Embolic Protection Devices in Transcatheter Aortic Valve Implantation: A Narrative Review of Current Evidence
Abstract
1. Introduction
2. Understanding the Causes and Timing of Stroke in TAVI Procedures: The Role of TAVI-Induced Debris
3. Cerebral Embolic Protection Device Types
4. The Current Evidence for Using Specific Embolic Protection Devices During TAVI
4.1. Major Trials
4.2. Meta-Analyses
5. Current Controversies
5.1. Complete vs. Partial Cerebral Embolic Protection
5.2. Deflection vs. Capture of Debris During Transcatheter Cardiac Interventions
5.3. EPD Use During Non-Femoral TAVI Procedures
5.4. EPD Use During Surgical Aortic Valve Replacement
6. Emerging Cerebral Embolic Protection Technologies in TAVI
6.1. Emblok Embolic Protection System
6.2. ProtEmbo Cerebral Protection System
6.3. Emboliner Total Embolic Protection Device
6.4. CAPTIS Embolic Protection System
6.5. FLOWer Embolic Protection Device
6.6. POINT-GUARD Dynamic Cerebral Protection System
7. Future Directions and Contemporary Clinical Practice
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Teirstein, P.S.; Tchetche, D.; Huang, J.; et al. 4-Year Outcomes of Patients With Aortic Stenosis in the Evolut Low Risk Trial. J. Am. Coll. Cardiol. 2023, 82, 2163–2165. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Sondergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Avvedimento, M.; Angellotti, D.; Ilardi, F.; Leone, A.; Scalamogna, M.; Castiello, D.S.; Manzo, R.; Mariani, A.; Immobile Molaro, M.; Simonetti, F.; et al. Acute advanced aortic stenosis. Heart Fail. Rev. 2023, 28, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Ktenopoulos, N.; Karanasos, A.; Katsaros, O.; Apostolos, A.; Latsios, G.; Moulias, A.; Papafaklis, M.I.; Tsigkas, G.; Tsioufis, C.; Toutouzas, K.; et al. Coronary Artery Disease and Severe Aortic Stenosis: Contemporary Treatment Options for Patients Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2024, 13, 7625. [Google Scholar] [CrossRef]
- Oikonomou, G.; Apostolos, A.; Drakopoulou, M.; Simopoulou, C.; Karmpalioti, M.; Toskas, P.; Stathogiannis, K.; Xanthopoulou, M.; Ktenopoulos, N.; Latsios, G.; et al. Long-Term Outcomes of Aortic Stenosis Patients with Different Flow/Gradient Patterns Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2024, 13, 1200. [Google Scholar] [CrossRef]
- Apostolos, A.; Ktenopoulos, N.; Chlorogiannis, D.D.; Katsaros, O.; Konstantinou, K.; Drakopoulou, M.; Tsalamandris, S.; Karanasos, A.; Synetos, A.; Latsios, G.; et al. Mortality Rates in Patients Undergoing Urgent Versus Elective Transcatheter Aortic Valve Replacement: A Meta-analysis. Angiology 2024, 33197241245733. [Google Scholar] [CrossRef]
- Teitelbaum, M.; Kotronias, R.A.; Sposato, L.A.; Bagur, R. Cerebral Embolic Protection in TAVI: Friend or Foe. Interv. Cardiol. 2019, 14, 22–25. [Google Scholar] [CrossRef]
- Kroon, H.; von der Thusen, J.H.; Ziviello, F.; van Wiechen, M.; Ooms, J.F.W.; Kardys, I.; Schipper, M.; van Gils, L.; Daemen, J.; de Jaegere, P.; et al. Heterogeneity of debris captured by cerebral embolic protection filters during TAVI. EuroIntervention 2021, 16, 1141–1147. [Google Scholar] [CrossRef]
- Vlastra, W.; Jimenez-Quevedo, P.; Tchetche, D.; Chandrasekhar, J.; de Brito, F.S., Jr.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A.; Ribichini, F.; et al. Predictors, Incidence, and Outcomes of Patients Undergoing Transfemoral Transcatheter Aortic Valve Implantation Complicated by Stroke. Circ. Cardiovasc. Interv. 2019, 12, e007546. [Google Scholar] [CrossRef]
