Impact of Ectopic Pregnancy on the Outcomes of the Subsequent Pregnancy: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
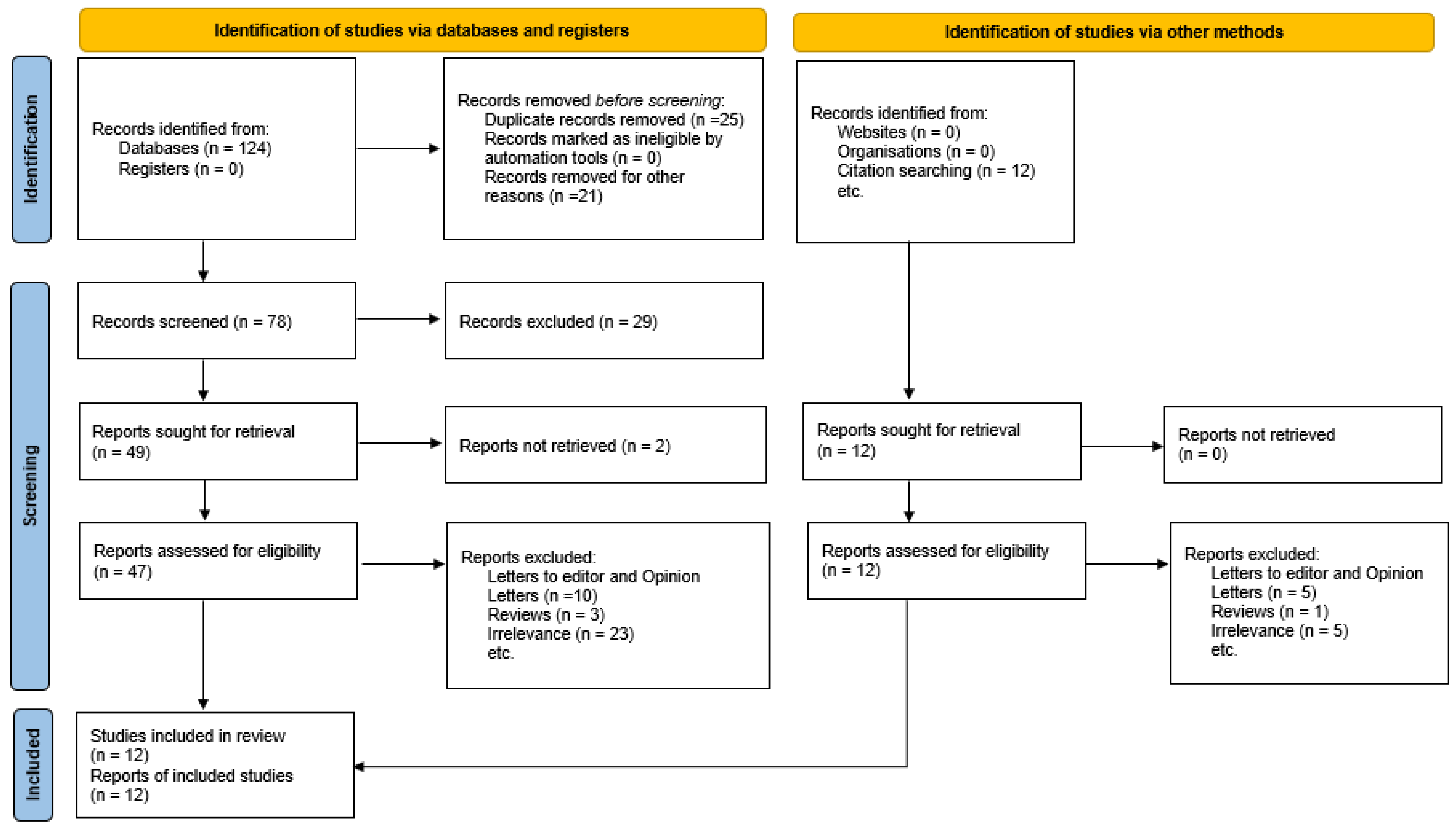
2. Materials and Methods
2.1. Eligibility Criteria, Information Sources, Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis
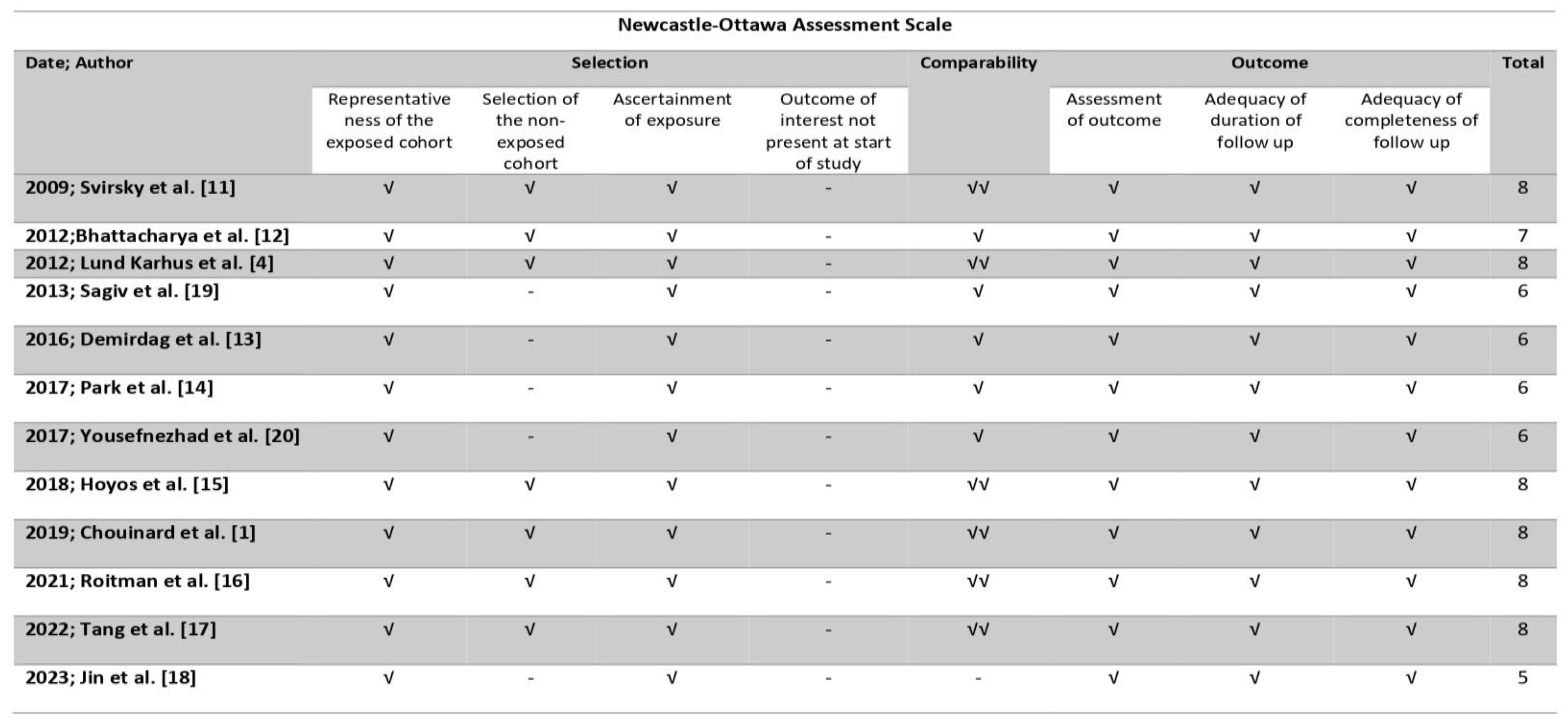
2.5. Assessment of Risk of Bias
| Year | Author | Type of Study | Inclusion Criteria | Exclusion Criteria | Sample Size | Outcome Investigated |
|---|---|---|---|---|---|---|
| 2009 | Svirsky et al. [11] | Register-based retrospective cohort (2000–2006) | Women receiving MTX treatment for prior EP. Available subsequent pregnancy outcome information. | Missing data. | 125 | Duration of the pregnancy. Rate of late induced abortion. Rate of early and late missed abortions. U/S detection of abnormalities. Neonatal weight and overall condition. |
| 2012 | Bhattacharya et al. [12] | Register-based retrospective cohort (1981–2000) | Women with an EP in their first pregnancy (exposed group). Women who had a prior live birth (group A). Women who had a prior miscarriage (group B). Women who had a prior abortion (group C). Available