Prophylactic Ureteral Catheterization for Preventing Ureteral Injury in Colorectal Cancer Surgery
Abstract
1. Introduction
2. Materials and Methods
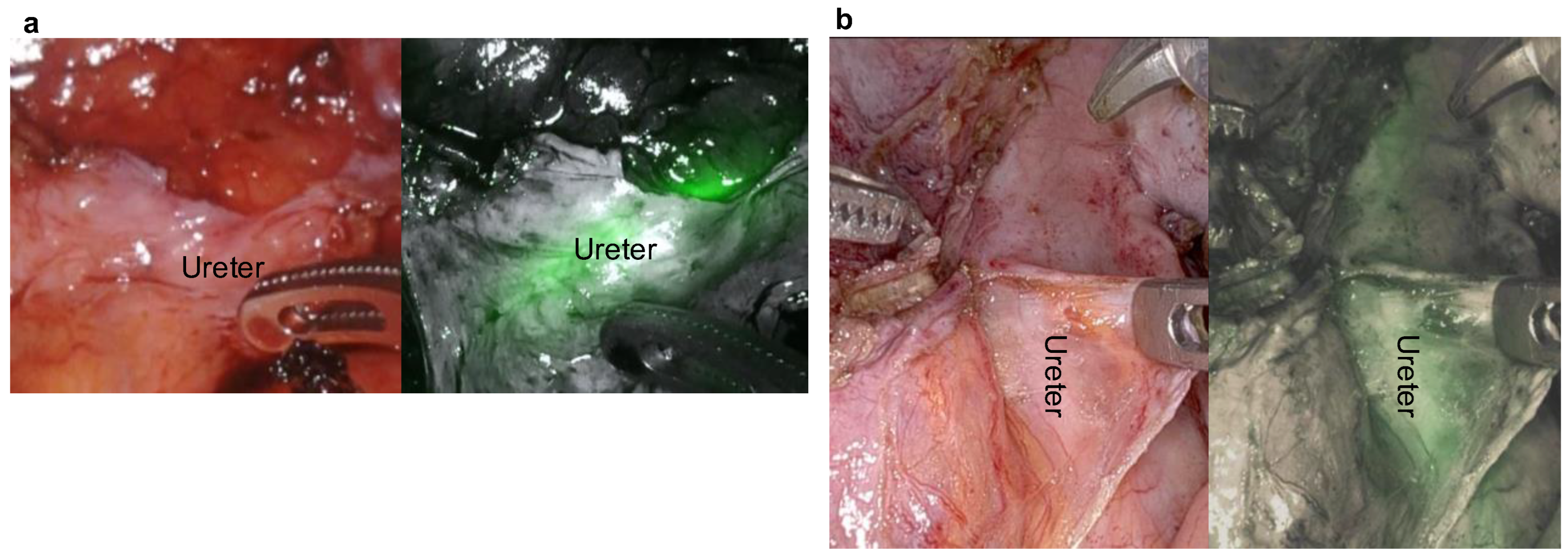
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APR | Abdominal Perineal Resection |
| CRC | Colorectal cancer |
| LAR | Low anterior resection |
| PUC | Prophylactic ureteral catheterization |
| TPE | Total Pelvic Exenteration |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Cancer Survival in Hospital-Based Cancer Registries. Cancer Information Service, National Cancer Center, Japan. Available online: https://hbcr-survival.ganjoho.jp (accessed on 22 March 2025).