- Katsaros, O.; Apostolos, A.; Ktenopoulos, N.; Koliastasis, L.; Kachrimanidis, I.; Drakopoulou, M.; Korovesis, T.; Karanasos, A.; Tsalamandris, S.; Latsios, G.; et al. Transcatheter Aortic Valve Implantation Access Sites: Same Goals, Distinct Aspects, Various Merits and Demerits. J. Cardiovasc. Dev. Dis. 2023, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Harmouch, W.; Karnkowska, B.; Thakker, R.; Rasmussen, P.; Shalaby, M.; Khalife, W.; Alwash, H.; Motiwala, A.; Kumfa, P.; Gilani, S.; et al. Cerebral Embolic Protection in Transcatheter Aortic Valve Implantation Using the Sentinel Cerebral Protection System: A Systematic Review and Meta-Analysis. Cardiol. Ther. 2024, 13, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Gasior, T. 2024 Update on Cerebral Embolic Protection After Transcatheter Aortic Valve Replacement. J. Clin. Med. 2024, 13, 7256. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Yakubov, S.J.; Deeb, G.M.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Crouch, J.; Merhi, W.; Wai Sang, S.L.; et al. 5-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients With Aortic Stenosis. J. Am. Coll. Cardiol. 2025, 85, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Pagnesi, M.; Martino, E.A.; Chiarito, M.; Mangieri, A.; Jabbour, R.J.; Van Mieghem, N.M.; Kodali, S.K.; Godino, C.; Landoni, G.; Colombo, A.; et al. Silent cerebral injury after transcatheter aortic valve implantation and the preventive role of embolic protection devices: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 221, 97–106. [Google Scholar] [CrossRef]
- Haussig, S.; Mangner, N.; Dwyer, M.G.; Lehmkuhl, L.; Lucke, C.; Woitek, F.; Holzhey, D.M.; Mohr, F.W.; Gutberlet, M.; Zivadinov, R.; et al. Effect of a Cerebral Protection Device on Brain Lesions Following Transcatheter Aortic Valve Implantation in Patients With Severe Aortic Stenosis: The CLEAN-TAVI Randomized Clinical Trial. JAMA 2016, 316, 592–601. [Google Scholar] [CrossRef]
- Ktenopoulos, N.; Koniari, I.; Mplani, V.; Artopoulou, E.; Tsigkas, G.; Gerakaris, A.; Kounis, N.; Velissaris, D. Effect of atrial fibrillation on cognitive function in heart failure patients. J. Geriatr. Cardiol. 2021, 18, 585–590. [Google Scholar] [CrossRef]
- Agrawal, A.; Isogai, T.; Shekhar, S.; Kapadia, S. Cerebral Embolic Protection Devices: Current State of the Art. US Cardiol. 2023, 17, e02. [Google Scholar] [CrossRef]
- Seeger, J.; Seppelt, P.; Iturbe-Orbe, M.; Leistner, D.; Wohrle, J.; Joner, M. Cerebral Embolic Protection in Patients Undergoing Left Atrial Appendage Closure. J. Cardiovasc. Dev. Dis. 2024, 12, 5. [Google Scholar] [CrossRef]
- Bjursten, H.; Norrving, B.; Ragnarsson, S. Late stroke after transcatheter aortic valve replacement: A nationwide study. Sci. Rep. 2021, 11, 9593. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Head, S.J.; Genereux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur. Heart J. 2012, 33, 2403–2418. [Google Scholar] [CrossRef]
- Camare, C.; Pucelle, M.; Negre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef]