subsequent pregnancy outcome information. | Missing data. | Exposed group: 2969 Group A: 667,299 Group B: 39705 Group C: 78,697 | Rate of clinical pregnancy. Rate of live birth. Rate of EP. Rate of miscarriage. Rate of termination. Rate of stillbirth. |
| 2013 | Lund Karhus et al. [4] | Historical controlled cohort study (1977–2009) | Women with an EP in their first pregnancy (exposed group). Women who had a prior live birth (group A). Women who had a prior miscarriage (group B). Women who had a prior abortion (group C). Available subsequent pregnancy outcome information. | Missing data. | Exposed group: 2917 Group A: 2917 Group B: 2917 Group C: 2917. | Rate of clinical pregnancy. Rate of live birth. Rate of EP. Rate of miscarriage. Rate of termination. Rate of stillbirth. |
| 2013 | Sagiv et al. [19] | Case series (1997–2007) | Women with an interstitial EP treated by MTX, laparoscopy or laparotomy. Available subsequent pregnancy outcome information. | Women >40 years old. Women with bilateral salpingectomy or bilateral tubal ligation. Missing data. | 14. | Fertility outcome. |
| 2016 | Demirdag et al. [13] | Register-based retrospective cohort (2007–2011) | Women with a tubal EP treated by MTX, surgery or expectantly. Available subsequent pregnancy outcome information. | Missing data. | 112. | Rates of IUP. Rates of EP. |
| 2017 | Park et al. [14] | Retrospective Cohort (2010–2016) | Women with a tubal EP treated by MTX, surgery, or expectantly. Available subsequent pregnancy outcome information. | Missing data. | 147. | Rate of clinical pregnancy. Rate of IU viable pregnancy. Rate of IU non-viable pregnancy. Rate of EP. |
| 2017 | Yousefnezhad et al. [20] | Cross-sectional study (2009–2013) | Women with an EP treated by MTX or surgery. Available subsequent pregnancy outcome information. | Missing data. | 114. | Fertility rate. Rate of term and preterm pregnancies. Rate of abortions. Rate of EP. |
| 2018 | Hoyos et al. [15] | Retrospective cohort (2000–2013) | Women with a prior interstitial ectopic pregnancy that underwent cornual wedge resection. Available subsequent pregnancy outcome information. | Missing data. | 19. | Rate of live birth. Rate of EP. Rate of abortion. Rate of adverse pregnancy outcomes. |