- Jacobs, M.; Verdeja, J.C.; Goldstein, H.S. Minimally invasive colon resection (laparoscopic colectomy). Surg. Laparosc. Endosc. 1991, 1, 144–150. [Google Scholar] [PubMed]
- Yamamoto, S.; Watanabe, M.; Hasegawa, H.; Kitajima, M. Prospective evaluation of laparoscopic surgery for rectosigmoidal and rectal carcinoma. Dis. Colon Rectum 2002, 45, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.; Poncet, G.; Voirin, D.; Laverriere, M.H.; Anglade, D.; Faucheron, J.L. Can adequate lymphadenectomy be obtained by laparoscopic resection in rectal cancer? Results of a case-control study in 200 patients. J. Gastrointest. Surg. 2010, 14, 1244–1247. [Google Scholar] [CrossRef]
- Kitano, S.; Inomata, M.; Mizusawa, J.; Katayama, H.; Watanabe, M.; Yamamoto, S.; Ito, M.; Saito, S.; Fujii, S.; Konishi, F.; et al. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): A phase 3, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2017, 2, 261–268. [Google Scholar] [CrossRef]
- Weber, P.A.; Merola, S.; Wasielewski, A.; Ballantyne, G.H. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis. Colon Rectum 2002, 45, 1689–1694, discussion 1695–1696. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Coratti, A.; Angelini, M.; Sbrana, F.; Cecconi, S.; Balestracci, T.; Caravaglios, G. Robotics in general surgery: Personal experience in a large community hospital. Arch. Surg. 2003, 138, 777–784. [Google Scholar] [CrossRef]
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Rautio, T.; Thomassen, N.; et al. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA 2017, 318, 1569–1580. [Google Scholar] [CrossRef]
- Gomez Ruiz, M.; Ballestero Diego, R.; Tejedor, P.; Cagigas Fernandez, C.; Cristobal Poch, L.; Suarez Pazos, N.; Castillo Diego, J. Robotic surgery for locally advanced T4 rectal cancer: Feasibility and oncological quality. Updates Surg. 2023, 75, 589–597. [Google Scholar] [CrossRef]
- Moriya, Y. Treatment strategy for locally recurrent rectal cancer. Jpn. J. Clin. Oncol. 2006, 36, 127–131. [Google Scholar] [CrossRef]
- Renehan, A.G. Techniques and outcome of surgery for locally advanced and local recurrent rectal cancer. Clin. Oncol. (R Coll. Radiol.) 2016, 28, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Paku, M.; Uemura, M.; Kitakaze, M.; Fujino, S.; Ogino, T.; Miyoshi, N.; Takahashi, H.; Yamamoto, H.; Mizushima, T.; Doki, Y.; et al. Impact of the preoperative prognostic nutritional index as a predictor for postoperative complications after resection of locally recurrent rectal cancer. BMC Cancer 2021, 21, 435. [Google Scholar] [CrossRef]
- Asoglu, O.; Karanlik, H.; Muslumanoglu, M.; Igci, A.; Emek, E.; Ozmen, V.; Kecer, M.; Parlak, M.; Kapran, Y. Prognostic and predictive factors after surgical treatment for locally recurrent rectal cancer: A single institute experience. Eur. J. Surg. Oncol. 2007, 33, 1199–1206. [Google Scholar] [CrossRef]
- McCarus, S.; Alexandre, A.F.; Kimura, T.; Feng, Q.; Han, W.; Shortridge, E.F.; Lima, R.B.; Schwartz, J.; Wexner, S.D. Abdominopelvic surgery: Intraoperative ureteral injury and prophylaxis in the United States, 2015–2019. Adv. Ther. 2023, 40, 3169–3185. [Google Scholar] [CrossRef]
- Souli, A.; Alves, A.; Tillou, X.; Menahem, B. Iatrogenic ureteral injury: What should the digestive surgeon know? J. Visc. Surg. 2024, 161, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Kyzer, S.; Gordon, P.H. The prophylactic use of ureteral catheters during colorectal operations. Am. Surg. 1994, 60, 212–216. [Google Scholar] [PubMed]
- Boyan, W.P., Jr.; Lavy, D.; Dinallo, A.; Otero, J.; Roding, A.; Hanos, D.; Dressner, R.; Arvanitis, M. Lighted ureteral stents in laparoscopic colorectal surgery; a five-year experience. Ann. Transl. Med. 2017, 5, 44. [Google Scholar] [CrossRef]