- Nombela-Franco, L.; Armijo, G.; Tirado-Conte, G. Cerebral embolic protection devices during transcatheter aortic valve implantation: Clinical versus silent embolism. J. Thorac. Dis. 2018, 10, S3604–S3613. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, P.; Al-Rashid, F.; Dottger, P.; Mori, K.; Plicht, B.; Wendt, D.; Bergmann, L.; Kottenberg, E.; Schlamann, M.; Mummel, P.; et al. Cerebral embolization during transcatheter aortic valve implantation: A transcranial Doppler study. Circulation 2012, 126, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Katsaros, O.; Ktenopoulos, N.; Korovesis, T.; Benetos, G.; Apostolos, A.; Koliastasis, L.; Sagris, M.; Milaras, N.; Latsios, G.; Synetos, A.; et al. Bicuspid Aortic Valve Disease: From Pathophysiology to Treatment. J. Clin. Med. 2024, 13, 4970. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; Schipper, M.E.; Ladich, E.; Faqiri, E.; van der Boon, R.; Randjgari, A.; Schultz, C.; Moelker, A.; van Geuns, R.J.; Otsuka, F.; et al. Histopathology of embolic debris captured during transcatheter aortic valve replacement. Circulation 2013, 127, 2194–2201. [Google Scholar] [CrossRef]
- Jimenez Diaz, V.A.; Kapadia, S.R.; Linke, A.; Mylotte, D.; Lansky, A.J.; Grube, E.; Settergren, M.; Puri, R. Cerebral embolic protection during transcatheter heart interventions. EuroIntervention 2023, 19, 549–570. [Google Scholar] [CrossRef]
- Bagur, R.; Sposato, L.A. Cerebral embolic protection during TAVI: The game is not over. EuroIntervention 2021, 16, 1123–1125. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Makkar, R.; Leon, M.; Abdel-Wahab, M.; Waggoner, T.; Massberg, S.; Rottbauer, W.; Horr, S.; Sondergaard, L.; Karha, J.; et al. Cerebral Embolic Protection during Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2022, 387, 1253–1263. [Google Scholar] [CrossRef]
- Varc-3 Writing, C.; Genereux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Leon, M.B.; Mehran, R.; Kuck, K.H.; Alu, M.C.; Braumann, R.E.; Kodali, S.; Kapadia, S.R.; Linke, A.; Makkar, R.; et al. Debris Heterogeneity Across Different Valve Types Captured by a Cerebral Protection System During Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Indja, B.; Woldendorp, K.; Vallely, M.P.; Grieve, S.M. Silent Brain Infarcts Following Cardiac Procedures: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e010920. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, H.; Schmermund, A.; Voigtlander, T.; Kahlert, P.; Erbel, R.; Mehta, R.H. Risk of stroke after transcatheter aortic valve implantation (TAVI): A meta-analysis of 10,037 published patients. EuroIntervention 2012, 8, 129–138. [Google Scholar] [CrossRef]
- Erdoes, G.; Basciani, R.; Huber, C.; Stortecky, S.; Wenaweser, P.; Windecker, S.; Carrel, T.; Eberle, B. Transcranial Doppler-detected cerebral embolic load during transcatheter aortic valve implantation. Eur. J. Cardiothorac. Surg. 2012, 41, 778–783; discussion 783–784. [Google Scholar] [CrossRef]
- Genereux, P.; Giustino, G. Stroke after transcatheter aortic valve replacement. EuroIntervention 2022, 18, e271–e272. [Google Scholar] [CrossRef]
- Urena, M.; Webb, J.G.; Cheema, A.; Serra, V.; Toggweiler, S.; Barbanti, M.; Cheung, A.; Ye, J.; Dumont, E.; DeLarochelliere, R.; et al. Impact of new-onset persistent left bundle branch block on late clinical outcomes in patients undergoing transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc. Interv. 2014, 7, 128–136. [Google Scholar] [CrossRef]