| 2019 | Chouinard et al. [1] | Population-based longitudinal cohort study (1989–2013) | Women with an EP in their first Pregnancy treated by surgery (exposed group). Women who had a prior live birth (unexposed group). Available subsequent pregnancy outcome information. | Missing data. | Exposed group: 15,823. Unexposed group: 110,1748. | Rate of live birth. Rate of EP. Rate of adverse pregnancy outcomes. |
| 2021 | Roitman et al. [16] | Retrospective cohort (1991–2004) | Women with an EP in their first pregnancy treated by surgery (group A). Pregnancy treated medically (group B). Women who had a prior live birth (unexposed group). Available subsequent pregnancy outcome information. | Multifetal pregnancies. Fetuses with congenital anomalies. Fetuses with chromosomal abnormalities. Missing data. | Group A: 1059. Group B: 365. Unexposed group: 242,258. | Rate of recurrent pregnancy loss. Rate of adverse pregnancy outcomes. |
| 2022 | Tang et al. [17] | Retrospective case–control (2014–2019) | Women with a prior interstitial ectopic pregnancy that underwent cornual wedge resection (exposed group). Women who had a prior live birth (unexposed group). All participants received frozen embryo transfer cycles of IVF/ICSI. Available subsequent pregnancy outcome information. | Women with uterine abnormities. Women with prior uterine surgery. Women with a personal history of diabetes, hypertension, or thyroid disorders. Missing data. | Exposed group: 75. Unexposed group: 375. | Rate of clinical pregnancy. Rate of live birth. Rate of EP. Rate of miscarriage. Rate of adverse pregnancy outcomes. |
| 2023 | Jin et al. [18] | Retrospective cohort (2009–2018) | Women with a prior treated CSP. Available subsequent pregnancy outcome information. | Missing data. | 51 | Subsequent fertility. Adverse pregnancy outcomes. |
3. Results
3.1. Study Selection and Characteristics
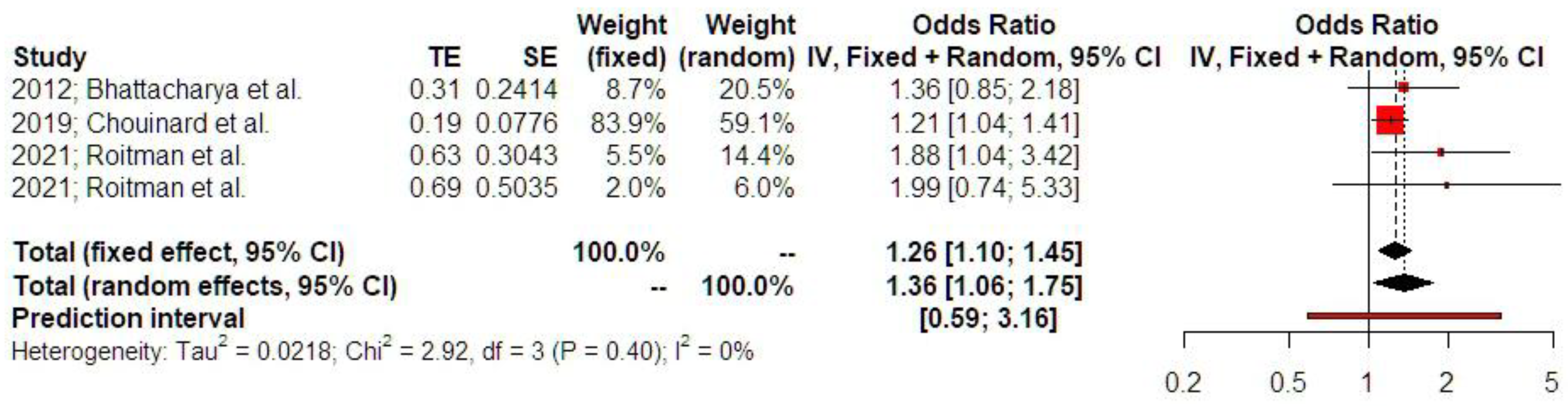
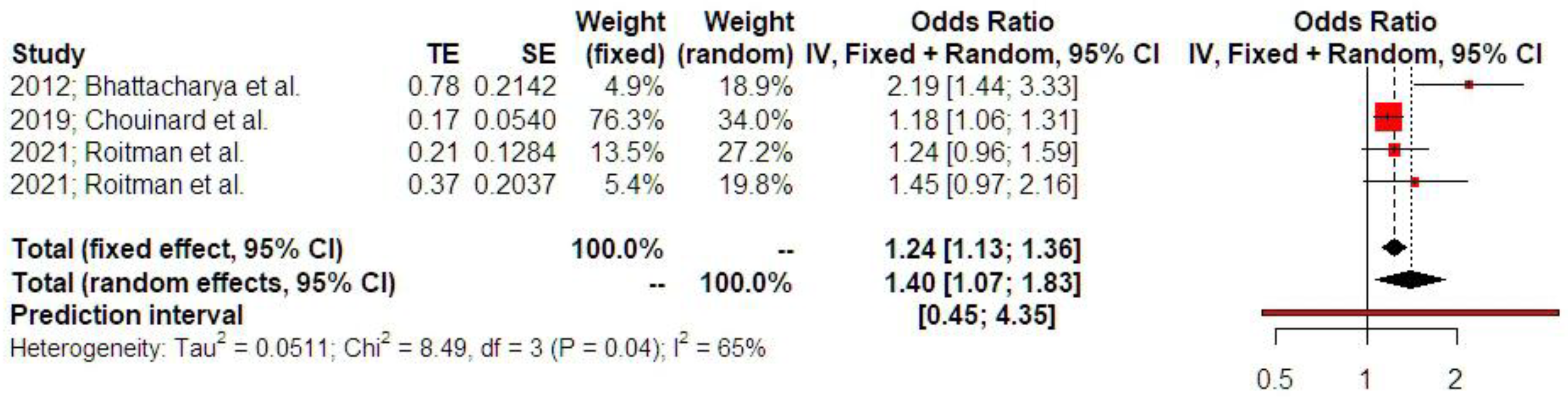
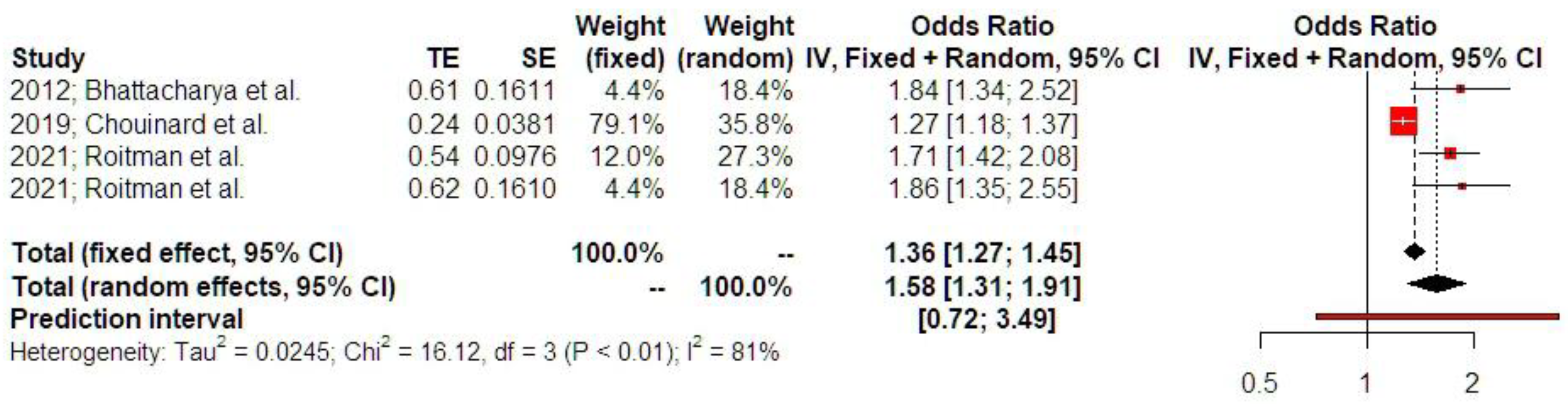
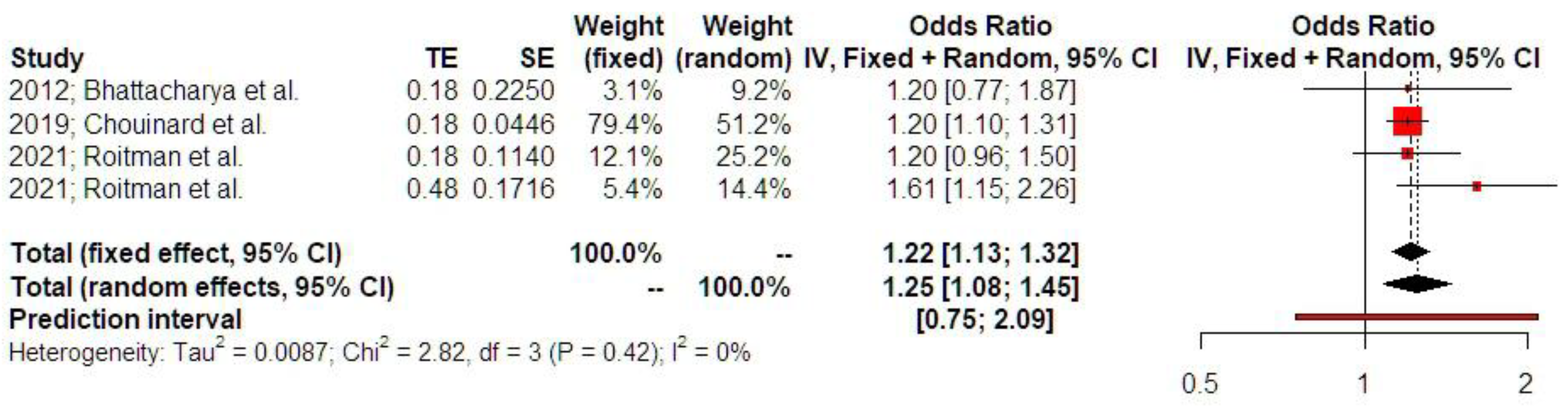
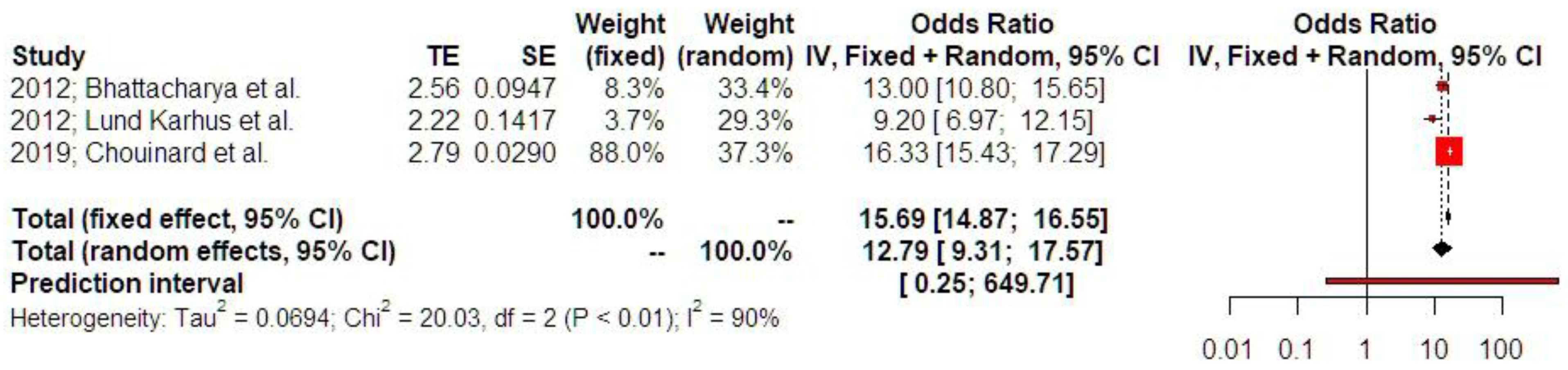
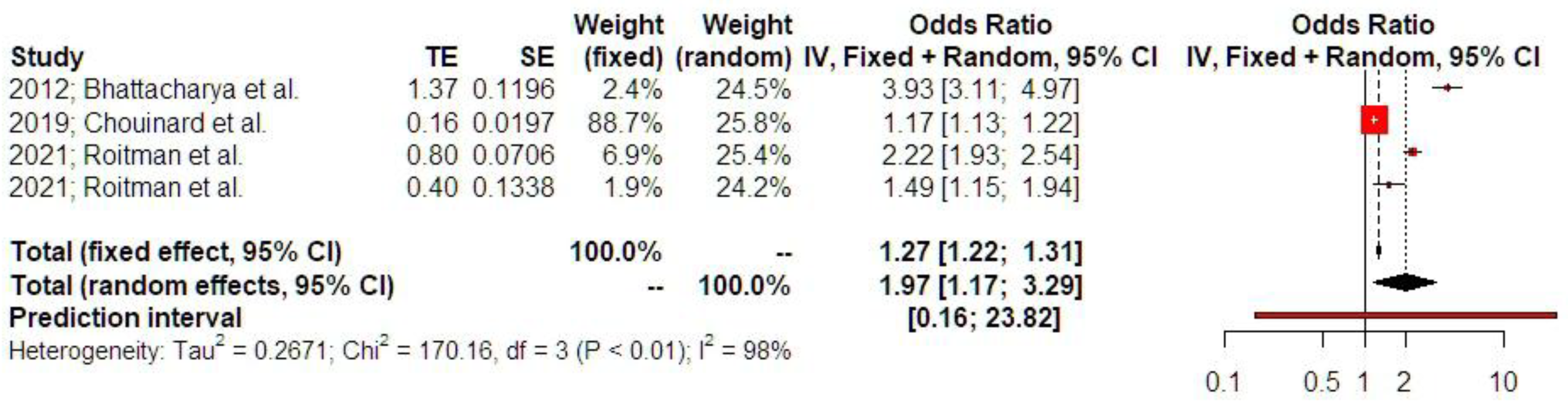
3.2. Synthesis of Results
4. Discussion
4.1. Principal Findings
4.2. Comparison to Existing Literature
4.2.1. Ectopic Pregnancy and Emergency Cesarean Section
4.2.2. Ectopic Pregnancy and Recurrence of Ectopic Pregnancy
4.2.3. Ectopic Pregnancy and Low Birth Weight
4.2.4. Ectopic Pregnancy and Preterm Labor
