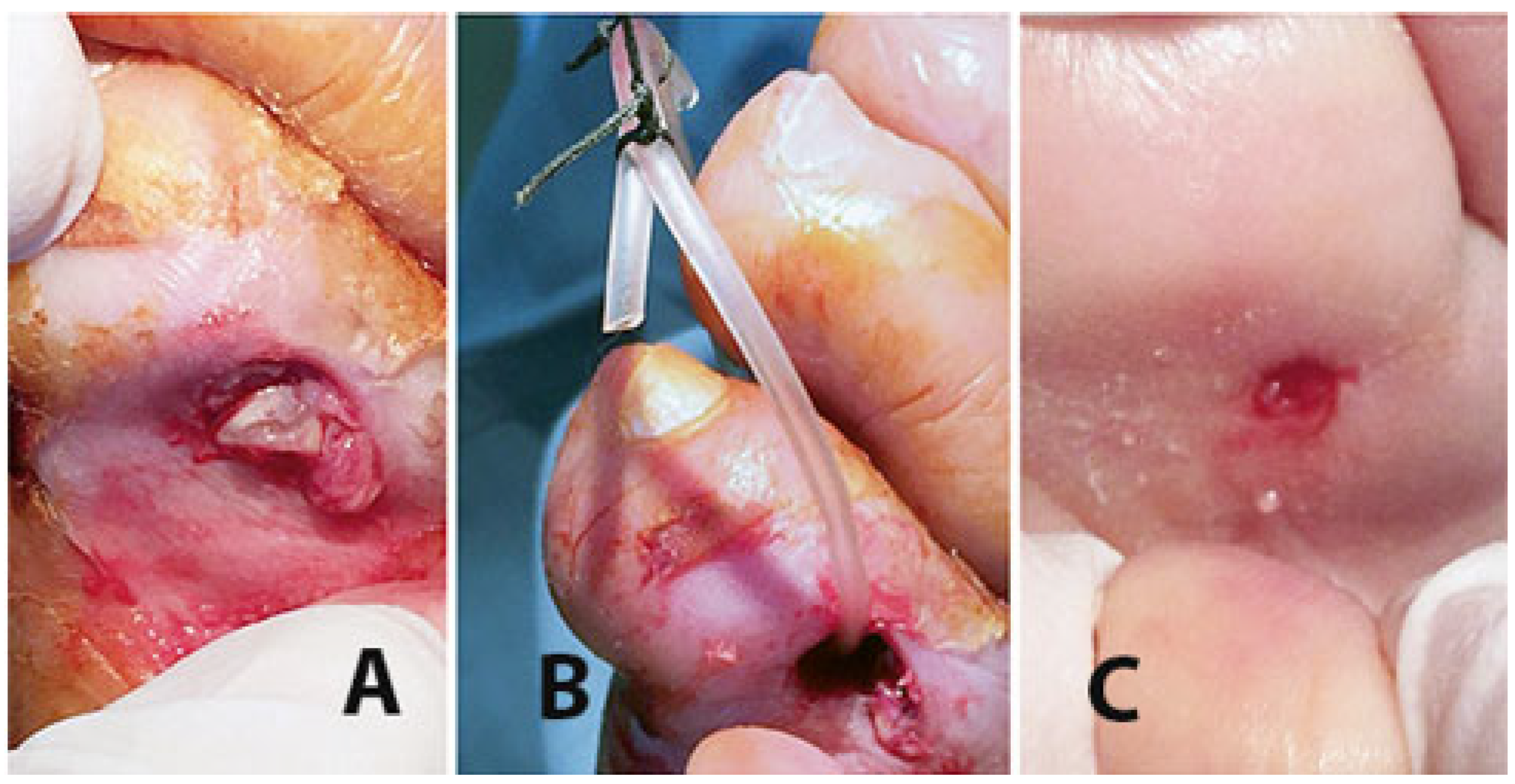
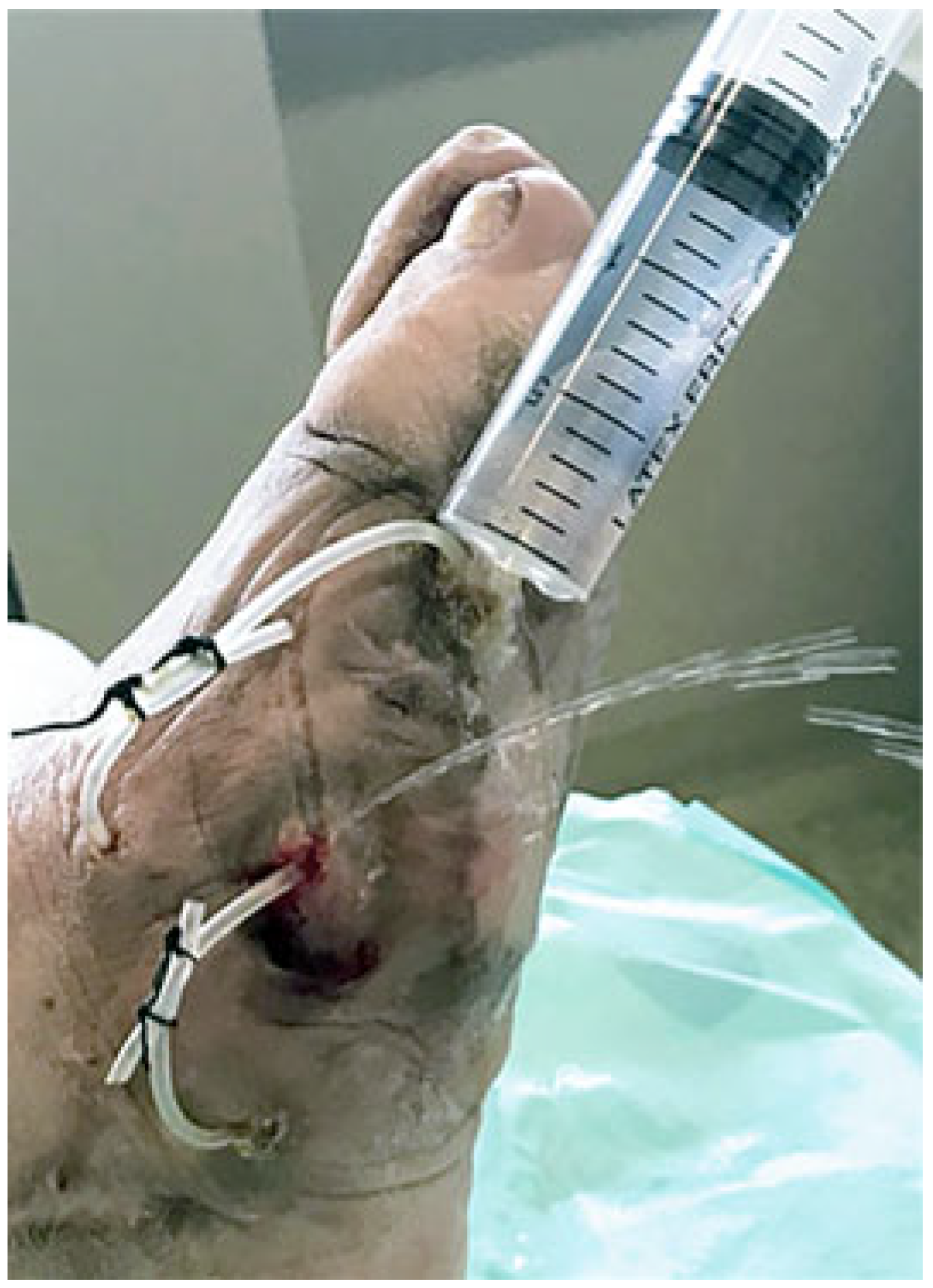
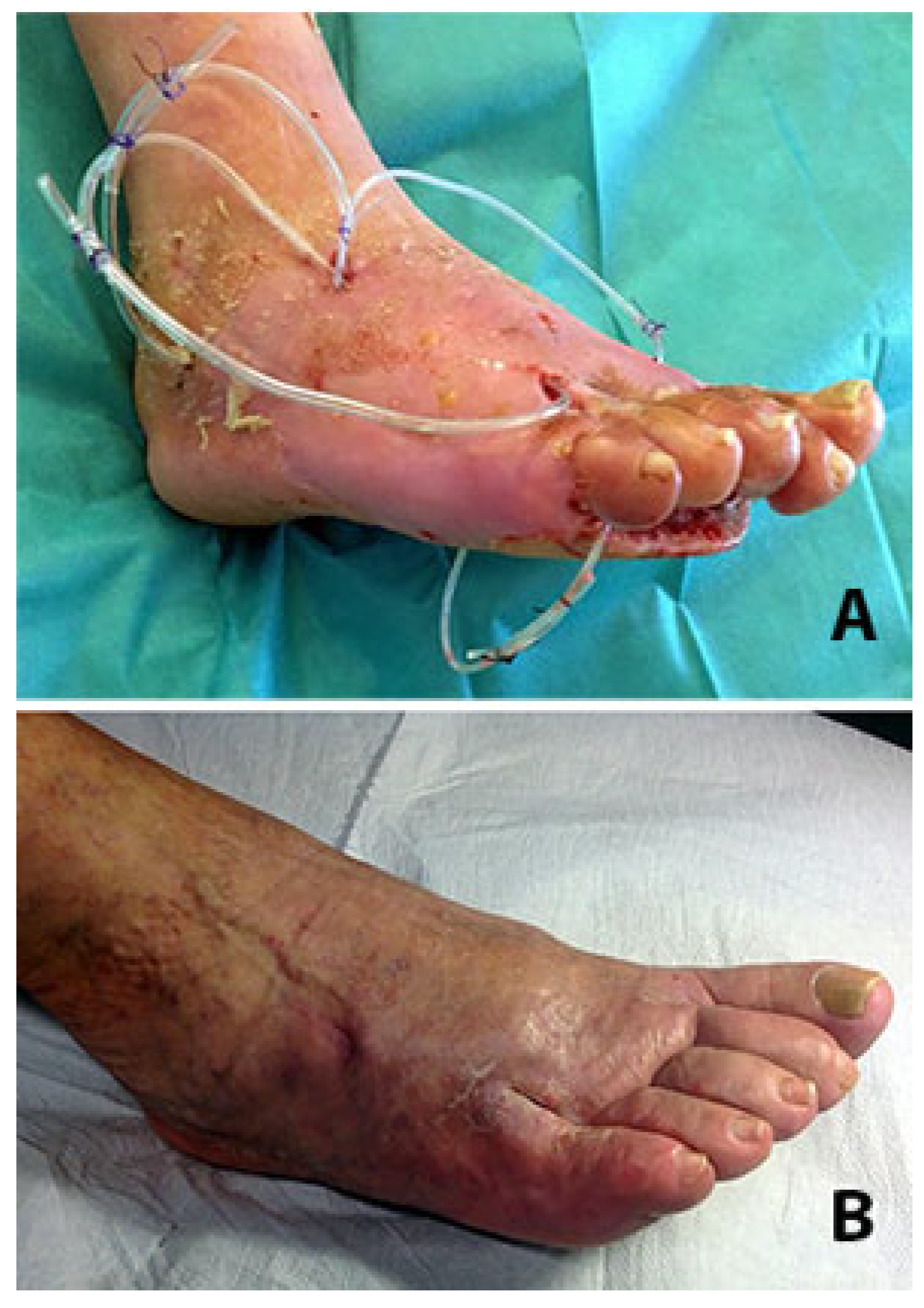
2. Surgical Technique
3. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Prompers, L.; Schaper, N.; Apelqvist, J.; Edmonds, M.; Jude, E.; Mauricio, D.; Uccioli, L.; Urbancic, V.; Bakker, K.; Holstein, P.; et al. Prediction of outcome in individuals with diabetic foot ulcers: Focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008, 51, 747–755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, S.M.; Johnson, B.L.; Samies, N.L.; Rawlinson, D.R.; Williamson, L.E.; Davis, S.A.; Kotrady, J.A.; York, J.W.; Langan, E.M.; Cull, D.L. Contemporary management of diabetic neuropathic foot ulceration: A study of 917 consecutively treated limbs. J. Am. Coll. Surg. 2011, 212, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Maida, C.; Pinto, A. Diabetic foot syndrome: Immune-inflammatory features as possible cardiovascular markers in diabetes. World J. Orthop. 2015, 6, 62–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hingorani, A.; LaMuraglia, G.M.; Henke, P.; Meissner, M.H.; Loretz, L.; Zinszer, K.M.; Driver, V.R.; Frykberg, R.; Carman, T.L.; Marston, W.; et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J. Vasc. Surg. 2016, 63 (Suppl. 2), 3S–21S. [Google Scholar] [CrossRef] [PubMed]
- Coxe, J.R. The Writings of Hippocrates and Galen; Lindsay and Blakinston: Philadelpia, PA, USA, 1846. [Google Scholar]