- Leone, A.; Castiello, D.S.; Angellotti, D.; Mariani, A.; Manzo, R.; Avvedimento, M.; Ilardi, F.; Piccolo, R.; Esposito, G.; Franzone, A. Incidence, predictors, and prognostic impact of temporary left bundle branch block after transcatheter aortic valve replacement. J. Electrocardiol. 2022, 74, 114–115. [Google Scholar] [CrossRef]
- Sherwood, M.W.; Xiang, K.; Matsouaka, R.; Li, Z.; Vemulapalli, S.; Vora, A.N.; Fanaroff, A.; Harrison, J.K.; Thourani, V.H.; Holmes, D.; et al. Incidence, Temporal Trends, and Associated Outcomes of Vascular and Bleeding Complications in Patients Undergoing Transfemoral Transcatheter Aortic Valve Replacement: Insights From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry. Circ. Cardiovasc. Interv. 2020, 13, e008227. [Google Scholar] [CrossRef]
- Perera, A.H.; Rudarakanchana, N.; Monzon, L.; Bicknell, C.D.; Modarai, B.; Kirmi, O.; Athanasiou, T.; Hamady, M.; Gibbs, R.G. Cerebral embolization, silent cerebral infarction and neurocognitive decline after thoracic endovascular aortic repair. Br. J. Surg. 2018, 105, 366–378. [Google Scholar] [CrossRef]
- Muralidharan, A.; Thiagarajan, K.; Van Ham, R.; Gleason, T.G.; Mulukutla, S.; Schindler, J.T.; Jeevanantham, V.; Thirumala, P.D. Meta-Analysis of Perioperative Stroke and Mortality in Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2016, 118, 1031–1045. [Google Scholar] [CrossRef]
- Vermeer, S.E.; Longstreth, W.T., Jr.; Koudstaal, P.J. Silent brain infarcts: A systematic review. Lancet Neurol. 2007, 6, 611–619. [Google Scholar] [CrossRef]
- Sayah, N.; Skalidis, I.; Mesnier, J.; Neylon, A.; Akodad, M.; Asgar, A. Cerebral Embolic Protection Devices: Are There Any Indications in Transcatheter Aortic Valve Replacement? J. Clin. Med. 2024, 13, 5471. [Google Scholar] [CrossRef]
- Gasior, T.; Mangner, N.; Bijoch, J.; Wojakowski, W. Cerebral embolic protection systems for transcatheter aortic valve replacement. J. Interv. Cardiol. 2018, 31, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Lansky, A.J.; Makkar, R.; Nazif, T.; Messe, S.; Forrest, J.; Sharma, R.; Schofer, J.; Linke, A.; Brown, D.; Dhoble, A.; et al. A randomized evaluation of the TriGuard HDH cerebral embolic protection device to Reduce the Impact of Cerebral Embolic LEsions after TransCatheter Aortic Valve ImplanTation: The REFLECT I trial. Eur. Heart J. 2021, 42, 2670–2679. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, P.G.; Kooistra, N.H.M.; Leenders, G.E.H.; Margolis, P.M.; Lansky, A.J.; Kraaijeveld, A.O.; Voskuil, M.; Stella, P.R. A pilot study with the TriGUARD 3 cerebral embolic protection device. EuroIntervention 2020, 16, e507–e509. [Google Scholar] [CrossRef] [PubMed]
- Jagielak, D.; Targonnski, R.; Ciecwierz, D. First-in-Human Use of the Next-generation ProtEmbo Cerebral Embolic Protection System During Transcatheter Aortic Valve-in-valve Implantation. Interv. Cardiol. 2021, 16, 1–4. [Google Scholar] [CrossRef]
- Fezzi, S.; Jagielak, D.; Targonski, R.; Schmidt, T.; Frerker, C.; Witkowski, A.R.; Lauterbach, M.; Leick, J.; Erglis, A.; Narbute, I.; et al. Final report of the PROTEMBO C Trial: A prospective evaluation of a novel cerebral protection device during TAVI. EuroIntervention 2024, 20, e264–e267. [Google Scholar] [CrossRef]
- Leite Filho, O.A.; Brandao, C.M.; Pomerantzeff, P.M.; Guedes, M.A.; Higuchi Mde, L.; Stolf, N.A. Particulate emboli capture by an intra-aortic filter device during aortic valve replacement. Rev. Bras. Cir. Cardiovasc. 2008, 23, 431–435. [Google Scholar] [CrossRef]