4.2.5. Ectopic Pregnancy and Pregnancy-Induced Hypertensive Disorders
4.2.6. Ectopic Pregnancy and Placental Abruption
4.3. Clinical Application
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| EP | Ectopic pregnancy |
| OR | Odds ratio |
| CI | Confidence interval |
| PI | Prediction interval |
| CS | Cesarean section |
| REM | Random effects model |
| NOS | Newcastle–Ottawa scale |
| TAS | Trial sequential analysis |
| HELLP | Hemolysis, elevated liver enzymes, and low platelets |
References
- Chouinard, M.; Mayrand, M.H.; Ayoub, A.; Healy-Profitós, J.; Auger, N. Ectopic pregnancy and outcomes of future intrauterine pregnancy. Fertil. Steril. 2019, 112, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Zane, S.B.; Kieke, B.A., Jr.; Kendrick, J.S.; Bruce, C. Surveillance in a time of changing health care practices: Estimating ectopic pregnancy incidence in the United States. Matern. Child Health J. 2002, 6, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Mann, L.M.; Kreisel, K.; Llata, E.; Hong, J.; Torrone, E.A. Trends in ectopic pregnancy diagnoses in United States emergency departments, 2006–2013. Matern. Child Health J. 2020, 24, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Lund Kårhus, L.; Egerup, P.; Wessel Skovlund, C.; Lidegaard, Ø. Long-term reproductive outcomes in women whose first pregnancy is ectopic: A national controlled follow-up study. Hum. Reprod. 2013, 28, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Egerup, P.; Kårhus, L.L.; Skovlund, C.W.; Lidegaard, Ø. Improving reproductive long-term prognosis for women with a first ectopic pregnancy. A national controlled follow-up study. Acta Obstet. Gynecol. Scand. 2014, 93, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, J.; Coste, J.; Fernandez, H.; Pouly, J.L.; Job-Spira, N. Sites of ectopic pregnancy: A 10-year population-based study of 1800 cases. Hum. Reprod. 2002, 17, 3224–3230. [Google Scholar] [CrossRef]
- Page, A.; Thomas, J.; Tulandi, T.; Zakhari, A.; Mills, K.; Po, L.; Shuman, M. Guideline No. 414: Management of pregnancy of unknown location and tubal and nontubal ectopic pregnancies. J. Obstet. Gynaecol. Can. 2021, 43, 614–630.e1. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 193: Tubal ectopic pregnancy. Obstet. Gynecol. 2018, 131, e91–e103. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 26 May 2025).