- Cavallini, M. Ulcer piercing: Cleansing of complicated diabetic neuropathic foot ulcers by positive pressure irrigation. J. Wound Care 2014, 23, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M. Wound piercing: A novel approach for deep cutaneous ulcer cleansing. Ann. Ital. Chir. 2014, 85, 506–510. [Google Scholar] [PubMed]
- Cavallini, M. Ulcer Piercing: A Novel Drainage Technique for Diabetic Patients with Complicated Foot. J. Endocrinol. Diabetes 2015, 2, 1–4. [Google Scholar] [CrossRef][Green Version]
- Cavallini, M. Mini-Invasive Drainage and Irrigation Avoid Exudate Stasis, Reduce Recurrence Risk and Allow Better Rehabilita-tion for Deep Infected Diabetic Foot Ulcers. Poster and oral presentation. In Proceedings of the EWMA 2020 Annual Conference, London, UK, 13–15 May 2020. [Google Scholar]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Dechert, T.A.; Ducale, A.E.; Ward, S.I.; Yager, D.R. Hyaluronan in human acute and chronic dermal wounds. Wound Repair Regen. 2006, 14, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.; Tecilazich, F.; Kafanas, A.; Doupis, J.; Gnardellis, C.; Leal, E.; Tellechea, A.; Pradhan, L.; Lyons, T.E.; Giurini, J.M.; et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012, 61, 2937–2947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCarty, S.M.; Cochrane, C.A.; Clegg, P.D.; Percival, S.L. The role of endogenous and exogenous enzymes in chronic wounds: A focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen. 2012, 20, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and inflammation in chronic wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trengove, N.J.; Bielefeldt-Ohmann, H.; Stacey, M.C. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000, 8, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, F.; Bai, J.; Wang, Z.; Qin, W. An observational study of the pH value during the healing process of diabetic foot ulcer. J. Tissue Viability 2024, 33, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Nouvong, A.; Ambrus, A.M.; Zhang, E.R.; Hultman, L.; Coller, H.A. Reactive oxygen species and bacterial biofilms in diabetic wound healing. Physiol. Genom. 2016, 48, 889–896. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.; Angelisanti, M.; Calzoni, C.; Somma, F.; Chiummariello, S. Treatment of ulcer and difficult wounds of the lower limbs: Our experience. Ann. Ital. Chir. 2012, 83, 135–144. [Google Scholar]
- Andros, G.; Armstrong, D.G.; Attinger, C.E.; Boulton, A.J.M.; Frykberg, R.G.; Joseph, W.S.; Lavery, L.A.; Morbach, S.; Niezgoda, J.A.; Toursarkissian, B. Consensus statement on negative pressure wound therapy (V.A.C. Therapy) for the management of diabetic foot wounds. Wounds 2006, 18, 1–32. [Google Scholar]
- Lone, A.M.; Zaroo, M.I.; Laway, B.A.; Pala, N.A.; Bashir, S.A.; Rasool, A. Vacuum-assisted closure versus conventional dressings in the management of diabetic foot ulcers: A prospective case–control study. Diabet. Foot Ankle 2014, 8, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boulton, A.J.; Armstrong, D.G.; Kirsner, R.S.; Attinger, C.E.; Lavery, L.A.; Lipsky, B.A.; Mills, J.L., Sr.; Steinberg, J.S. Diagnosis and Management of Diabetic Foot Complications; American Diabetes Association: Arlington, VA, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538977/ (accessed on 1 January 2025).