- Samim, M.; Agostoni, P.; Hendrikse, J.; Budde, R.P.; Nijhoff, F.; Kluin, J.; Ramjankhan, F.; Doevendans, P.A.; Stella, P.R. Embrella embolic deflection device for cerebral protection during transcatheter aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2015, 149, 799–805.e1–e2. [Google Scholar] [CrossRef][Green Version]
- Grubman, D.; Ahmad, Y.; Leipsic, J.A.; Blanke, P.; Pasupati, S.; Webster, M.; Nazif, T.M.; Parise, H.; Lansky, A.J. Predictors of Cerebral Embolic Debris During Transcatheter Aortic Valve Replacement: The SafePass 2 First-in-Human Study. Am. J. Cardiol. 2023, 207, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; El Faquir, N.; Rahhab, Z.; Rodriguez-Olivares, R.; Wilschut, J.; Ouhlous, M.; Galema, T.W.; Geleijnse, M.L.; Kappetein, A.P.; Schipper, M.E.; et al. Incidence and predictors of debris embolizing to the brain during transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2015, 8, 718–724. [Google Scholar] [CrossRef]
- Nazif, T.M.; Moses, J.; Sharma, R.; Dhoble, A.; Rovin, J.; Brown, D.; Horwitz, P.; Makkar, R.; Stoler, R.; Forrest, J.; et al. Randomized Evaluation of TriGuard 3 Cerebral Embolic Protection After Transcatheter Aortic Valve Replacement: REFLECT II. JACC Cardiovasc. Interv. 2021, 14, 515–527. [Google Scholar] [CrossRef]
- Jagielak, D.; Targonski, R.; Frerker, C.; Abdel-Wahab, M.; Wilde, J.; Werner, N.; Lauterbach, M.; Leick, J.; Grygier, M.; Misterski, M.; et al. Safety and performance of a novel cerebral embolic protection device for transcatheter aortic valve implantation: The PROTEMBO C Trial. EuroIntervention 2022, 18, 590–597. [Google Scholar] [CrossRef]
- Reddy, P.; Merdler, I.; Ben-Dor, I.; Satler, L.F.; Rogers, T.; Waksman, R. Cerebrovascular events after transcatheter aortic valve implantation. EuroIntervention 2024, 20, e793–e805. [Google Scholar] [CrossRef]
- Bagur, R.; Solo, K.; Alghofaili, S.; Nombela-Franco, L.; Kwok, C.S.; Hayman, S.; Siemieniuk, R.A.; Foroutan, F.; Spencer, F.A.; Vandvik, P.O.; et al. Cerebral Embolic Protection Devices During Transcatheter Aortic Valve Implantation: Systematic Review and Meta-Analysis. Stroke 2017, 48, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Fei, G.; Rizwan Amanullah, M.; Lim, S.T.; Abdul Aziz, Z.; Govindasamy, S.; Chao, V.T.T.; Ewe, S.H.; Ho, K.W.; Yap, J. Safety and efficacy of cerebral embolic protection in transcatheter aortic valve implantation: An updated meta-analysis. AsiaIntervention 2024, 10, 51–59. [Google Scholar] [CrossRef]
- Heuts, S.; Gabrio, A.; Veenstra, L.; Maesen, B.; Kats, S.; Maessen, J.G.; Walton, A.S.; Nanayakkara, S.; Lansky, A.J.; van ’t Hof, A.W.J.; et al. Stroke reduction by cerebral embolic protection devices in transcatheter aortic valve implantation: A systematic review and Bayesian meta-analysis. Heart 2024, 110, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.K.; Ahmad, Y.; Arnold, A.D.; Howard, J.P. Cerebral Embolic Protection Devices During Transcatheter Aortic Valve Replacement: A Meta-analysis of Randomized Controlled Trials. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101031. [Google Scholar] [CrossRef]