- Svirsky, R.; Rozovski, U.; Vaknin, Z.; Pansky, M.; Schneider, D.; Halperin, R. The safety of conception occurring shortly after methotrexate treatment of an ectopic pregnancy. Reprod. Toxicol. 2009, 27, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; McLernon, D.J.; Lee, A.J.; Bhattacharya, S. Reproductive outcomes following ectopic pregnancy: Register-based retrospective cohort study. PLoS Med. 2012, 9, e1001243. [Google Scholar] [CrossRef] [PubMed]
- Demirdag, E.; Guler, I.; Abay, S.; Oguz, Y.; Erdem, M.; Erdem, A. The impact of expectant management, systemic methotrexate and surgery on subsequent pregnancy outcomes in tubal ectopic pregnancy. Ir. J. Med. Sci. 2017, 186, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Park, E.H.G.; Mohammadi-Zaniani, G.; Pronin, S.; Elderfield, C.H.J.; Duncan, W.C. Subsequent pregnancy outcome of tubal ectopic pregnancies treated by methotrexate and salpingectomy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Allsworth, J.E.; Awonuga, A.O.; Vilchez, G.; Adekola, H.; Rodriguez-Kovacs, J.; Malik, M.; Hoyos, L.R. Outcomes in subsequent pregnancies after wedge resection for interstitial ectopic pregnancy: A retrospective cohort study. J. Matern. Fetal Neonatal Med. 2019, 32, 2354–2360. [Google Scholar] [CrossRef]
- Roitman, M.S.; Wainstock, T.; Sheiner, E.; Leibson, T.; Pariente, G. Ectopic pregnancy: Perinatal outcomes of future gestations and long-term neurological morbidity of the offspring. Arch. Gynecol. Obstet. 2021, 304, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Du, T.; Huang, J.; Lin, J.; Tang, S.; Zhao, M.; Kuang, Y. Effect of previous wedge resection for interstitial pregnancy on pregnancy and neonatal outcomes following frozen-thawed embryo transfer (FET) cycles of IVF/ICSI: A retrospective study. Reprod. Biol. Endocrinol. 2022, 20, 23. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.; Zhang, P.; Zheng, L.; Qi, F. Subsequent fertility after cesarean scar pregnancy: A retrospective analysis. BMC Pregnancy Childbirth 2023, 23, 337. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, R.; Debby, A.; Keidar, R.; Kerner, R.; Golan, A. Interstitial pregnancy management and subsequent pregnancy outcome. Acta Obstet. Gynecol. Scand. 2013, 92, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Yousefnezhad, A.; Pirdehghan, A.; Roshandel Rad, M.; Eskandari, A.; Ahmadi, S. Comparison of the pregnancy outcomes between the medical and surgical treatments in tubal ectopic pregnancy. Int. J. Reprod. Biomed. 2018, 16, 31–34. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papageorgiou, D.; Sapantzoglou, I.; Zachariou, E.; Antsaklis, P.; Daskalakis, G.; Pergialiotis, V. Impact of Ectopic Pregnancy on the Outcomes of the Subsequent Pregnancy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4112. https://doi.org/10.3390/jcm14124112
Papageorgiou D, Sapantzoglou I, Zachariou E, Antsaklis P, Daskalakis G, Pergialiotis V. Impact of Ectopic Pregnancy on the Outcomes of the Subsequent Pregnancy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(12):4112. https://doi.org/10.3390/jcm14124112
Chicago/Turabian StylePapageorgiou, Dimitrios, Ioakeim Sapantzoglou, Eleftherios Zachariou, Panagiotis Antsaklis, Georgios Daskalakis, and Vasilios Pergialiotis. 2025. "Impact of Ectopic Pregnancy on the Outcomes of the Subsequent Pregnancy: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 12: 4112. https://doi.org/10.3390/jcm14124112
APA StylePapageorgiou, D., Sapantzoglou, I., Zachariou, E., Antsaklis, P., Daskalakis, G., & Pergialiotis, V. (2025). Impact of Ectopic Pregnancy on the Outcomes of the Subsequent Pregnancy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(12), 4112. https://doi.org/10.3390/jcm14124112