- Van Battum, P.; Schaper, N.; Prompers, L.; Apelqvist, J.; Jude, E.; Piaggesi, A.; Bakker, K.; Edmonds, M.; Holstein, P.; Jirkovska, A.; et al. Differences in minor amputation rate in diabetic foot disease throughout Europe are in part explained by differences in disease severity at presentation. Diabet. Med. 2011, 28, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M. Mini-invasive drainage technique to treat diabetic foot infection. In Proceedings of the 8th International Symposium on the Diabetic Foot, The Hague, The Netherlands, 22–25 May 2019; Poster p4.01. Available online: https://isdf.nl/wp-content/uploads/2019/09/Abstractbook-ISDF2019.pdf (accessed on 28 May 2025).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallini, M. Minimally Invasive Drainage for Diabetic Foot Phlegmon. J. Clin. Med. 2025, 14, 3918. https://doi.org/10.3390/jcm14113918
Cavallini M. Minimally Invasive Drainage for Diabetic Foot Phlegmon. Journal of Clinical Medicine. 2025; 14(11):3918. https://doi.org/10.3390/jcm14113918
Chicago/Turabian StyleCavallini, Marco. 2025. "Minimally Invasive Drainage for Diabetic Foot Phlegmon" Journal of Clinical Medicine 14, no. 11: 3918. https://doi.org/10.3390/jcm14113918
APA StyleCavallini, M. (2025). Minimally Invasive Drainage for Diabetic Foot Phlegmon. Journal of Clinical Medicine, 14(11), 3918. https://doi.org/10.3390/jcm14113918