- Hassinger, T.E.; Mehaffey, J.H.; Mullen, M.G.; Michaels, A.D.; Elwood, N.R.; Levi, S.T.; Hedrick, T.L.; Friel, C.M. Ureteral stents increase risk of postoperative acute kidney injury following colorectal surgery. Surg. Endosc. 2018, 32, 3342–3348. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Khubchandani, I.T. Prophylactic ureteric catheters in colon surgery- How safe are they? Report of three cases. Dis. Colon Rectum 1990, 33, 508–510. [Google Scholar] [CrossRef]
- Higgins, C.C. Ureteral injuries during surgery. A review of 87 cases. JAMA 1967, 199, 82–88. [Google Scholar] [CrossRef]
- Tsujinaka, S.; Wexner, S.D.; DaSilva, G.; Sands, D.R.; Weiss, E.G.; Nogueras, J.J.; Efron, J.; Vernava, A.M., 3rd. Prophylactic ureteric catheters in laparoscopic colorectal surgery. Tech. Coloproctol. 2008, 12, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Shahait, A.D.; Mesquita-Neto, J.W.B.; Girten, K.; Weaver, D.; Gruber, S.A.; Gamal, M. Iatrogenic ureteral injury and prophylactic stent use in veterans undergoing colorectal surgery. J. Surg. Res. 2021, 265, 272–277. [Google Scholar] [CrossRef]
- Ryu, S.; Hara, K.; Okamoto, A.; Kitagawa, T.; Marukuchi, R.; Ito, R.; Nakabayashi, Y. Fluorescence ureteral navigation during laparoscopic surgery for clinically suspected stage T4 colorectal cancer: A cohort study. Surg. Oncol. 2022, 40, 101672. [Google Scholar] [CrossRef]
- Hamada, M.; Matsumi, Y.; Sekimoto, M.; Kurokawa, H.; Kita, M.; Kinoshita, H. Image navigation surgery with the fluorescent ureteral catheter of recurrent tumors in the pelvic cavity. Dis. Colon Rectum 2022, 65, e72–e76. [Google Scholar] [CrossRef]
- Ferrara, M.; Kann, B.R. Urological Injuries during Colorectal Surgery. Clin. Colon Rectal Surg. 2019, 32, 196–203. [Google Scholar] [CrossRef]
- Halabi, W.J.; Jafari, M.D.; Nguyen, V.Q.; Carmichael, J.C.; Mills, S.; Pigazzi, A.; Stamos, M.J. Ureteral injuries in colorectal surgery: An analysis of trends, outcomes, and risk factors over a 10-year period in the United States. Dis. Colon Rectum 2014, 57, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Marcelissen, T.A.T.; Den Hollander, P.P.; Tuytten, T.R.A.H.; Sosef, M.N. Incidence of iatrogenic ureteral injury during open and laparoscopic colorectal surgery: A Single Center experience and review of the literature. Surg. Laparosc. Endosc. Percutan. Tech. 2016, 26, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Palaniappa, N.C.; Telem, D.A.; Ranasinghe, N.E.; Divino, C.M. Incidence of iatrogenic ureteral injury after laparoscopic colectomy. Arch. Surg. 2012, 147, 267–271. [Google Scholar] [CrossRef]
- Moretto, S.; Saita, A.; Scoffone, C.M.; Talso, M.; Somani, B.K.; Traxer, O.; Angerri, O.; Knoll, T.; Liatsikos, E.; Herrmann, T.R.W.; et al. Ureteral stricture rate after endoscopic treatments for urolithiasis and related risk factors: Systematic review and meta-analysis. World J. Urol. 2024, 42, 234. [Google Scholar] [CrossRef]
- Moretto, S.; Saita, A.; Scoffone, C.M.; Talso, M.; Somani, B.K.; Traxer, O.; Angerri, O.; Liatsikos, E.; Ulvik, Y.; Cracco, C.M.; et al. An international Delphi survey and consensus meeting to define the risk factors for ureteral stricture after endoscopic treatment for urolithiasis. World J. Urol. 2024, 42, 412. [Google Scholar] [CrossRef]
- Wetherell, J.; Mitchell, J.; Sothilingam, N.; Micheal, J.; Guver, A.; Felton, J.; Wolf, J.H. Prophylactic ureteral catheterization performed by the colorectal surgeon: A retrospective analysis. J. Gastrointest. Surg. 2024, 28, 1917–1918. [Google Scholar] [CrossRef] [PubMed]
- Merola, J.; Arnold, B.; Luks, V.; Ibarra, C.; Resio, B.; Davis, K.A.; Pei, K.Y. Prophylactic ureteral stent placement vs no ureteral stent placement during open colectomy. JAMA Surg. 2018, 153, 87–90. [Google Scholar] [CrossRef]
- Alexandre, A.F.; Kimura, T.; Feng, Q.; Han, W.; Shortridge, E.; Schwartz, J.; Wexner, S.D. Effectiveness and cost of stenting in ureteral injury in colorectal surgeries in the US: 2015–2019. JSLS 2023, 27, e2023.00023. [Google Scholar] [CrossRef]