- Elfaituri, M.K.; Khaled, T.; BenGhatnsh, A.; Faraj, H.A.A.; Msherghi, A. Abstract 15586: Efficacy and Safety of Cerebral Embolic Protection Devices in Transcatheter Aortic-Valve Replacement: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Circulation 2023, 148 (Suppl. 1). [Google Scholar] [CrossRef]
- Ndunda, P.M.; Vindhyal, M.R.; Muutu, T.M.; Fanari, Z. Clinical Outcomes of Sentinel Cerebral Protection System Use During Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Cardiovasc. Revasc. Med. 2020, 21, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Testa, L.; Latib, A.; Casenghi, M.; Gorla, R.; Colombo, A.; Bedogni, F. Cerebral Protection During Transcatheter Aortic Valve Implantation: An Updated Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008463. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, J.; Lu, L.; Qiu, J.; Fu, Y.; Zheng, J. The efficacy of different types of cerebral embolic protection device during transcatheter aortic valve implantation: A meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1205943. [Google Scholar] [CrossRef] [PubMed]
- Ktenopoulos, N.; Katsaros, O.; Apostolos, A.; Drakopoulou, M.; Tsigkas, G.; Tsioufis, C.; Davlouros, P.; Toutouzas, K.; Karanasos, A. Emerging Transcatheter Therapies for Valvular Heart Disease: Focus on Mitral and Tricuspid Valve Procedures. Life 2024, 14, 842. [Google Scholar] [CrossRef]
- Synetos, A.; Ktenopoulos, N.; Katsaros, O.; Vlasopoulou, K.; Korovesis, T.; Drakopoulou, M.; Apostolos, A.; Koliastasis, L.; Toutouzas, K.; Tsioufis, C. New Therapeutic Advances in the Management of Tricuspid Valve Regurgitation. J. Clin. Med. 2024, 13, 4599. [Google Scholar] [CrossRef]
- Palmerini, T.; Saia, F.; Kim, W.K.; Renker, M.; Iadanza, A.; Fineschi, M.; Bruno, A.G.; Ghetti, G.; Vanhaverbeke, M.; Sondergaard, L.; et al. Vascular Access in Patients With Peripheral Arterial Disease Undergoing TAVR: The Hostile Registry. JACC Cardiovasc. Interv. 2023, 16, 396–411. [Google Scholar] [CrossRef]
- Alwaqfi, N.; AlBarakat, M.M.; Qariouti, H.; Ibrahim, K.; Alzoubi, N. Stroke after heart valve surgery: A single center institution report. J. Cardiothorac. Surg. 2024, 19, 518. [Google Scholar] [CrossRef]
- Alabbadi, S.; Bowdish, M.E.; Sallam, A.; Tam, D.Y.; Hasan, I.; Kumaresan, A.; Alzahrani, A.H.; Iribarne, A.; Egorova, N.; Chikwe, J. Transcatheter versus surgical aortic valve replacement in patients younger than 65 years in the United States. J. Thorac. Cardiovasc. Surg. 2025, S0022-5223(25)00002-9. [Google Scholar] [CrossRef]
- Mack, M.J.; Acker, M.A.; Gelijns, A.C.; Overbey, J.R.; Parides, M.K.; Browndyke, J.N.; Groh, M.A.; Moskowitz, A.J.; Jeffries, N.O.; Ailawadi, G.; et al. Effect of Cerebral Embolic Protection Devices on CNS Infarction in Surgical Aortic Valve Replacement: A Randomized Clinical Trial. JAMA 2017, 318, 536–547. [Google Scholar] [CrossRef]
- Latib, A.; Mangieri, A.; Vezzulli, P.; Spagnolo, P.; Sardanelli, F.; Fellegara, G.; Pagnesi, M.; Giannini, F.; Falini, A.; Gorla, R.; et al. First-in-Man Study Evaluating the Emblok Embolic Protection System During Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2020, 13, 860–868. [Google Scholar] [CrossRef]
- Mylotte, D.; Narbute, I.; Neylon, A.; Schäfer, U.; Werner, N.; Virmani, R.; Kolodgie, F.; Zivadinov, R.; Erglis, A. TCT-43: First-In-Human Experience of a Novel Transradial Device for Embolic Deflection During Transcatheter Aortic Valve Replacement. JACC 2018, 72 (Suppl. 13), B19. [Google Scholar] [CrossRef]