- Summerton, D.J.; Kitrey, N.D.; Lumen, N.; Serafetinidis, E.; Djakovic, N.; European Association of Urology. EAU guidelines on iatrogenic trauma. Eur. Urol. 2012, 62, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Mandovra, P.; Kalikar, V.; Patankar, R.V. Real-Time Visualization of Ureters Using Indocyanine Green During Laparoscopic Surgeries: Can We Make Surgery Safer? Surg. Innov. 2019, 26, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Wherry, D.; Marohn, M. Use of ultrasound to identify the ureter. Surg. Endosc. 1993, 7, 467. [Google Scholar] [CrossRef]
- Cellina, M.; Cè, M.; Rossini, N.; Cacioppa, L.M.; Ascenti, V.; Carrafiello, G.; Floridi, C. Computed Tomography Urography: State of the Art and Beyond. Tomography 2023, 9, 909–930. [Google Scholar] [CrossRef]
- Slooter, M.D.; Janssen, A.; Bemelman, W.A.; Tanis, P.J.; Hompes, R. Currently available and experimental dyes for intraoperative near-infrared fluorescence imaging of the ureters: A systematic review. Tech. Coloproctol. 2019, 23, 305–313. [Google Scholar] [CrossRef]
- Dip, F.D.; Nahmod, M.; Anzorena, F.S.; Moreira, A.; Sarotto, L.; Ampudia, C.; Kalaskar, S.N.; Ferraina, P.; Rosenthal, R.J.; Wexner, S.D. Novel technique for identification of ureters using sodium fluorescein. Surg. Endosc. 2014, 28, 2730–2733. [Google Scholar] [CrossRef]
- Mahalingam, S.M.; Dip, F.; Castillo, M.; Roy, M.; Wexner, S.D.; Rosenthal, R.J.; Low, P.S. Intraoperative ureter visualization using a novel near-infrared fluorescent dye. Mol. Pharm. 2018, 15, 3442–3447. [Google Scholar] [CrossRef]

| Indications | No. of Patients (%) |
|---|---|
| Colon cancer * | 12 (28.6) |
| Rectal cancer | 14 (33.3) |
| Local recurrence of colon cancer | 3 (7.1) |
| Local recurrence of rectal cancer | 13 (31.0) |
| Procedures | No. of Patients (%) |
|---|---|
| Tumor resection | 7 (16.7) |
| Left colectomy | 2 (4.8) |
| Sigmoid colectomy | 8 (19.0) |
| LAR | 9 (21.4) |
| APR | 15 (35.7) |
| TPE | 1 (2.4) |
| Indications | No. of Patients (%) |
|---|---|
| History of pelvic surgery by laparotomy | 24 (47.6) |
| Tumor proximity to ureter | 11 (26.2) |
| History of radiotherapy | 8 (19.0) |
| Inflammation in pelvic cavity | 7 (16.7) |
| Morbid obesity | 1 (2.4) |
| Side of Catheterization | Unilateral | Bilateral |
|---|---|---|
| No. of patients (%) | 17 (40) (R: 3, L: 14) | 25 (60) |
| Time (min, range) | 8 (4–21) | 13 (5–27) |
| Indications | No. of Patients (%) |
|---|---|
| Hematuria | 6 (14.3) |
| Dysuria | 5 (12.0) |
| Urinary tract infection | 4 (9.5) |
| Hydronephrosis | 1 (2.4) |
| Ureteral injury | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohnuma, S.; Kanehara, K.; Sato, Y.; Ono, T.; Murakami, M.; Kajiwara, T.; Suzuki, H.; Karasawa, H.; Watanabe, K.; Kawamorita, N.; et al. Prophylactic Ureteral Catheterization for Preventing Ureteral Injury in Colorectal Cancer Surgery. J. Clin. Med. 2025, 14, 4123. https://doi.org/10.3390/jcm14124123
Ohnuma S, Kanehara K, Sato Y, Ono T, Murakami M, Kajiwara T, Suzuki H, Karasawa H, Watanabe K, Kawamorita N, et al. Prophylactic Ureteral Catheterization for Preventing Ureteral Injury in Colorectal Cancer Surgery. Journal of Clinical Medicine. 2025; 14(12):4123. https://doi.org/10.3390/jcm14124123
Chicago/Turabian StyleOhnuma, Shinobu, Keigo Kanehara, Yukihiro Sato, Tomoyuki Ono, Megumi Murakami, Taiki Kajiwara, Hideyuki Suzuki, Hideaki Karasawa, Kazuhiro Watanabe, Naoki Kawamorita, and et al. 2025. "Prophylactic Ureteral Catheterization for Preventing Ureteral Injury in Colorectal Cancer Surgery" Journal of Clinical Medicine 14, no. 12: 4123. https://doi.org/10.3390/jcm14124123
APA StyleOhnuma, S., Kanehara, K., Sato, Y., Ono, T., Murakami, M., Kajiwara, T., Suzuki, H., Karasawa, H., Watanabe, K., Kawamorita, N., Ito, A., Kamei, T., & Unno, M. (2025). Prophylactic Ureteral Catheterization for Preventing Ureteral Injury in Colorectal Cancer Surgery. Journal of Clinical Medicine, 14(12), 4123. https://doi.org/10.3390/jcm14124123