- Danenberg, H.; Vaknin-Assa, H.; Makkar, R.; Virmani, R.; Manevich, L.; Codner, P.; Patel, V.; Finn, A.V.; Landes, U.; Rubinshtein, R.; et al. First-in-human study of the CAPTIS embolic protection system during transcatheter aortic valve replacement. EuroIntervention 2023, 19, e948–e952. [Google Scholar] [CrossRef] [PubMed]
- Full Arch Cerebral Embolic Protection of the Brain (Point-GuardTM) During Cardiovascular Procedures. 2019. Available online: https://www.tctmd.com/slide/full-arch-cerebral-embolic-protection-brain-point-guardtm-during-cardiovascular-procedures (accessed on 20 March 2025).
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev. Esp. Cardiol. (Engl. Ed.) 2022, 75, 524. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Lowenstern, A.; Hung, A.; Manandhar, P.; Wegermann, Z.K.; Kapadia, S.R.; Lindman, B.R.; Goel, K.; Levack, M.; Barker, C.M.; Reed, S.D.; et al. Association of Transcatheter Aortic Valve Replacement Reimbursement, New Technology Add-on Payment, and Procedure Volumes With Embolic Protection Device Use. JAMA Cardiol. 2022, 7, 945–952. [Google Scholar] [CrossRef]
- Megaly, M.; Sorajja, P.; Cavalcante, J.L.; Pershad, A.; Gossl, M.; Abraham, B.; Omer, M.; Elbadawi, A.; Garcia, S. Ischemic Stroke With Cerebral Protection System During Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2020, 13, 2149–2155. [Google Scholar] [CrossRef]
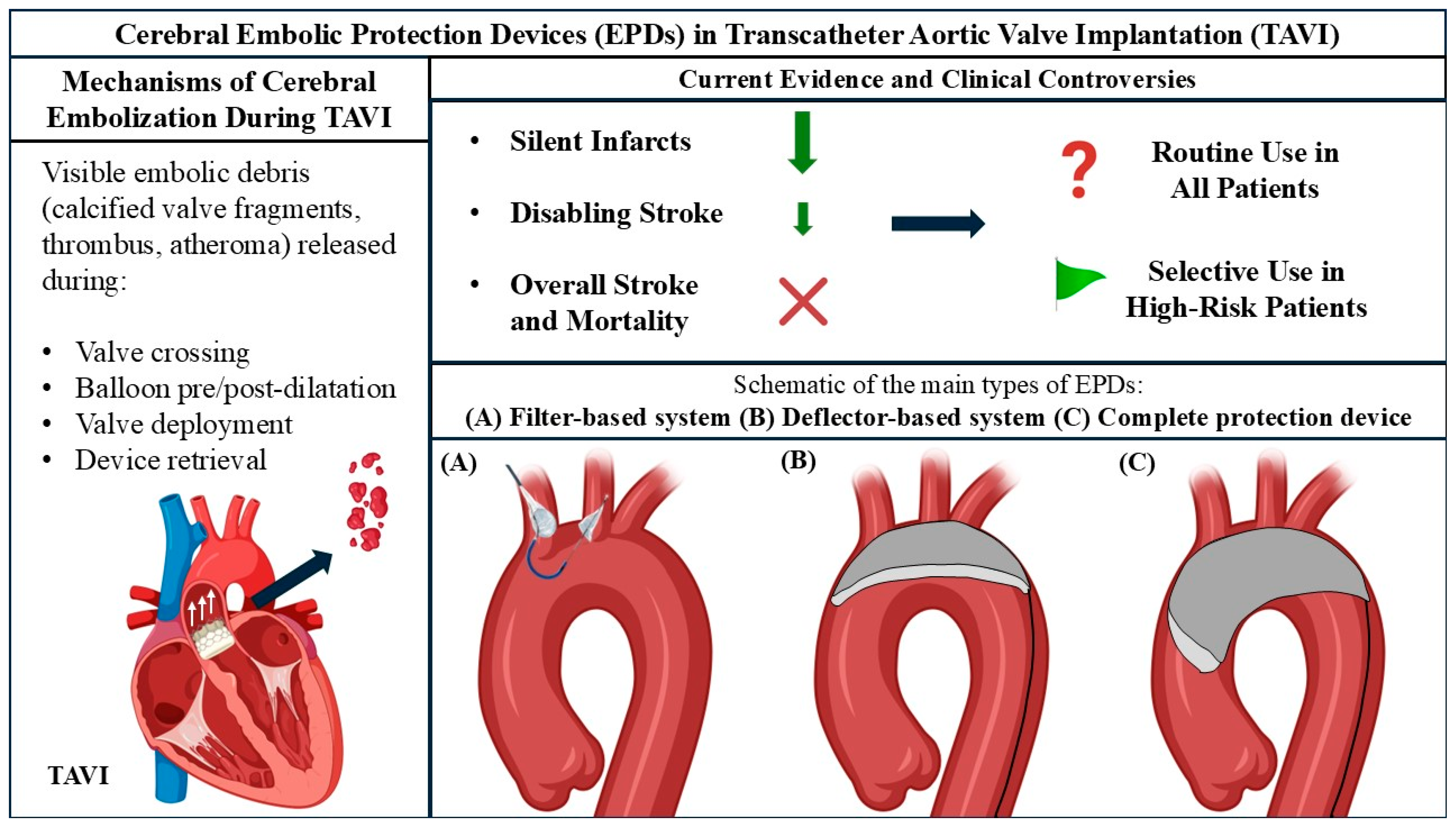
| Device | Cerebral Embolic Protection | Function | Pore Size | Access Site | Access Size | |
|---|---|---|---|---|---|---|
| ProtEmbo Cerebral Protection System |  | Complete | Deflect | 60 μm | Left radial | 6 Fr |
| Sentinel Cerebral Protection System | 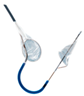 | Partial | Partial capture | 140 μm | Right radial | 6 Fr |
| EMBOL-X Device |  | Complete | Capture and removal | 120 μm | Middle sternotomy | 14 Fr |
| TriGUARD 3 Cerebral Protection Device | 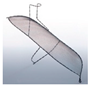 | Complete | Deflect | 115 × 145 μm | Femoral | 8 Fr |
| Embrella Embolic Deflector | 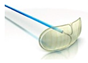 | Partial | Deflect | 100 μm | Radial/ brachial | 6 Fr |
| Emblok Embolic Protection System |  | Complete | Capture and removal | 125 μm | Femoral (contralateral) | 11 Fr |
| Emboliner Total Embolic Protection |  | Complete | Capture and removal | 150 μm | Femoral (contralateral) | 10 Fr |
| POINT-GUARD | 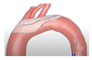 | Complete | Deflect | 105 µm | Femoral | 10 Fr |
| CAPTIS Full-Body Embolic Protection Device | 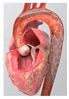 | Complete | Capture and removal | 115 × 145 μm | Femoral | 16 Fr |
| FLOWer |  | Complete | Capture and removal | 70 μm | Femoral | 12 Fr |
| First Author and Year | Device(s) Evaluated | Patient Population | Primary Outcomes | Main Results | Conclusions |
|---|---|---|---|---|---|
| Harmouch et al. (2024) [12] | Sentinel CPS | TAVI patients | Periprocedural stroke | No difference in overall stroke, decreased disabling stroke (RR 0.41, p = 0.02) | Sentinel CPS associated with decreased disabling stroke |
| Tan et al. (2024) [57] | Sentinel CPS |
TAVI patients | Stroke, mortality | Overall: reduced mortality, all stroke, disabling stroke. Randomized data: reduced disabling stroke | Sentinel CPS associated with lower rates of mortality, all stroke, and disabling stroke (overall), disabling stroke (randomized) |
| Heuts et al. (2024) [58] | Various EPD |
TAVI patients | Disabling stroke | RR 0.56 (95% CI, 0.28 to 1.19), unlikely clinically relevant | Beneficial EPD effect likely not clinically relevant for disabling stroke |
| Reddy et al. (2023) [59] | Various EPD |
TAVI patients | Stroke, mortality | No significant difference in all stroke, disabling stroke, non-disabling stroke, or mortality. Sentinel reduced disabling stroke | No significant difference in clinical endpoints with EPDs overall |
| Elfaituri et al. (2023) [60] | Various EPD |
TAVI patients | Total lesion volume, stroke, mortality | No significant difference in total lesion volume, stroke, disabling stroke, or mortality | EPDs did not significantly alter important clinical outcomes |
| Ndunda et al. (2020) [61] | Sentinel CPS |
TAVI patients | Mortality, all stroke, disabling stroke | Significant reductions in mortality, all stroke, and disabling stroke | Sentinel CPS associated with improved outcomes |
| Testa et al. (2018) [62] | Various EPD |
TAVI patients | MACE, mortality, stroke | Reduced MACE, mortality, and stroke with EPD use | EPD use associated with better clinical outcomes |
| Pagnesi et al. (2016) [16] | Various EPD |
TAVI patients | Clinically evident stroke, mortality, silent lesions | Smaller volume of silent lesions, no reduction in number of lesions, very low evidence for clinical stroke/mortality reduction | EPDs may reduce silent lesion volume but lack strong evidence for clinical stroke/mortality benefit |
| Wang et al. (2024) [63] | Various EPD |
TAVI patients | Stroke | I&LCCA type reduced stroke risk, TMCA type did not | I&LCCA EPDs may be more effective than TMCA EPDs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latsios, G.; Ktenopoulos, N.; Apostolos, A.; Koliastasis, L.; Kachrimanidis, I.; Vlachakis, P.K.; Katsaros, O.; Mantzouranis, E.; Tsalamandris, S.; Drakopoulou, M.; et al. Embolic Protection Devices in Transcatheter Aortic Valve Implantation: A Narrative Review of Current Evidence. J. Clin. Med. 2025, 14, 4098. https://doi.org/10.3390/jcm14124098
Latsios G, Ktenopoulos N, Apostolos A, Koliastasis L, Kachrimanidis I, Vlachakis PK, Katsaros O, Mantzouranis E, Tsalamandris S, Drakopoulou M, et al. Embolic Protection Devices in Transcatheter Aortic Valve Implantation: A Narrative Review of Current Evidence. Journal of Clinical Medicine. 2025; 14(12):4098. https://doi.org/10.3390/jcm14124098
Chicago/Turabian StyleLatsios, George, Nikolaos Ktenopoulos, Anastasios Apostolos, Leonidas Koliastasis, Ioannis Kachrimanidis, Panayotis K. Vlachakis, Odysseas Katsaros, Emmanouil Mantzouranis, Sotirios Tsalamandris, Maria Drakopoulou, and et al. 2025. "Embolic Protection Devices in Transcatheter Aortic Valve Implantation: A Narrative Review of Current Evidence" Journal of Clinical Medicine 14, no. 12: 4098. https://doi.org/10.3390/jcm14124098
APA StyleLatsios, G., Ktenopoulos, N., Apostolos, A., Koliastasis, L., Kachrimanidis, I., Vlachakis, P. K., Katsaros, O., Mantzouranis, E., Tsalamandris, S., Drakopoulou, M., Synetos, A., Aggeli, C., Tsioufis, K., & Toutouzas, K. (2025). Embolic Protection Devices in Transcatheter Aortic Valve Implantation: A Narrative Review of Current Evidence. Journal of Clinical Medicine, 14(12), 4098. https://doi.org/10.3390/jcm14124098













